Abstract
Background
Perceived HIV risk may impact willingness to initiate PrEP among people who inject drugs (PWID).
Methods
We analyzed baseline data from PrEP eligible PWID in Baltimore, MD. Risk perception was assessed by PWID relative to the average risk of their age group categorized as: higher-than, lower-than, or about average. Participants were informed of PrEP for HIV prevention and asked about their willingness to use daily PrEP. Associations of PrEP indication (categorized as injection risk only vs any sexual risk), perceived HIV risk and non-willingness to use PrEP was assessed using generalized linear models.
Results
Among 489 participants, 61 % were male, 66 % were Black and mean age was 46 years. One-third (35 %) of the participants were aware of PrEP and <1 % had used PrEP in the prior 30 days. Overall, 30 % of PWID reported lower-than-average perceived HIV risk and 18 % reported non-willingness to use PrEP. Participants with injection risk only were more likely (aOR: 2.75; 95 %CI: 1.60 – 4.73) to report having lower-than-average perceived HIV risk compared to those with any sexual risk. Participants with lower-than-average perceived risk were more likely to report non-willingness to use PrEP compared to those with higher perceived risk (adjusted PR: 1.91; 95 %CI: 1.18 – 3.10).
Conclusion
A considerable proportion of PWID eligible for PrEP reported having low risk of HIV acquisition despite being eligible for PrEP. Consistent and tailored PrEP messaging that addresses drug use HIV risk perception may be critical to increasing PrEP uptake among PWID.
Keywords: Pre-exposure prophylaxis, People who inject drugs, HIV, Risk perception, United States
Highlights
-
•
HIV PrEP awareness and use continue to be low among people who inject drugs.
-
•
Risk category for PrEP indication is associated with HIV risk perception among PWID.
-
•
Addressing drug use risk perception may be critical to increasing PrEP uptake.
1. Introduction
People who inject drugs (PWID) continue to be at increased risk of HIV infection. As of 2021, there were approximately 13.2 million PWID worldwide, with 1 in 8 (12 %) of them living with HIV. Globally, injection drug use was responsible for about 10 % of all new HIV infections (UNODC, 2023). In the United States (U.S.), the Centers for Disease Control and Prevention (CDC) estimates that about 7 % of new HIV infections in 2021 were linked to injection drug use (CDC, 2021). PWID have a risk of acquiring HIV that is 35 times higher compared to people who do not inject drugs (UNODC, 2023). Previous data suggest that this increased risk results from risky injection behavior, such as needle and injection equipment sharing, as well as high-risk sexual behavior (Mistler et al., 2021, Williams et al., 2019, Broz et al., 2014, Jo et al., 2020).
Despite proven efficacy for HIV prevention (McCormack et al., 2016, Molina et al., 2015, Choopanya et al., 2013), previous data suggest that Pre-Exposure Prophylaxis (PrEP) uptake among PWID has been suboptimal in the U.S. (McFarland et al., 2020, Sherman et al., 2019, Corcorran et al., 2022). Risk perception is a critical component of behavior change and is defined by Ferrer and Klein as “an individual’s perceived susceptibility to a threat”. Perceptions of risk, whether accurate or inaccurate, can significantly impact health outcomes (Ferrer and Klein, 2015). There is evidence that gaps exist between perception of risk and the objective assessment of risk (Rutledge et al., 2018, Dubin et al., 2019). For individuals at risk for HIV, low self-perceived risk of acquiring HIV has the potential to affect the PrEP care cascade – a framework describing the multiple stages of PrEP engagement (Mayer et al., 2020). Qualitative data among PWID suggests that low perceived risk of HIV may influence interest in PrEP and could potentially be a barrier to PrEP utilization (Bazzi et al., 2018, Biello et al., 2018). In addition, studies among predominantly White PWID enrolled in opioid treatment programs in Connecticut and Massachusetts found an association between high perceived HIV risk and progress along the PrEP cascade such as PrEP awareness, and willingness to use PrEP among PWID (Ni et al., 2021, Stein et al., 2014, Shrestha et al., 2017). Among men who have sex with men (MSM), HIV risk perception has emerged as a critical step of this cascade, influencing PrEP awareness, interest/willingness, PrEP use, adherence, and retention in PrEP care (Nunn et al., 2017, Yellin et al., 2023). Less is known about HIV risk perception and its impact on PrEP willingness among predominantly minority PWID.
CDC indication categories for PrEP use include sexual or injection drug use practices that increase risk of acquiring HIV. Prior research among PWID has explored individual, social and structural factors that contribute to suboptimal uptake (Pleuhs et al., 2022, Schneider et al., 2021, Walters et al., 2022, Dubov et al., 2023), but less is known about the role of PrEP indication category (sexual, injection drug use, or both) on HIV risk perception. To address this gap, this study sought to describe the PrEP cascade and assess the association of PrEP indication category, low perceived HIV risk and unwillingness to use PrEP among PrEP eligible PWID. This analysis utilized quantitative data from a large sample of street-recruited, PWID who use various drug types, regardless of their drug treatment status to improve generalizability of our study findings.
2. Methodology
2.1. Study setting, sample, and recruitment
We analyzed baseline data from individuals enrolled in the INSITE Study, an interventional cluster-randomized trial among people who inject drugs in Baltimore, MD. A detailed description of this study has previously been published (Page et al., 2024). Briefly, 720 PWID were recruited across 12 neighborhood sites or clusters in Baltimore routinely visited by the Baltimore City Health Department syringe service program (SSP). Participants were included in the study if they were 1) 18 years and older, 2) living with HIV and reported a history of injection drug use, 3) not living with HIV and injected drugs for 4 days or more in the previous 30 days, 4) not living with HIV and shared a needle/syringe in the prior 6 months. Neighborhood sites (clusters) were randomly assigned to an experimental arm that consisted of availability of an integrated care van (ICV) to complement the city's mobile SSP or a control arm where no additional services were provided. The goal of the integrated care van is to provide a range of services to PWID including HIV and HCV testing and linkage, PrEP, medication assisted treatment, wound care, case management services and on-site medical management. At enrollment, a biometric capture was completed and data on sociodemographic characteristics including quality of life, drug and alcohol use, injection- and sex-related risk behaviors were collected from study participants using Research Electronic Data Capture (REDCap). Blood samples were collected from study participants for testing of HIV antibody, HIV RNA and tenofovir diphosphate (TFV-DP) concentration. For these analyses, we restricted our sample to PWID who were 18 years or older, not living with HIV, and met at least one of the CDC criteria for PrEP eligibility (sexual risk defined as having more than one sexual partner and inconsistent condom use; injection risk defined as sharing needles or injection drug equipment). All participants included in the study provided written informed consent. The study was approved by the Johns Hopkins Medicine institutional review board.
2.2. Study measures
The PrEP cascade included PrEP awareness, ever discussing PrEP with a health professional, any prescription of PrEP ever and PrEP use in the prior 30 days. PrEP awareness was determined based on whether participants had heard of PrEP before study enrollment. For participants who were aware of PrEP, discussions related to PrEP with a health professional, prior PrEP prescriptions and PrEP use in the prior 30 days was determined based on “Yes” or “No” responses to the questions “Have you ever talked to a doctor or another medical provider about taking PrEP?”, “Have you ever been prescribed PrEP to prevent HIV (a medicine called Truvada)?” and “When is the last time you took PrEP?”, respectively. PrEP use in the prior 30 days was confirmed quantitatively using dried blood spot samples with TFV-DP values at baseline. The following cut-offs were used for adherence interpretation of TFV-DP values: <450 fmol/punches, <2 doses/week; 450–949 fmol/punches, 2–3 doses/week; 950–1799 fmol/punches, 4–6 doses/week; and ≥1800 fmol/punches, 7 doses/week (Yager et al., 2020).
PrEP indication category was determined using CDC’s eligibility criteria for PrEP and dichotomized for this analysis as injection risk only (defined as having a HIV-positive injecting partner or sharing injection equipment) and any sexual risk (defined as having one or more of the following 1) an HIV-positive sexual partner, 2) a history of a bacterial STI in past 6 months, or 3) a history of inconsistent or no condom use with sexual partner(s)).
HIV risk perception was a trichotomous variable based on response to the following question “Compared with other people your age in Baltimore, would you say your risk of getting HIV is lower than average, about average, or higher than average?”.
Willingness to use PrEP was assessed after participants were told the following: “There is evidence from clinical studies that people who are HIV-negative, but are at risk for HIV, can reduce their risk by taking PrEP every day (a medication called Truvada).” Participants were then asked, “Assuming PrEP didn't cost you any money, would you be willing to take a medicine every day to reduce your risk of getting HIV?” Responses were reported as “No chance”, “Very little chance”, “Some chance”, or “Very good chance”. For this analysis, responses were dichotomized, and participants were classified as unwilling to use PrEP if they reported “no chance” or “very little chance” of using PrEP.
Sociodemographic characteristics included in our analyses to describe our study sample were age, race (White, Black, other), ethnicity (Hispanic, non-Hispanic), sexual orientation (heterosexual, gay, bisexual, lesbian, other), birth sex (male, female), educational level (high school and above, less than high school), marital status (married, never married, divorced/separated, widowed), history of incarceration, SSP use prior to the study survey (“never”, within the last 7 days, “within the last 30 days”, “within the last 6 months”, and “more than 6 months ago”) and having health insurance assessed by self-report. We categorized housing based on prior work by Gonzalez Corro et al. as “stable”, “unstable” and “undomiciled” (Gonzalez Corro et al., 2024). For behavioral risk factors, we included age at first injection drug use, injection drug type in the prior six months and hazardous alcohol use. Hazardous alcohol use was defined based on the results of the AUDIT-C (score ≥3 for female and ≥4 for males) (Bush et al., 1998).
2.3. Statistical analysis
Descriptive statistics were used to summarize participants' sociodemographic and behavioral risk characteristics and presented as frequencies and proportions. The student’s t-test evaluated differences in continuous variables, respectively. Pearson’s chi-square and Fisher’s exact tests were used to assess differences in categorical variables. Multinomial logistic regression was used to estimate the association of PrEP indication category and HIV risk perception. Among PrEP eligible PWID, we used a log-binomial regression to estimate the association of HIV risk perception and unwillingness to use PrEP. We chose log-binomial regression because logistic regression would have overestimated the risk of the outcome (>10 % of our sample were unwilling to use PrEP). Covariates included in our adjusted models were age, race, birth sex, sexual orientation and educational level. Covariates were selected a priori based on their hypothesized relationship with the exposure and outcome variable. Odds ratios (for multinomial logistic regression), prevalence ratios (for log-binomial regression) and 95 % confidence intervals (CI) were estimated using robust variance estimator. All analyses were done using Stata version 18.0 (StataCorp LLC, College Station, TX).
3. Results
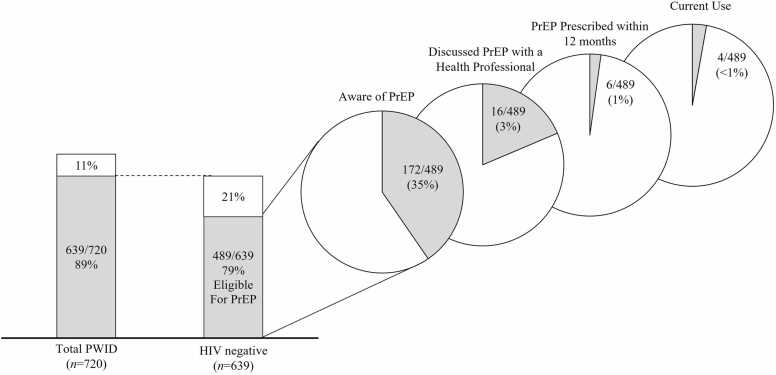
Of the 720 individuals enrolled in the INSITE study, 639 (89 %) had a negative HIV test result and 489 (68 %) were eligible for PrEP at baseline.
3.1. Participant characteristics
Among 489 PWID eligible for PrEP in this study, 61 % were male, 89 % were heterosexual, 67 % were Black and 97 % were non-Hispanic. The mean age of participants was 46 years old (standard deviation [SD] = 11). Two-thirds (65 %) of the participants had a high school diploma and reported never being married. The mean age that participants first injected drugs was 23 (SD = 8). The most commonly reported injection drugs used in the 6 months prior to the survey were heroin and cocaine used concurrently/speedball (76 %), heroin alone (97 %) and cocaine alone (68 %). Less than a third of participants reported injecting fentanyl (31 %), other prescription opioids (32 %), benzodiazepines (25 %), or amphetamines (18 %). Over half (57 %) of the participants met the criteria for hazardous alcohol use. The majority reported prior incarceration (88 %). SSP use prior to the study survey was reported as follows: within 7 days (38 %), within 30 days (21 %), within 6 months (13 %), more than 6 months ago (11 %), and never (17 %). A majority of participants reported being undomiciled (9 %) or having unstable housing (46 %) (Table 1).
Table 1.
Participant characteristics, self-perceived risk of HIV and PrEP indication category among PWID eligible for PrEP in Baltimore MD.
| Sociodemographic characteristicsa | Total | PrEP Indication Category |
HIV Risk Perceptionc |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Any Sexual Risk | Only Injection Risk | p-value | Higher than average | About average | Lower than average |
|||
| N=489 | N=178 | N=311 | N=163 | N=174 | N=148 | |||
| Age, mean (SD) | 46 (11) | 44 (10) | 47 (11) | 0.002⁎ | 45 (11) | 45 (11) | 48 (10) | 0.021⁎ |
| Birth Sex | <0.001⁎ | 0.014⁎ | ||||||
| Male | 294 (61) | 80 (45) | 211 (71) | 84 (52) | 113 (65) | 97 (66) | ||
| Female | 191 (39) | 98 (55) | 85 (29) | 79 (48) | 61 (35) | 51 (34) | ||
| Sexual Orientation | <0.001⁎ | 0.16 | ||||||
| Heterosexual | 433 (89) | 137 (77) | 296 (95) | 138 (85) | 162 (93) | 129 (87) | ||
| Homosexual/Bisexual/Otherb | 56 (11) | 41 (23) | 15 (5) | 25 (15) | 12 (7) | 19 (13) | ||
| Race | 0.14 | 0.019⁎ | ||||||
| White | 136 (28) | 56 (31) | 80 (26) | 47 (29) | 61 (35) | 27 (18) | ||
| Black | 328 (67) | 110 (62) | 218 (70) | 108 (66) | 106 (61) | 111 (75) | ||
| Othersf | 25 (5) | 12 (7) | 13 (4) | 8 (5) | 7 (4) | 10 (7) | ||
| Hispanic | 13 (3) | 8 (4) | 5 (2) | 0.078 | 4 (2) | 5 (3) | 4 (3) | 0.97 |
| Educational Level | 0.52 | 0.44 | ||||||
| Less than High School | 171 (35) | 59 (33) | 112 (36) | 57 (35) | 66 (38) | 46 (31) | ||
| High School and Above | 318 (65) | 119 (67) | 199 (64) | 106 (65) | 108 (62) | 102 (69) | ||
| Marital Status | 0.001⁎ | 0.46 | ||||||
| Married | 61 (12) | 12 (7) | 49 (16) | 21 (13) | 21 (12) | 18 (12) | ||
| Never Married | 317 (65) | 129 (72) | 188 (60) | 101 (62) | 117 (67) | 96 (65) | ||
| Divorced/Separated | 85 (17) | 33 (19) | 52 (17) | 30 (18) | 32 (18) | 23 (16) | ||
| Widowed | 26 (5) | 4 (2) | 22 (7) | 11 (7) | 4 (2) | 11 (7) | ||
| Housingd | 0.40 | 0.21 | ||||||
| Stable | 217 (44) | 83 (47) | 134 (43) | 70 (43) | 77 (44) | 67 (45) | ||
| Unstable | 227 (46) | 76 (43) | 151 (49) | 80 (49) | 74 (43) | 72 (49) | ||
| Undomiciled | 45 (9) | 19 (11) | 26 (8) | 13 (8) | 23 (13) | 9 (6) | ||
| Alcohol Usec | 0.057 | 0.25 | ||||||
| No/low Risk | 211 (43) | 65 (37) | 146 (47) | 79 (48) | 68 (39) | 62 (42) | ||
| Moderate/high risk | 277 (57) | 113 (63) | 164 (53) | 84 (52) | 106 (61) | 85 (57) | ||
| History of Incarceration | 428 (88) | 153 (86) | 275 (88) | 0.43 | 141 (87) | 152 (87) | 131 (89) | 0.87 |
| Age at 1st IDU, mean (SD) | 23 (8) | 22 (7) | 23 (8) | 0.25 | 22 (7) | 23 (8) | 24 (9) | 0.069 |
| Have health insurance | 436 (90) | 158 (90) | 278 (89) | 0.89 | 145 (90) | 154 (89) | 134 (91) | 0.90 |
| SSP Use | 0.561 | 0.424 | ||||||
| Never | 81 (17) | 35 (20) | 46 (15) | 26 (16) | 33 (19) | 21 (14) | ||
| Within the last 7 days | 187 (38) | 64 (36) | 123 (39) | 66 (40) | 58 (32) | 61 (31) | ||
| Within the last 30 days | 103 (21) | 40 (23) | 63 (20) | 25 (15) | 41 (24) | 37 (25) | ||
| Within the last 6 months | 66 (14) | 20 (11) | 46 (15) | 28 (17) | 21 (12) | 16 (11) | ||
| More than 6 months ago | 52 (11) | 19 (10) | 33 (11) | 18 (12) | 21 (12) | 13 (9) | ||
| Injection drug typee | ||||||||
| Heroin and Cocaine (speedball) | 373 (76) | 142 (80) | 231 (74) | 122 (75) | 133 (76) | 115 (78) | ||
| Heroin alone | 476 (97) | 174 (98) | 302 (97) | 158 (97) | 171 (98) | 143 (97) | ||
| Cocaine alone | 331 (68) | 130 (73) | 201 (65) | 110 (67) | 123 (71) | 95 (64) | ||
| Fentanyl | 152 (31) | 61 (34) | 91 (29) | 53 (33) | 48 (28) | 49 (33) | ||
| Prescription opioids (excluding Fentanyl) | 156 (32) | 60 (34) | 96 (31) | 52 (32) | 51 (29) | 51 (34) | ||
| Amphetamine/ Methamphetamine | 86 (18) | 36 (20) | 50 (16) | 25 (15) | 33 (19) | 26 (18) | ||
| Benzodiazepines | 122 (25) | 46 (26) | 76 (24) | 48 (29) | 35 (20) | 37 (25) | ||
Abbreviations: IQR, Interquartile range; SD, standard deviation
Variable distribution are reported as n (column %) unless otherwise specified
Others included Lesbian and Queer
Variables with missing responses HIV risk perception = 4; alcohol use = 1
Stable housing defined as owning a home (condo or house), renting an apartment/house (alone or with others) or a single room; unstable housing defined as staying with family or friends, in a drug treatment/residential program, or in a shelter; undomiciled defined as being homeless (living on street, park, abandoned building) or other (Gonzalez Corro et al., 2024).
Not mutually exclusive
Others included Asian, American Indian/Alaskan Native, Native Hawaiian or Pacific Islander and more than one race
Significant P-values <0.05
3.2. PrEP cascade
Among the 489 PWID eligible for PrEP, 317 (65 %) were unaware of PrEP, 172 (35 %) were aware of PrEP, 16 (3 %) had ever discussed PrEP with a health professional, 6 (1 %) were ever prescribed and 4 (<1 %) had used PrEP in the prior 30 days (Fig. 1). Half (2/4) of the participants that reported recent PrEP use had TFV-DP concentrations <450 fmol/punch consistent with less than two doses/week of emtricitabine co-formulated with tenofovir disoproxil fumarate (F/TDF) over the preceding 12 weeks. One participant had TFV-DP concentrations between 450 – 949 fmol/punch consistent with 2–3 F/TDF doses/week, while the other had concentrations between 950 – 1799 fmol/punch consistent 4–6 F/TDF doses/week over the preceding 12 weeks.
Fig. 1.
PrEP eligibility and PrEP cascade among people who inject drugs in Baltimore, MD (2018).
3.3. PrEP indication category and HIV risk perception
Overall, 64 % of PrEP eligible PWID had risk of HIV acquisition only through injection drug use and 36 % of participants had risk of HIV acquisition through sex (9 % had sexual risk alone and 27 % had both injection and sexual risk). Participants reported their HIV risk perception as higher than average (33 %), about average (36 %) and lower than average (30 %).
In unadjusted analysis, participants with only injection risk were more likely to report lower-than-average (vs higher-than-average) perceived risk of HIV infection (OR: 2.80; 95 % CI: 1.70 – 4.61; p < 0.001) compared to those with any sexual risk. Adjusted multinomial regression revealed that PWID with only injection risk were more likely to report lower-than-average (vs higher-than-average) perceived risk (aOR: 2.75; 95 % CI: 1.60 – 4.73; p < 0.001) compared to those with any sexual risk (Table 2). The association between PrEP indication category and average (vs higher-than-average) perceived risk was not significant in both unadjusted (OR: 1.04; 95 % CI: 0.68 – 1.61 p = 0.849) and adjusted analyses (aOR: 0.83; 95 % CI: 0.53 – 1.31; p = 0.435).
Table 2.
Association of PrEP indication category with lower than and about average HIV Risk perception relative to higher-than-average perceived risk of HIV infection.
| HIV risk perception among PWIDa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lower-than-average risk perception |
About average risk perception |
||||||||
| OR | 95 % CI | aORb | 95 % CI | OR | 95 % CI | aORb | 95 % CI | ||
| PrEP Indication category | |||||||||
| Any sexual risk | ref | - | ref | ||||||
| Injection risk only | 2.80⁎ | 1.70 – 4.61 | 2.75⁎ | 1.60 – 4.73 | 1.04 | 0.68 – 1.61 | 0.83 | 0.53 – 1.32 | |
| Age | 1.02 | 0.99 – 1.04 | 0.99 | 0.97 – 1.02 | |||||
| Race | |||||||||
| White | ref | - | - | - | ref | - | - | - | |
| Black | 1.45 | 0.78 – 2.72 | 0.74 | 0.45 – 1.24 | |||||
| Others | 2.50 | 0.87 – 7.24 | 0.77 | 0.27 – 2.26 | |||||
| Birth sex | |||||||||
| Male | ref | - | - | - | ref | - | - | - | |
| Female | 0.63 | 0.38 – 1.05 | 0.62* | 0.39 – 0.98 | |||||
| Educational level | |||||||||
| Less than High School | ref | - | - | - | ref | - | - | - | |
| High school and above | 1.27 | 0.78 – 2.08 | 0.86 | 0.54 – 1.36 | |||||
| Sexual orientation | |||||||||
| Heterosexual | ref | - | - | - | ref | - | - | - | |
| Homosexual/Bisexual/Other | 1.70 | 0.78 – 3.70 | ref | 0.47 | 0.22 – 1.02 | ||||
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence intervals; ref, reference group
Lower-than average and about average risk perception compared to higher-than-average HIV risk perception
Multinomial logistic regression adjusted for age, race, birth sex, sexual orientation, educational level
Significant P value: <0.05
3.4. HIV risk perception and unwillingness to use PrEP
Overall, 18 % of PWID eligible for PrEP reported unwillingness to use PrEP. In unadjusted analysis, PWID with lower-than-average perceived risk were more likely to be unwilling to use PrEP (PR: 1.93; 95 % CI: 1.23 – 3.01; p = 0.004) compared to PWID with higher-than-average perceived risk. This association remained unchanged in an adjusted log-binomial regression model (adjusted PR: 1.91; 95 % CI: 1.18 – 3.10; p = 0.008) for unwillingness to use PrEP in participants with lower-than-average perceived HIV risk compared to those with higher-than-average perceived HIV risk (Table 3).
Table 3.
Association of HIV risk perception and unwillingness to use PrEP.
| PR | 95 % CI | adjusted PRa | 95 % CI | |
|---|---|---|---|---|
| HIV Risk perception | ||||
| Higher-than-average risk | ref | - | - | - |
| About average risk | 0.76 | 0.44 – 1.32 | 0.75 | 0.43 – 1.32 |
| Lower-than-average risk | 1.93⁎ | 1.23 – 3.01 | 1.90⁎ | 1.20 – 3.02 |
| Age | 0.99 | 0.96 – 1.01 | ||
| Race | ||||
| White | ref | |||
| Black | 1.40 | 0.77 – 2.55 | ||
| Others | 1.52 | 0.70 – 3.30 | ||
| Birth sex | ||||
| Male | ref | |||
| Female | 1.10 | 0.73 – 1.66 | ||
| Educational level | ||||
| Less than High School | ref | |||
| High school and above | 1.21 | 0.79 – 1.86 | ||
| Sexual orientation | ||||
| Heterosexual | ref | |||
| Homosexual/Bisexual/Other | 0.62 | 0.30 – 1.28 |
Abbreviations: PR, prevalence ratio; CI, confidence intervals; ref, reference group
Log-binomial regression adjusted for age, race, birth sex, sexual orientation, educational level
Significant P value: <0.05
The associations between unwillingness to use PrEP and average (compared to higher-than-average) perceived HIV was not significant in unadjusted (PR: 0.76; 95 % CI: 0.44 – 1.32; p = 0.324) and adjusted analyses (aPR: 0.75; 95 % CI: 0.43 – 1.32; p = 0.324).
4. Discussion
Our study demonstrated that a considerable proportion (65 %) of PrEP eligible PWID were unaware of PrEP, and less than 1 % had used PrEP in the prior 30 days. PWID at risk for HIV through unsafe injection behavior were more likely to underestimate their HIV risk compared to those with any sexual risk, highlighting a critical gap in risk assessment (subjective vs. objective). This low perceived risk was significantly associated with unwillingness to use PrEP among PWID and may contribute to critical gaps in engagement across the PrEP cascade including low PrEP awareness, missed opportunities for discussions about PrEP with health professionals, and low PrEP prescription rates and use among PWID accessing SSP services highlighted in our study.
While most of our study participants reported at least an average perceived risk of HIV, a considerable proportion reported low perceived risk, despite meeting the eligibility criteria for PrEP. This underestimation of risk aligns with findings of a study in a Southwestern US city, where PWID accessing a mobile outreach service similarly had lower perceived risk for HIV acquisition despite reporting high risk behaviors (Champion and Recto, 2023). There may be several reasons for the low perceived risk among PWID observed in our study. One possible explanation for this low perceived risk is the concept of “risk multiplexity”. Previous studies have suggested that the presence of multiple intersecting risk factors heightened individuals' awareness of their vulnerability to HIV (Koku and Felsher, 2020). However, for some PWID, focusing on only one type of risk—such as injection-related risk—may lead to an underestimation of their overall HIV risk. Another contributing factor may be the success of harm reduction interventions, such as syringe service programs (SSPs), which have been associated with significant reduction in HIV transmission among PWID (Platt et al., 2017, Fernandes et al., 2017). Over time, this reduction in HIV transmission rates may be accompanied by low perception of HIV risk. However, in the absence of high coverage of harm reduction or HIV prevention services, the introduction of HIV into networks of PWID was found to be associated with rapid spread of HIV among PWID as illustrated by recent outbreaks in Indiana and West Virginia (Atkins et al., 2020, Conrad et al., 2015, Furukawa et al., 2022). A third potential reason for low perceived risk may relate to how HIV prevention efforts have evolved over the years. While network-based interventions among PWID to promote behavior changes or increase PrEP uptake have been on the rise, PrEP marketing, education programs or campaigns aimed at improving risk perception and PrEP use have historically been contextualized to sexual and gender minority groups (Bazzi et al., 2018). This type of marketing could be a potential barrier to PrEP uptake among PWID at high risk of HIV as qualitative data from Boston and Providence reveals that participants often link PrEP with being MSM (Biello et al., 2018). Indeed, these traditional approaches at PrEP marketing may not adequately address the needs of PWID who only face injection-related risks. Our findings suggest that behavior change programs focused on HIV risk may need to be expanded to include tailored PrEP messaging related to risky injection behavior and contextualized to PWID.
In our analysis, the significant association between perceived HIV risk among PWID and unwillingness to use PrEP is consistent with prior data. For instance, although not restricted to PrEP eligible PWID, a study in Connecticut revealed that individuals with higher perceived HIV risk were over twice as likely to be willing to initiate PrEP, compared to those with no perceived risk for HIV (Ni et al., 2021). Similarly, another study in Connecticut among people with opioid use disorder enrolled in a methadone program found this likelihood to be four to eight times higher (Shrestha et al., 2017). Prior research suggests that while interventions such as receipt of sterile syringes from SSPs may contribute to this low perceived HIV risk by mitigating needle sharing, PWID frequently engaged in high-risk behaviors, such as needle sharing and condomless sex, when faced with pressing socioeconomic and psychological needs (Biello et al., 2018). Therefore, consistent messaging is critical to ensure that PWID are accurately aware of their HIV risks. Taken together, these findings underscore the importance of accurate risk perception on willingness to use PrEP among PWID.
Our finding of low PrEP awareness (35 %) is consistent with an earlier survey among PWID accessing SSP services in Baltimore, where only 28 % of respondents eligible for PrEP were aware of PrEP (Sherman et al., 2019). Though not specifically among PWID eligible for PrEP, data from the CDC National HIV Behavioral Surveillance system estimates that in 2022, 35 % of PWID sampled across 20 US cities were aware of PrEP. Unfortunately, among those aware of PrEP, only a small number of participants had discussed PrEP with a health professional, suggesting missed opportunities for linkage to PrEP among PWID. Multiple factors across socioecological levels including competing health needs and other priorities that arise due to drug use or dependence, PrEP-related stigma and concerns of medication side effects, may all contribute to this gap (Biello et al., 2018). Further, previous data suggest that provider-related factors including provider knowledge about PrEP, attitude towards PWID, implicit bias and prescription practices are potential barriers to PrEP care for PWID (Biello et al., 2018, Dubov et al., 2023). In a quantitative study conducted in San Francisco, only 15 % of the 340 PWID who had seen a health provider in the past 12 months reported discussing PrEP (Vincent and McFarland, 2022). In another study among PWID accessing mobile syringe exchange program services in New Jersey, barriers to PrEP use included partner disclosure issues (51 %), lack of health insurance (33 %) and feelings of embarrassment (45 %) or anxiety (52 %) (Roth et al., 2018). Consequently, it is unsurprising that we found low PrEP use among participants in our study which also aligns with data from a review by Mistler et al., where PrEP uptake ranged from non-existent to 3 % among PWID (Mistler et al., 2021). Our data suggest that gaps persist across stages of the PrEP care cascade, ranging from factors contributing to inaccurate perceived HIV risk to those preventing PrEP use.
While the majority of PWID in our study expressed willingness to use PrEP, data among MSM suggests that willingness to use PrEP differs from the intention to use PrEP (Rendina et al., 2017). This discrepancy may be another potential reason for low PrEP uptake in our study. We recognize that increased risk perception or willingness to use PrEP alone may not be sufficient to change behavior that is required to improve PrEP uptake. Thus, longitudinal studies may be needed to explore the association between risk perception, willingness to use PrEP and actual PrEP use among PWID.
There are key limitations to consider from our study. First, due to the cross-sectional design of our study, causality cannot be established between PrEP indication category and HIV risk perception or between HIV risk perception and willingness to use PrEP. Second, our study design involving the use of self-reported measures for assessing main study outcomes was susceptible to social desirability and recall bias. While the outcome and exposure measures used in this study are based on prior research, the lack of a validated measure for risk perception and willingness to use PrEP is a limitation. In addition, the categorization of risk perception and willingness in our analysis simplifies the complexity of these measures. This approach may not capture the nuanced ways in which individuals assess their risk or willingness to use PrEP and may oversimplify the diversity of experiences among participants. Lastly, the study sample was recruited adjacent to SSP sites which might affect the generalizability of the findings to all PWID. Given that this setting may attract individuals who are more likely to engage with harm reduction services, our sample might not reflect the broader population of PWID, particularly those who are less connected to health or harm reduction services.
5. Conclusion
Overall, our findings suggest that considerable gaps still exist across the PrEP cascade among PWID. Strategies such as tailored PrEP messaging depicting the experiences of PWID and benefit of PrEP for HIV prevention among PWID may improve perception of HV risk from drug use among PWID. Examples include educational campaigns such as infomercials, posters or brochures on social media, websites, or at locations PWID access services such as SSPs. In addition, approaches that leverage information sharing within social networks of PWID may be effective in spreading accurate information about HIV risk and utility of PrEP for PWID. Indeed, drug use HIV risk perception may be critical to improving PrEP uptake among PWID.
Role of funding source
The research presented in this paper was made possible through grants from the National Institute on Drug Abuse (R01DA045556, K24DA035684) of the National Institutes of Health and the Johns Hopkins Center for AIDS Research (P30AI094189). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Tarfa Verinumbe: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Susan G. Sherman: Funding acquisition, Writing – review & editing. Oluwaseun Falade-Nwulia: Conceptualization, Methodology, Writing – review & editing. Miles Landry: Data curation, Writing – review & editing. Kathleen R. Page: Funding acquisition, Writing – review & editing. Katie Zook: Data curation, Project administration, Writing – review & editing. Brian Weir: Methodology, Writing – review & editing. Gregory M. Lucas: Funding acquisition, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- Atkins A., McClung R.P., Kilkenny M., Bernstein K., Willenburg K., Edwards A., Lyss S., Thomasson E., Panneer N., Kirk N., Watson M., Adkins E., DiNenno E., Hogan V., Neblett Fanfair R., Napier K., Ridpath A.D., Perdue M., Chen M., Oster A.M. Notes from the field: outbreak of human immunodeficiency virus infection among persons who inject drugs - cabell county, West Virginia, 2018-2019. MMWR Morb. Mortal Wkly Rep. 2020;69(16):499–500. doi: 10.15585/mmwr.mm6916a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi A.R., Biancarelli D.L., Childs E., Drainoni M.L., Edeza A., Salhaney P., Mimiaga M.J., Biello K.B. Limited knowledge and mixed interest in pre-exposure prophylaxis for HIV prevention among people who inject drugs. AIDS Patient Care STDS. 2018;32(12):529–537. doi: 10.1089/apc.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello K.B., Bazzi A.R., Mimiaga M.J., Biancarelli D.L., Edeza A., Salhaney P., Childs E., Drainoni M.L. Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm. Reduct. J. 2018;15(1):55. doi: 10.1186/s12954-018-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz D., Wejnert C., Pham H.T., DiNenno E., Heffelfinger J.D., Cribbin M., Krishna N., Teshale E.H., Paz-Bailey G. HIV infection and risk, prevention, and testing behaviors among injecting drug users - National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR Surveill Summ. 2014;63(6):1–51. [PubMed] [Google Scholar]
- Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch. Intern. Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- CDC . C. Publication; 2021. HIV Surveillance Report 2021. [Google Scholar]
- Champion J.D., Recto P. An assessment of HIV Risk, perceptions of risk, and potential adherence to preexposure prophylaxis among HIV-negative people with injection drug use who access mobile outreach services. J. Addict. Nurs. 2023;34(2):101–110. doi: 10.1097/jan.0000000000000522. [DOI] [PubMed] [Google Scholar]
- Choopanya K., Martin M., Suntharasamai P., Sangkum U., Mock P.A., Leethochawalit M., Chiamwongpaet S., Kitisin P., Natrujirote P., Kittimunkong S., Chuachoowong R., Gvetadze R.J., McNicholl J.M., Paxton L.A., Curlin M.E., Hendrix C.W., Vanichseni S. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/s0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- Conrad C., Bradley H.M., Broz D., Buddha S., Chapman E.L., Galang R.R., Hillman D., Hon J., Hoover K.W., Patel M.R., Perez A., Peters P.J., Pontones P., Roseberry J.C., Sandoval M., Shields J., Walthall J., Waterhouse D., Weidle P.J., Duwve J.M. Community outbreak of HIV infection linked to injection drug use of oxymorphone-Indiana, 2015. MMWR Morb. Mortal. Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- Corcorran M.A., Scott J.D., Tinsley J., Wald A., Glick S.N. Awareness and correlates of HIV pre-exposure prophylaxis (PrEP) among HIV-negative people who access syringe services in seattle, Washington. Subst Use Misuse. 2022;57(3):337–343. doi: 10.1080/10826084.2021.2012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S., Goedel W.C., Park S.H., Hambrick H.R., Schneider J.A., Duncan D.T. Perceived Candidacy for Pre-exposure Prophylaxis (PrEP) Among Men Who Have Sex with Men in Paris, France. AIDS Behav. 2019;23(7):1771–1779. doi: 10.1007/s10461-018-2279-y. [DOI] [PubMed] [Google Scholar]
- Dubov A., Krakower D.S., Rockwood N., Montgomery S., Shoptaw S. Provider implicit bias in prescribing HIV pre-exposure prophylaxis (PrEP) to people who inject drugs. J. Gen. Intern. Med. 2023;38(13):2928–2935. doi: 10.1007/s11606-023-08040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R.M., Cary M., Duarte G., Jesus G., Alarcão J., Torre C., Costa S., Costa J., Carneiro A.V. Effectiveness of needle and syringe Programmes in people who inject drugs - An overview of systematic reviews. BMC Public Health. 2017;17(1):309. doi: 10.1186/s12889-017-4210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer R., Klein W.M. Risk perceptions and health behavior. Curr. Opin. Psychol. 2015;5:85–89. doi: 10.1016/j.copsyc.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N.W., Weimer M., Willenburg K.S., Kilkenny M.E., Atkins A.D., Paul McClung R., Hansen Z., Napier K., Handanagic S., Carnes N.A., Kemp Rinderle J., Neblett-Fanfair R., Oster A.M., Smith D.K. Expansion of Preexposure Prophylaxis Capacity in Response to an HIV Outbreak Among People Who Inject Drugs-Cabell County, West Virginia, 2019. Public Health Rep. 2022;137(1):25–31. doi: 10.1177/0033354921994202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Corro L.A., Zook K., Landry M., Rosecrans A., Harris R., Gaskin D., Falade-Nwulia O., Page K.R., Lucas G.M. An analysis of social determinants of health and their implications for Hepatitis C virus treatment in people who inject drugs: the case of Baltimore. Open Forum Infect Dis. 2024;11(4) doi: 10.1093/ofid/ofae107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y., Bartholomew T.S., Doblecki-Lewis S., Rodriguez A., Forrest D.W., Tomita-Barber J., Oves J., Tookes H.E. Interest in linkage to PrEP among people who inject drugs accessing syringe services; Miami, Florida. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koku Emmanuel, Felsher Marisa. The Effect of Social Networks and Social Constructions on HIV Risk Perceptions. AIDS and Behavior. 2020;24:206–221. doi: 10.1007/s10461-019-02637-y. [DOI] [PubMed] [Google Scholar]
- Mayer K.H., Agwu A., Malebranche D. Barriers to the wider use of pre-exposure prophylaxis in the united states: a narrative review. Adv Ther. 2020;37(5):1778–1811. doi: 10.1007/s12325-020-01295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S., Dunn D.T., Desai M., Dolling D.I., Gafos M., Gilson R., Sullivan A.K., Clarke A., Reeves I., Schembri G., Mackie N., Bowman C., Lacey C.J., Apea V., Brady M., Fox J., Taylor S., Antonucci S., Khoo S.H., Gill O.N. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/s0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland W., Lin J., Santos G.M., Arayasirikul S., Raymond H.F., Wilson E. Low PrEP awareness and use among people who inject drugs, San Francisco, 2018. AIDS Behav. 2020;24(5):1290–1293. doi: 10.1007/s10461-019-02682-7. [DOI] [PubMed] [Google Scholar]
- Mistler C.B., Copenhaver M.M., Shrestha R. The pre-exposure prophylaxis (PrEP) care cascade in people who inject drugs: a systematic review. AIDS Behav. 2021;25(5):1490–1506. doi: 10.1007/s10461-020-02988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Capitant C., Spire B., Pialoux G., Cotte L., Charreau I., Tremblay C., Le Gall J.M., Cua E., Pasquet A., Raffi F., Pintado C., Chidiac C., Chas J., Charbonneau P., Delaugerre C., Suzan-Monti M., Loze B., Fonsart J., Delfraissy J.F. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N. Engl. J. Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- Ni Z., Altice F.L., Wickersham J.A., Copenhaver M.M., DiDomizio E.E., Nelson L.E., Shrestha R. Willingness to initiate pre-exposure prophylaxis (PrEP) and its use among opioid-dependent individuals in drug treatment. Drug Alcohol Depend. 2021;219 doi: 10.1016/j.drugalcdep.2020.108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A.S., Brinkley-Rubinstein L., Oldenburg C.E., Mayer K.H., Mimiaga M., Patel R., Chan P.A. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31(5):731–734. doi: 10.1097/qad.0000000000001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K.R., Weir B.W., Zook K., Rosecrans A., Harris R., Grieb S.M., Falade-Nwulia O., Landry M., Escobar W., Ramirez M.P., Saxton R.E., Clarke W.A., Sherman S.G., Lucas G.M. Integrated care van delivery of evidence-based services for people who inject drugs: a cluster-randomized trial. Addiction. 2024 doi: 10.1111/add.16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L., Minozzi S., Reed J., Vickerman P., Hagan H., French C., Jordan A., Degenhardt L., Hope V., Hutchinson S., Maher L., Palmateer N., Taylor A., Bruneau J., Hickman M. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9) doi: 10.1002/14651858.CD012021.pub2. Cd012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleuhs B., Mistler C.B., Quinn K.G., Dickson-Gomez J., Walsh J.L., Petroll A.E., John S.A. Evidence of potential discriminatory HIV pre-exposure prophylaxis (PrEP) prescribing practices for people who inject drugs among a small percentage of providers in the U.S. J Prim Care Community Health. 2022;13 doi: 10.1177/21501319211063999. 21501319211063999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina H.J., Whitfield T.H.F., Grov C., Starks T.J., Parsons J.T. Distinguishing hypothetical willingness from behavioral intentions to initiate HIV pre-exposure prophylaxis (PrEP): findings from a large cohort of gay and bisexual men in the U.S. Social Sci. Med. 2017;172:115–123. doi: 10.1016/j.socscimed.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.M., Aumaier B.L., Felsher M.A., Welles S.L., Martinez-Donate A.P., Chavis M., Van Der Pol B. An exploration of factors impacting preexposure prophylaxis eligibility and access among syringe exchange users. Sex Transm Dis. 2018;45(4):217–221. doi: 10.1097/olq.0000000000000728. [DOI] [PubMed] [Google Scholar]
- Rutledge R., Madden L., Ogbuagu O., Meyer J.P. HIV risk perception and eligibility for pre-exposure prophylaxis in women involved in the criminal justice system. AIDS Care. 2018;30(10):1282–1289. doi: 10.1080/09540121.2018.1447079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K.E., White R.H., O'Rourke A., Kilkenny M.E., Perdue M., Sherman S.G., Grieb S.M., Allen S.T. Awareness of and interest in oral pre-exposure prophylaxis (PrEP) for HIV prevention and interest in hypothetical forms of PrEP among people who inject drugs in rural West Virginia. AIDS Care. 2021;33(6):721–728. doi: 10.1080/09540121.2020.1822506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S.G., Schneider K.E., Park J.N., Allen S.T., Hunt D., Chaulk C.P., Weir B.W. PrEP awareness, eligibility, and interest among people who inject drugs in Baltimore, Maryland. Drug Alcohol Depend. 2019;195:148–155. doi: 10.1016/j.drugalcdep.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R., Karki P., Altice F.L., Huedo-Medina T.B., Meyer J.P., Madden L., Copenhaver M. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug Alcohol Depend. 2017;173:107–116. doi: 10.1016/j.drugalcdep.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Thurmond P., Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS Behav. 2014;18(9):1694–1700. doi: 10.1007/s10461-014-0778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC . U. N. publication; 2023. World Drug Report 2023. [Google Scholar]
- Vincent W., McFarland W. Missed opportunities for healthcare providers to discuss HIV preexposure prophylaxis with people who inject drugs. Int J Drug Policy. 2022;110 doi: 10.1016/j.drugpo.2022.103873. [DOI] [PubMed] [Google Scholar]
- Walters S.M., Frank D., Van Ham B., Jaiswal J., Muncan B., Earnshaw V., Schneider J., Friedman S.R., Ompad D.C. PrEP care continuum engagement among persons who inject drugs: rural and urban differences in stigma and social infrastructure. AIDS Behav. 2022;26(4):1308–1320. doi: 10.1007/s10461-021-03488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.E., Dangerfield D.T., 2nd, Kral A.H., Wenger L.D., Bluthenthal R.N. Correlates of sexual coercion among people who inject drugs (PWID) in Los Angeles and San Francisco, CA. J. Urban Health. 2019;96(3):469–476. doi: 10.1007/s11524-018-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J., Castillo-Mancilla J., Ibrahim M.E., Brooks K.M., McHugh C., Morrow M., McCallister S., Bushman L.R., MaWhinney S., Kiser J.J., Anderson P.L. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following tenofovir alafenamide: the TAF-DBS Study. J Acquir Immune Defic Syndr. 2020;84(3):323–330. doi: 10.1097/qai.0000000000002354. [DOI] [PubMed] [Google Scholar]
- Yellin H., Levy M.E., Magnus M., Kuo I., Siegel M. HIV risk perception, willingness to use PrEP, and PrEP uptake among young men who have sex with men in Washington, DC. AIDS Behav. 2023;27(9):2844–2854. doi: 10.1007/s10461-023-04008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]