Abstract
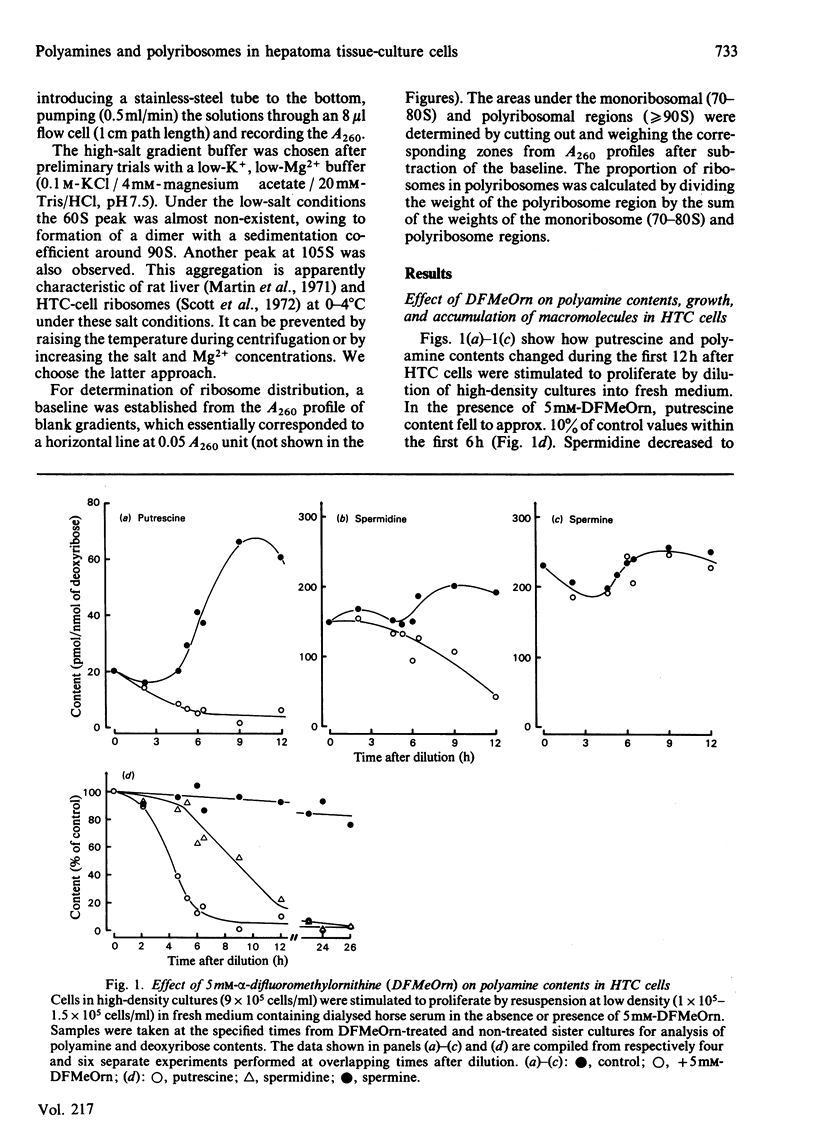
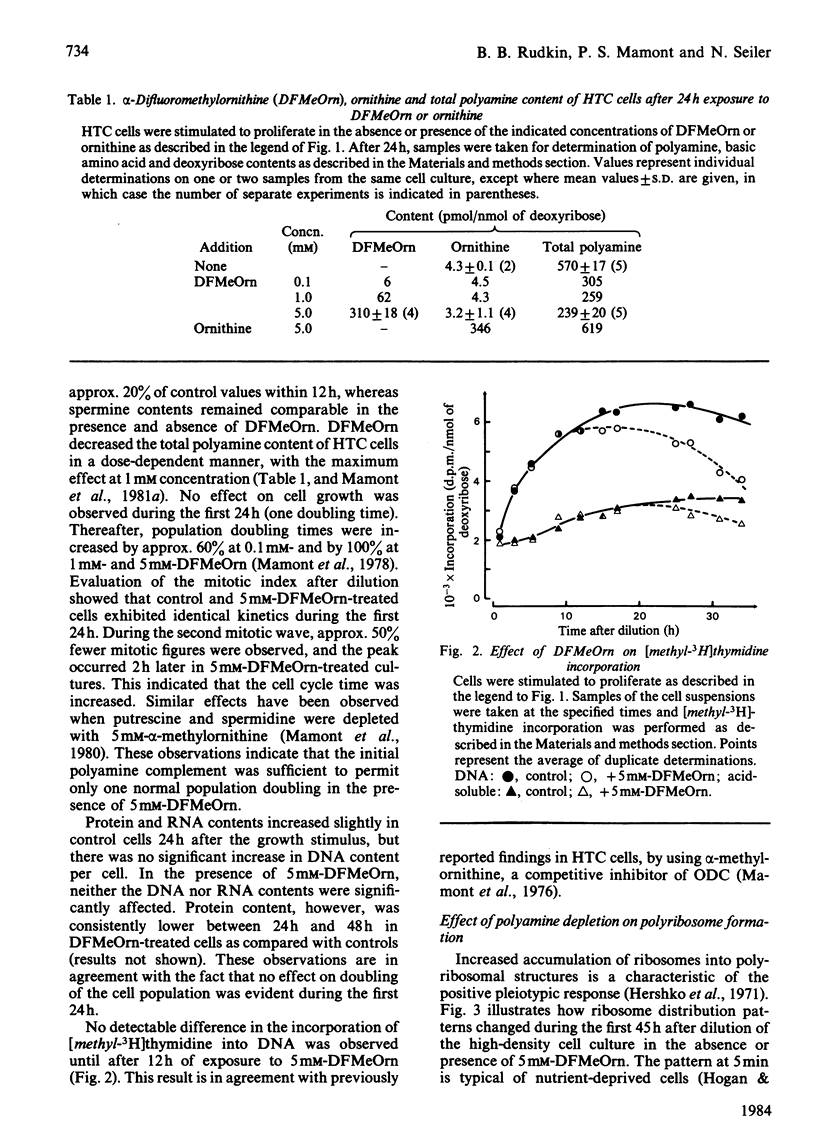
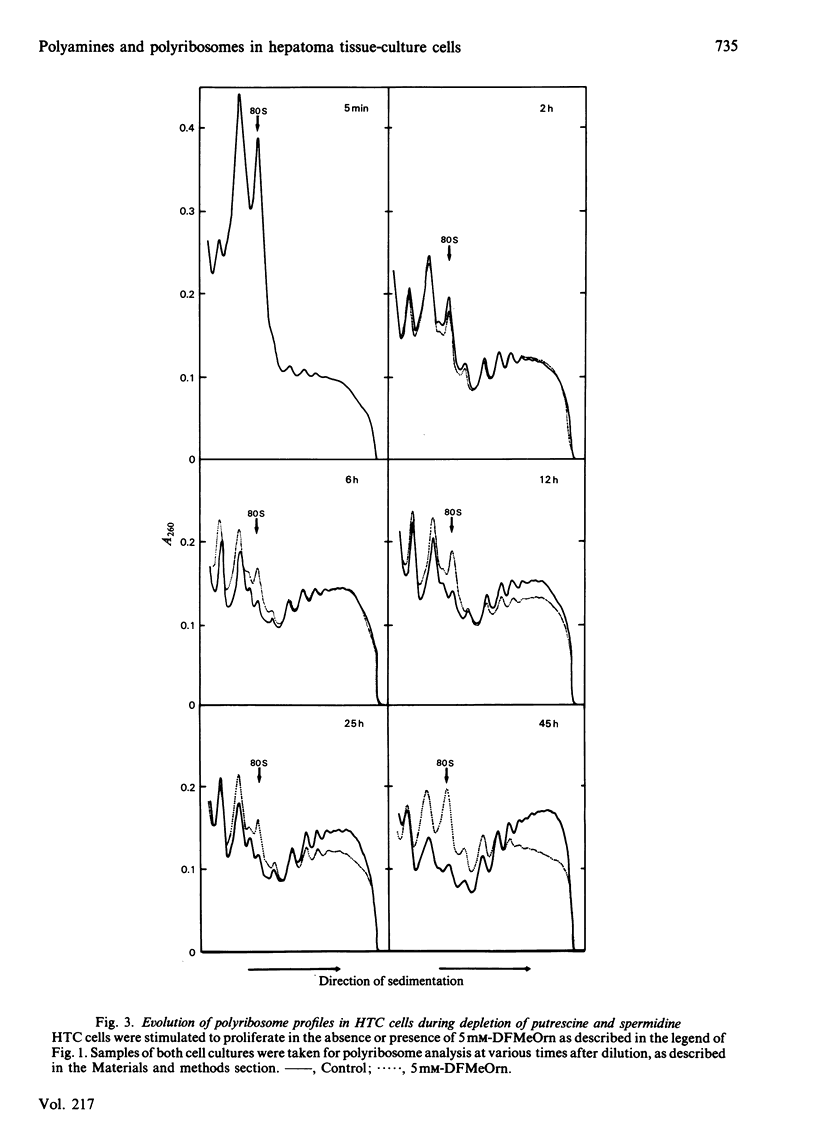
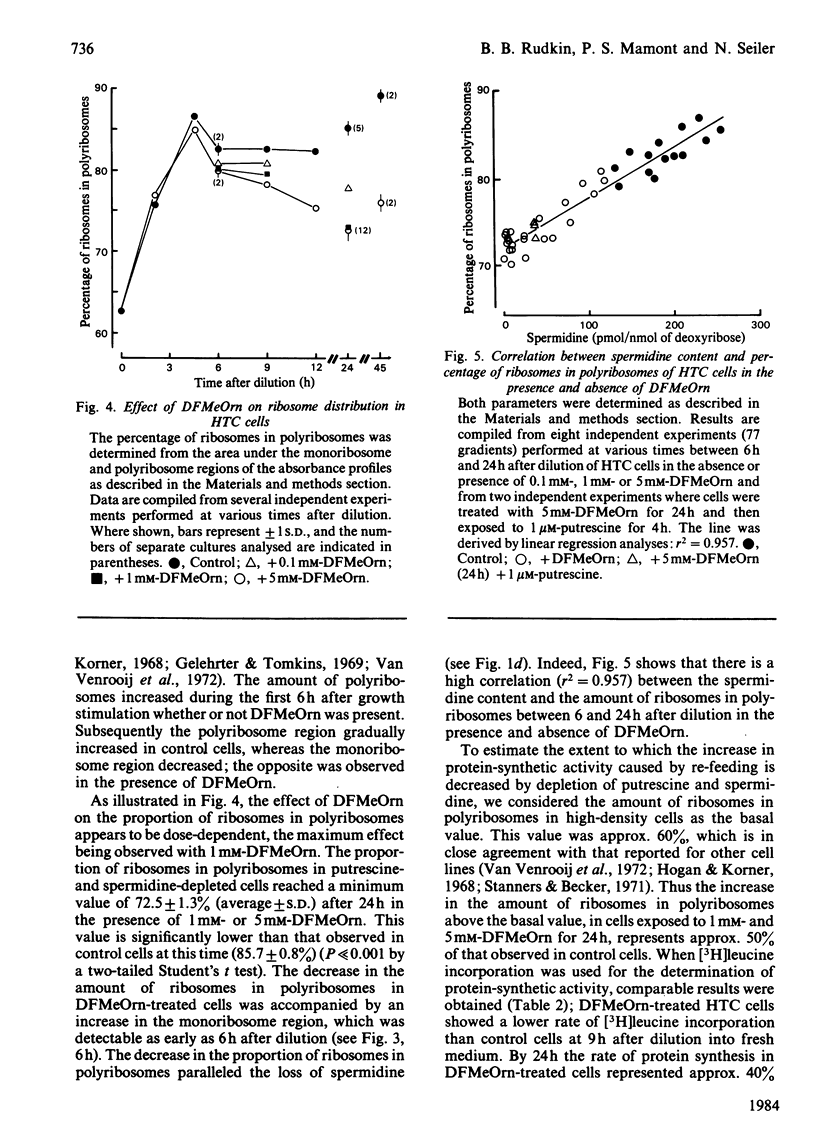
Hepatoma tissue-culture (HTC) cells were exposed to DL-alpha-difluoromethylornithine (DFMeOrn), a specific irreversible inhibitor of ornithine decarboxylase. Concomitantly with the decrease in spermidine, a decrease in the amount of ribosomes in polyribosomes was observed. Spermine concentrations remained essentially comparable with those in cells not exposed to this inhibitor. Exposure of putrescine- and spermidine-depleted HTC cells to spermidine or spermine rapidly reversed the effect of DFMeOrn on polyribosome profiles, whereas addition of putrescine to the cell culture medium had an effect only after its transformation into spermidine and spermine. The results show that the perturbation of polyribosome formation in DFMeOrn-treated HTC cells is due to spermidine deficiency and that a normal polyamine complement is required for optimal protein-synthetic activity in these cells. The results also indicate that protein synthesis is perturbed before DNA synthesis during depletion of putrescine and spermidine in HTC cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A. K., Pihl A. Variable rate of polypeptide chain elongation in vitro. Effect of spermidine. Eur J Biochem. 1980 May;106(1):257–262. doi: 10.1111/j.1432-1033.1980.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Erwin B. G., Pegg A. E. Uptake of alpha-difluoromethylornithine by mouse fibroblasts. Biochem Pharmacol. 1982 Sep 1;31(17):2820–2823. doi: 10.1016/0006-2952(82)90140-x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. Control of tyrosine aminotransferase synthesis in tissue culture by a factor in serum. Proc Natl Acad Sci U S A. 1969 Oct;64(2):723–730. doi: 10.1073/pnas.64.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J., Schechter P. J., Hanke N. F., de Smet Y., Agid Y., Tell G., Koch-Weser J. Concentration gradients of free and total gamma-aminobutyric acid and homocarnosine in human CSF: comparison of suboccipital and lumbar sampling. J Neurochem. 1982 Dec;39(6):1618–1622. doi: 10.1111/j.1471-4159.1982.tb07995.x. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hogan B. L., Korner A. Ribosomal subunits of Landschütz ascites cells during changes in polysome distribution. Biochim Biophys Acta. 1968 Nov 20;169(1):129–138. doi: 10.1016/0005-2787(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Hovi T. Suppression of the formation of polyamines and macromolecules by DL-alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) in phytohaemagglutinin-activated human lymphocytes. Biochem J. 1979 Jan 15;178(1):109–117. doi: 10.1042/bj1780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kojima M., Watanabe Y., Maeda K., Hirose S. Stimulation of polypeptide synthesis by spermidine at the level of initiation in rabbit reticulocyte and wheat germ cell-free systems. Biochem Biophys Res Commun. 1980 Nov 28;97(2):480–486. doi: 10.1016/0006-291x(80)90288-0. [DOI] [PubMed] [Google Scholar]
- Kramer G., Odom O. W., Hardesty B. Polyamines in eukaryotic peptide initiation. Methods Enzymol. 1979;60:555–566. doi: 10.1016/s0076-6879(79)60053-8. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Kornfeld B., Fullerton P., Evans R. Protein synthesis and the presence of absence of a measurable G1 in cultured Chinese hamster cells. J Cell Physiol. 1980 Sep;104(3):461–467. doi: 10.1002/jcp.1041040318. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
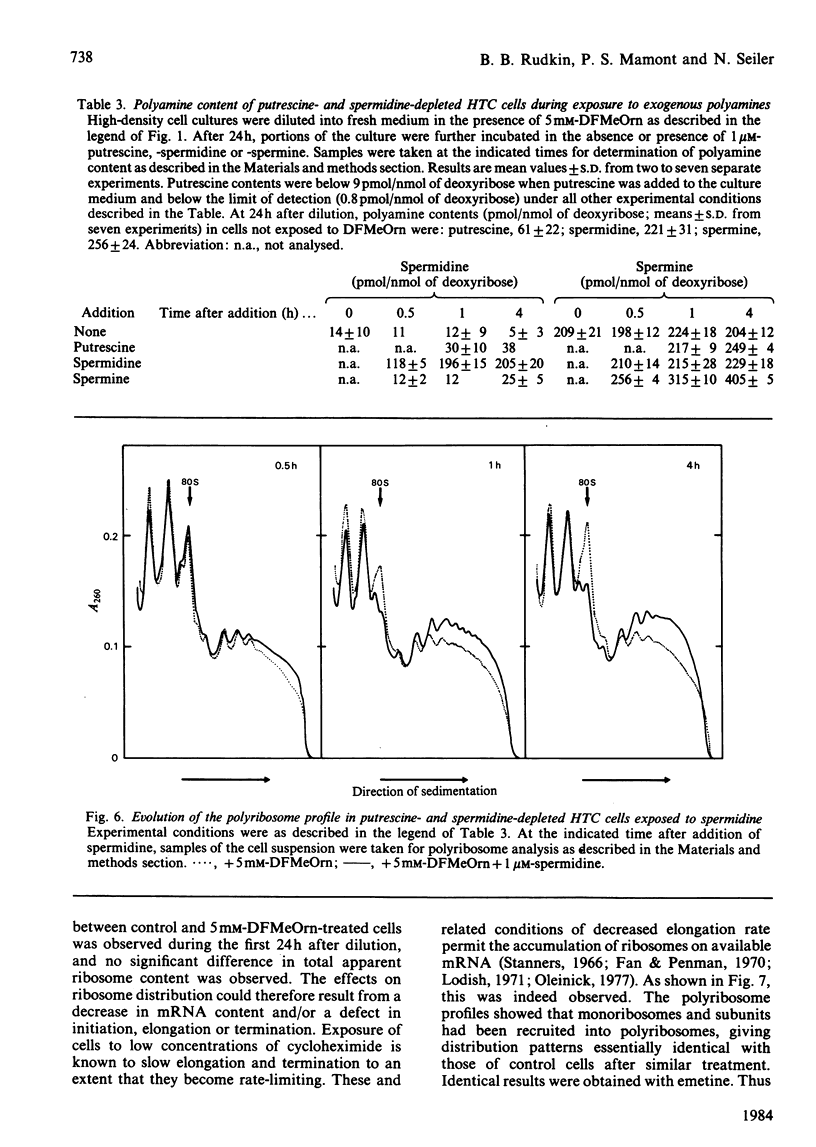
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Seiler N., Siat M., Joder-Ohlenbusch A. M., Knödgen B. Metabolism of acetyl derivatives of polyamines in cultured polyamine-deficient rat hepatoma cells. Med Biol. 1981 Dec;59(5-6):347–353. [PubMed] [Google Scholar]
- Oleinick N. L. Initiation and elongation of protein synthesis in growing cells: differential inhibition by cycloheximide and emetine. Arch Biochem Biophys. 1977 Jul;182(1):171–180. doi: 10.1016/0003-9861(77)90296-x. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., James L. J. Selective killing of transformed baby hamster kidney (BHK) cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4994–4998. doi: 10.1073/pnas.72.12.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Raymondjean M., Bogdanovsky D., Bachner L., Kneip B., Schapira G. Regulation of messenger RNA by a ribonucleic factor in the presence of polyamines. FEBS Lett. 1977 Apr 15;76(2):311–315. doi: 10.1016/0014-5793(77)80175-0. [DOI] [PubMed] [Google Scholar]
- Reichman M., Penman S. The mechanism of inhibition of protein synthesis by 5-azacytidine in HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):282–289. doi: 10.1016/0005-2787(73)90145-7. [DOI] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Hampaï A. Column chromatography of amino acids with fluorescence detection. J Chromatogr. 1973 Aug 29;83:353–356. doi: 10.1016/s0021-9673(00)97051-1. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Shields R., Tomkins G. M. Mechanism of hormonal induction of tyrosine aminotransferase studied by measurement of the concentration of growing enzyme molecules. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2937–2941. doi: 10.1073/pnas.69.10.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
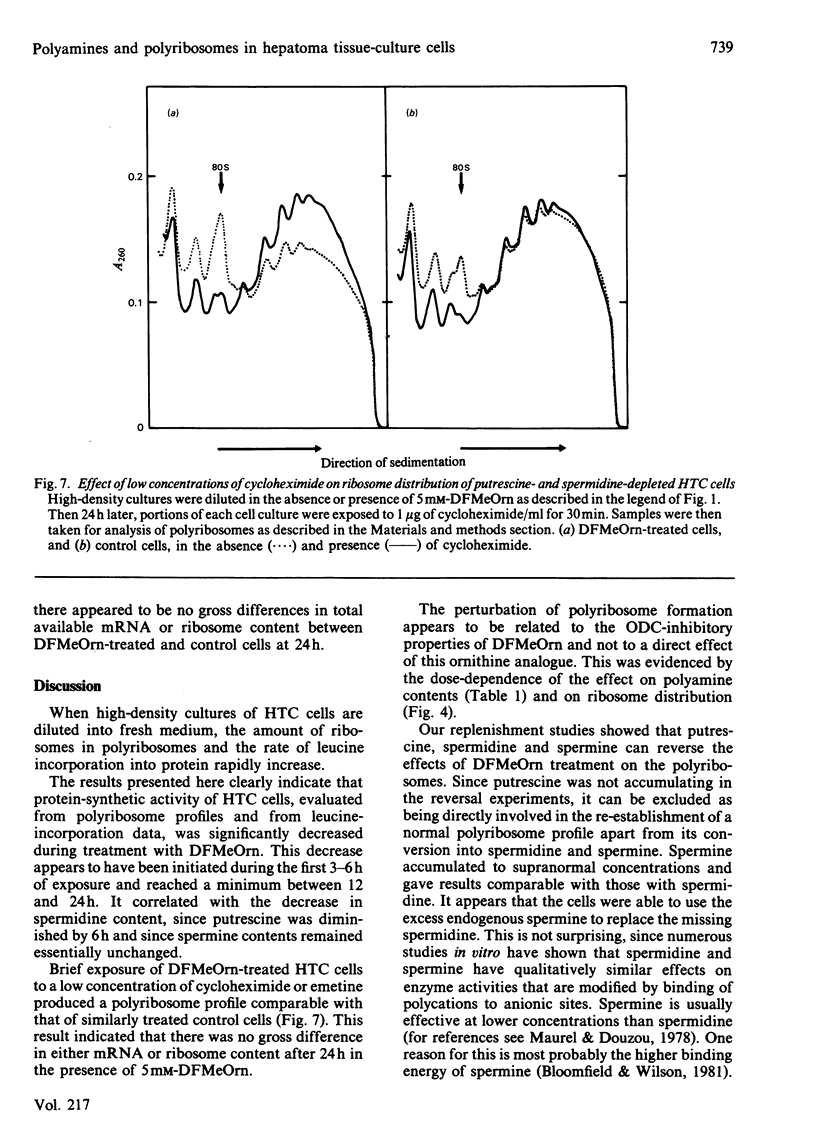
- Seiler N., Schmidt-Glenewinkel T. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J Neurochem. 1975 Apr;24(4):791–795. [PubMed] [Google Scholar]
- Stanners C. P., Becker H. Control of macromolecular synthesis in proliferating and resting Syrian hamster cells in monolayer culture. I. Ribosome function. J Cell Physiol. 1971 Feb;77(1):31–42. doi: 10.1002/jcp.1040770105. [DOI] [PubMed] [Google Scholar]
- Stanners C. P. The effect of cycloheximide on polyribosomes from hamster cells. Biochem Biophys Res Commun. 1966 Sep 8;24(5):758–764. doi: 10.1016/0006-291x(66)90390-1. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tuomi K., Raina A., Mäntyjärvi R. Synthesis of Semliki-forest virus in polyamine-depleted baby-hamster kidney cells. Biochem J. 1982 Jul 15;206(1):113–119. doi: 10.1042/bj2060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Danzin C., Mamont P. Reversed-phase ion-pair liquid chromatographic procedure for the simultaneous analysis of S-adenosylmethionine, its metabolites and the natural polyamines. J Chromatogr. 1982 Feb 12;227(2):349–368. doi: 10.1016/s0378-4347(00)80389-8. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. The effect of high ionic strength on monomers, polyribosomes, and puromycin-treated polyribosomes. Biochim Biophys Acta. 1970 Mar 19;204(1):221–229. doi: 10.1016/0005-2787(70)90505-8. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Henshaw E. C., Hirsch C. A. Effects of deprival of glucose or individual amino acids on polyribosome distribution and rate of protein synthesis in cultured mammalian cells. Biochim Biophys Acta. 1972 Jan 18;259(1):127–137. doi: 10.1016/0005-2787(72)90480-7. [DOI] [PubMed] [Google Scholar]


