Abstract
Chickpea is considered a rich source of nutrients, especially protein and dietary fibre. Besides, chickpea has potential benefits for the maintenance of gut health by improving intestinal integrity and serving as a source of energy for the gut microbiota. Moreover, chickpea consumption has been found to possess anti-cancer, anti-inflammatory, and antioxidant activity. On undergoing certain treatments like soaking, dehulling, roasting, and germination, the anti-nutritional profile of chickpeas can be reduced. Observing these benefits, this review explores the impact of chickpea and its components on maintaining gut health, emphasizing various benefits. Besides, the paper comprehensively covers the nutritional composition of chickpeas and factors influencing the bioavailability of its components concerning gut health. Additionally, it outlines the mechanisms through which chickpeas influence gastrointestinal health, providing valuable insights into complex processes and potential therapeutic applications. Furthermore, the review identifies contributions that can guide future research, encouraging further exploration of chickpeas' role in gut health and the development of interventions. As a result of the presented review, chickpeas can be used as an affordable source of food, which is nutritionally stable and prevents gastrointestinal diseases.
Keywords: Chickpea, Gut health, dietary fibre, functional food, Germination, anti-nutritional profile
Graphical abstract
1. Introduction
Chickpea (Cicer arietinum L.), popularly known as garbanzo bean or Bengal gram, belongs taxonomically to the Fabaceae family, specifically to the monogeneric tribe Cicereae [1]. Chickpeas are one of the oldest cultivated crops and their versatility, nutritional value, and adaptability have contributed to their widespread popularity, utilized in various culinary forms. Though it is believed to have originated in Middle Eastern countries and spread to eastern and western parts of the world, the majority of chickpea production is in Australia, Eastern Africa, and South Asia, with 80–85 percent of the desi type grown worldwide. Moreover, it ranks as the third most popular pulse in the world, with an annual production of 13.3 million tons, which is grown over 50 countries, especially in North Africa, Asia, America, Southern Europe and Australia (Zhang et al., 2017). Among these, Southern and South-East Asian countries dominate in chickpea production, leading to 80 % of regional contribution [2]. Specifically, in African Sub-Saharan regions and India, chickpeas are recognized as a major source of protein and amino acids, especially for reducing the risk of malnutrition and contributing to food safety worldwide [2]. More importantly, on account of all pulses cultivated in India, chickpea represents 45 % of total pulse production, contributing about 75 percent of global production. Similarly, India has produced over 66.19 % of chickpea globally during 2018, totalling 17.20 million tonnes as well as, covering about 32.51 % of cultivation area (around 29.03 million ha) (Malik, 2021). Furthermore, chickpea consumption in India has risen steadily and is cultivated in 25 states [3]. For instance, Andhra Pradesh is the highest chickpea yielding state in India, averaging 1.4 metric tons per ha [2]. Indeed, chickpeas contain a healthy balance of protein, carbohydrates, fats, vitamins, and minerals, making them a good food component to incorporate into a daily diet for better nutritional status in developing countries [4]. Besides, it holds a good amount of dietary fiber, which is beneficial in reducing the chances of several serious ailments such as gastrointestinal diseases, diabetes, coronary artery diseases, stroke, and even cancer [5]. Moreover, chickpea has significant probiotic effect by promoting growth of beneficial bacteria, which is primarily due to their high levels of dietary fiber and resistant starch that nourishes gut microbiota (Sidhu et al., 2020). Chickpea has become recognized as a functional food due to its various health benefits including managing cholesterol, controlling diabetes, anti-cancerous and aiding weight loss [6]. Functional foods can be defined as natural or industrially processed foods that when consumed regularly can positively affect health beyond normal nutrition (Granato et al., 2020). The consumption of functional foods can be associated with benefits like disease prevention, increased nutrient intake and anti-inflammatory properties (Tripathy et al., 2019).
There is a growing demand for plant-based proteins, i.e., legumes and pulses because of its nutritional benefits towards various diseases [7]. For instance, the phenolic compounds and fermentable fiber present in chickpeas make it a prime substance for maintaining the functioning of gut microbiota as well as preventing other gut related diseases [8]. Moreover, gut microbiota is crucial in regulating the metaboloic responses in the host physiology and is considered as a vital metabolic organ [9]. Indeed, effective gut health can be achieved through maintaining a well-balanced, non-dysbiotic microbial community structure, along with preserving an intact colonic mucus and epithelial barrier. Any disruptions in these elements are linked to the onset of various gut associated diseases, such as obesity, colon cancer, and inflammatory bowel disease, among others. As a solution, the consumption of chickpeas improves mucus production in the gut, inhibits cancer cell proliferation, microbiome composition modulation, and attenuation of inflammation [10]. Furthermore, chickpea based diet helps enhance the colonic micro-environment by producing short chain fatty acids and improved metagenomic function [8]. Apart from this, the peptides present in chickpea protein prevent colonic cancer by possessing antiproliferative and antioxidant activity [10].
Coming to the anti-nutritional factors present in chickpeas, it include tannin, phytic acid, and protease inhibitors that hinder the nutritional quality and absorption of nutrients [11]. Some of the domestic processing methods utilized for reducing the effect of these anti-nutritional factors include soaking, dehulling, germinating, and roasting, enhancing the protein digestibility and sensory properties of chickpeas [12]. Besides, incorporating chickpeas into a balanced diet is part of ongoing efforts to promote not only general health but also the well-being of the gastrointestinal system. These legumes serve as a natural and nutritious means to support gut health, aligning with a broader initiative to emphasize the connection between diet and the maintenance of a healthy digestive tract. Overall, existing efforts in research and nutritional studies underscore the potential benefits of incorporating chickpeas into the diet for gut health. These efforts focus on understanding the specific mechanisms through which chickpeas influence the gut microbiota and exploring their role in preventing or managing gut-related disorders.
Considering the aforementioned benefits, this review aims to summarize the impact of chickpeas and its components on maintaining gut health. Moreover, the contribution of this work is given in Table 1 which outlines the novelty of the study in comparison with related works and are summarized as.
-
•
This study summarizes a comprehensive knowledge about the nutritional composition of chickpea and different factors affecting the nutritional bio-availability of chickpea components, fostering a favorable environment for beneficial gut microbes.
-
•
Besides, this work summarizes the mechanisms through which chickpeas influence gut health and provides valuable insights into the intricate processes involved, offering potential avenues for therapeutic applications.
-
•
Building the relationship between chickpeas and gut health, the findings emphasize the inclusion of chickpeas as a beneficial component in promoting and maintaining optimal gut health.
-
•
Further, the identified contributions pave the way for future research, encouraging further exploration of chickpeas' role in gut health and the development of interventions.
Table 1.
Comparison of our Proposed Work with State of the Arts.
| Reference | Importance of gut health | Mechanism of nutrients on gut health | Functional foods for gut health | Chickpea and gut health | Future Direction |
Objectives |
|---|---|---|---|---|---|---|
| [13,14] | ✓ | ✓ | To obtain relative in- formation regarding the importance of gut health and effect of diet on the host gut. | |||
| [[15], [16]]. | ✓ | To explore the mechanism of nutrients on maintaining gut health. | ||||
| [[5], [17]], | ✓ | To compare the effect of different functional foods on gut health. | ||||
| [18] | ✓ | ✓ | To investigate the effect of chickpea consumption on gut microbiota and its mechanism. | |||
| [19] | ✓ | To provide insights of the essential nutrients and its futuristic perspectives on modulating gut microbiome. | ||||
| Proposed work | ✓ | ✓ | ✓ | ✓ | ✓ | Added below |
2. Nutritional composition: unfolding the benefits
Chickpeas is considered a good source of several nutritional components including carbohydrates, protein, fat, dietary fiber, vitamins, and minerals, often preferred for maintaining the gut microenvironment [8]. Bringing together these benefits, this section highlights key nutritional components present in chickpeas as listed in Table 2. Chickpeas contain smaller amounts of digestible carbohydrates and larger amounts of unavailable carbohydrates, compared to other legumes. It is worth noting that the major proportion of carbohydrates present in chickpeas is starch which has slow digestibility [20]. Also, chickpeas are rich in amylose, amylopectin, and cellulose whereas sugars like oligosaccharides are found in smaller proportions which ultimately benefits maintaining gut health [7]. Moreover, bifidobacteria and lactobacillus comes under generally recognized as safe (GRAS) [21]. Other parallel research reveals that the alpha-galactosidase present in pulses like chickpeas helps to increase bifidobacteria and lactobacilli levels, improve bowel movement, and prevent the gut from carcinogenic agents [22]. Chickpea is also a popular source of protein and hence possesses several nutritional properties including a balance of amino acids and other biological activities [23]. Moreover, Chickpea protein has the highest bioavailability in the human body and its hydrolysates have properties including angiotensin-converting enzyme inhibition and antioxidant activity [6]. Besides, recent studies confirm that chickpea protein albumin, globulin, and glutelin can decrease the starch digestibility time, thus preventing gut related diseases. Additionally, studies have shown that chickpea protein albumin, globulin, and glutelin can decrease the starch digestibility time, thus preventing gut related diseases [24]. Chickpea protein is valuable in plant-based diets as it is a great source of essential amino acids, and hence plays a major role in human nutrition. It is found to be a rich source of non-essential amino acids such as aspartic acid and glutamic acid along with modest amounts of histidine, serine, proline, alanine, glycine, and cysteine [25]. Researchers stated that the alpha-galactosidase present in chickpeas helps to increase bifidobacteria and lactobacilli levels, improve bowel movement, and protect the gut from carcinogenic agents [22]. Fermented black chickpea flour is a rich source of amino acid and resistant starch, significantly increased after fermenting via Lactiplanti Bacillus plantarum T0A10. More importantly, the total resistant starch content of chickpeas can be increased by treating it with the pullulanase enzyme [26]. In relation to gut health, the resistant starch content in chickpeas gets fermented by the gut microbiota, which helps in the production of short chain fatty acids [27].
Table 2.
Nutritional composition of Desi and Kabuli type of Chickpea.
| Nutrients | Desi Type | Kabuli type | Reference |
|---|---|---|---|
| Carbohydrate (%) | 51.74–70.17 | 61.70–72.8 | [28,29] |
| Protein (%) | 20.29–22.37 | 23.68–24.51 | [28,29] |
| Amino Acids (mg/100g) | |||
| Total essential amino acid | 33.59 | 32.63 | [29,30] |
| Total essential amino acid | 51.57 | 54.55 | |
| Fat (%) | 3.62–4.62 | 4.12–5.03 | [28] |
| Dietary Fibre (%) | 18.73–23.8 | 21.86 | [29] |
| Vitamin (mg/100g) | |||
| Thiamine | 0.33–0.36 | 0.31–0.33 | [30] |
| Riboflavin | 0.21 | 0.26 | |
| Niacin | 1.72 | 1.22 | |
| Pantothenic acid | 1.09 | 1.02 | |
| Minerals (mg/100g) | |||
| Calcium | 148–178 | 145–189 | [29,[18], [31]] |
| Phosphorus | 355 | 35 | |
| Manganese | 1.15–1.32 | 1.39–1.42 | |
| Iron | 5.2–5.3 | 4.6–6.4 | |
| Copper | 1.16–1.30 | 1.20–130 | |
| Zinc | 3.32–6.17 | 2.53–4.18 | |
Chickpeas fat includes sterols, lipids, tocopherols (phytosterols), and fatty acids like monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) [32]. The fat of chickpea seeds is characterized by the high content of essential unsaturated fatty acids: linoleic acid, oleic acid, and linolenic acid, as well as saturated fatty acids such as palmitic acid and stearic acid. Incorporation of chickpeas in diet could help in enhancing the polyunsaturated fatty acids content in the human body [33]. As per the studies done on the basis of clinical trials, essential fatty acids in chickpeas are observed to maintain cellular integrity, leading to a healthy gut barrier ([32]. Chickpea is considered a good source of water-soluble vitamins such as B2, B5, and B6 [20]. The most important minerals contained in chickpeas are calcium, phosphorus, magnesium, iron, copper, zinc, sodium, and potassium, most of the seed calcium is located in the seed coat [34]. Being a good source of zinc and iron, chickpea consumption also helps in the prevention of mineral malnutrition. In a complementary study, the administration of iron was observed to be effective in balancing the gut microbiota and beneficial for patients with Inflammatory Bowel Disease (IBD) and colorectal cancer [35]. Moreover, an in vitro study stated that supplementing chickpeas is favorable for gut health, as minerals like calcium in it have synergistic effects with specific bacterial species. Vitamin B4 has a positive influence on gut related diseases including constipation, ulcers, dyspepsia, and other gastrointestinal disturbances [34]. Another variety of chickpeas, known as Kabuli Chana, has various nutrients including lignin, cellulose, fat, and dietary fiber [36]. The Chickpea hull comprises dietary fiber and bound and free phenolics [37]. Chickpea hull has a greater amount of dietary fiber which is more than 70 %. Recently, the two major commercial types of chickpeas; Kabuli and Desi were evaluated, in which the nutritional quality of Kabuli chickpea puree was compared with Apulian black chickpea puree. On one hand, black chickpea puree contains a high content of fiber and bioactive compounds, while Kabuli chickpea puree contains high lipid concentration. Besides, it has been exhibited dietary fiber variations in different genotypes of chickpeas, under processing techniques including soaking and cooking. While cooking, the cellulose as well as lignin contents accelerate while hemicellulose content decreases [38].
3. Mechanism: modulating gut microbiota composition and Diversity
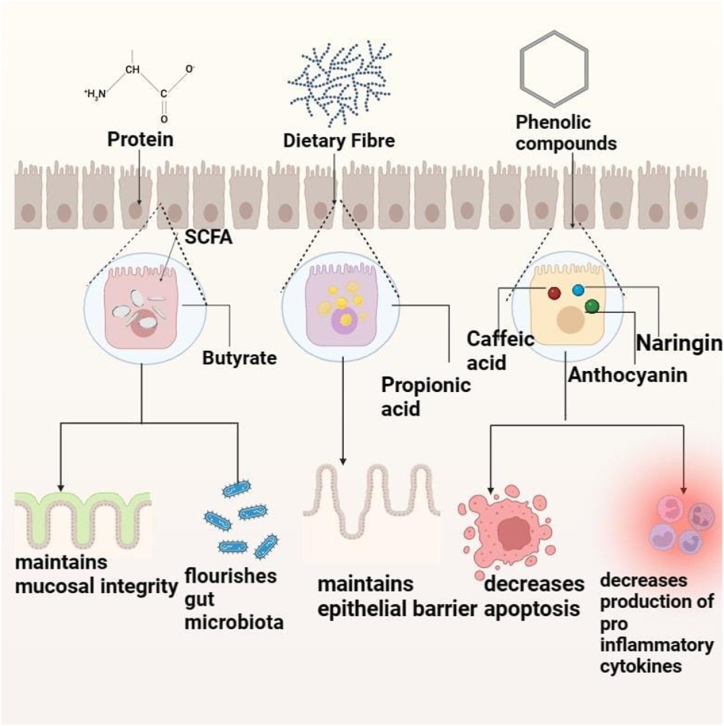
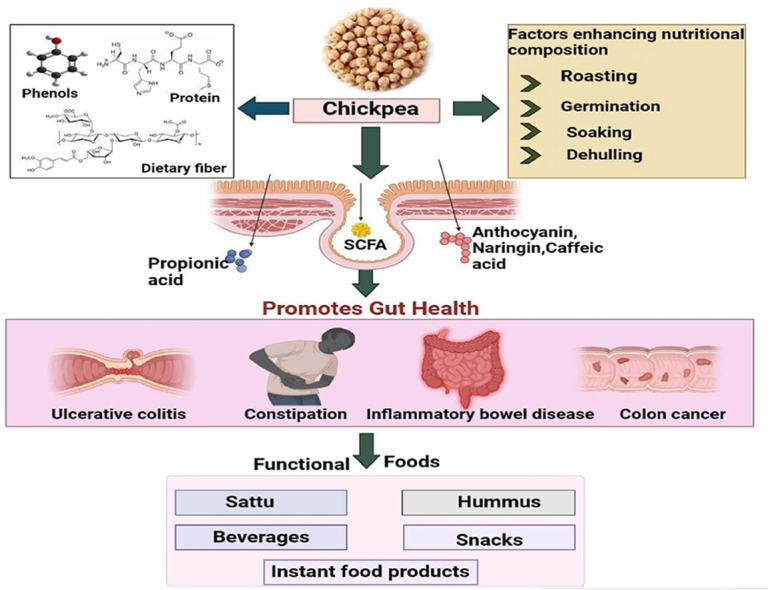
Gut health refers to the mutual and beneficial relationship between the gut microbiota and the immune system, which aims to diminish harmful inflammatory responses and strengthen the integrity of the mucosal barrier of the gut [5]. This section discusses the mechanism by which chickpea components like dietary fiber, protein, and phenolic compounds modulate colonic microbiota and gut health as illustrated in Fig. 1.
Fig. 1.
Chickpeas mechanism of action by promoting digestion, diversifying gut bacteria, and providing anti-inflammatory and antioxidative benefits through fiber, protein, and phenolic compounds.
3.1. Dietary fiber
Chickpea contains a high content of dietary fiber, considered effective for maintaining the gut microbiome, regular bowel movements, and relieving constipation [39]. Indeed, the gut microbiota produces metabolites like short-chain fatty acids (SCFA) in order to utilize the dietary fiber as human indigenous enzymes can-not digest it properly [40]. Being a rich source of dietary fiber, chickpea helps in maintaining the health of the colonic microbiome and epithelial barrier which prevents several gut-related diseases [8]. Researchers have shown that dietary fibers present in chickpeas increase propionic acid levels and improve Lactobacillus and Bacteroides [41]. Further, intestinal mucosa production can be effectively increased by dietary fiber and SCFA from the intestinal epithelium [40]. Another parallel work on chickpeas has shown that dietary fiber can mechanically decrease the interaction time between mucosal cells and carcinogens, proteolytic fermentation time due to bulky fecal, and can improve viscosity. Furthermore, Bacillus and Bacteroides in the gut can be increased in abundance due to increased consumption of chickpea dietary fiber, which positively affects the gut microbiota homeostasis [41].
3.2. Protein
Due to the presence of protease inhibitors in plants, majorly in pulses and legumes like chickpeas, the digestibility of protein decreases, and the undigested protein gets fermented by the microbiota in the colon. The substantial amount of undigested protein helps to improve the number of species capable of protein fermentation which ultimately flourishes the gut microbiota [42]. Precursors of SCFA such as propionate and butyrate, release as an end product of amino acid and peptide fermentation, which benefit gut health [40]. Apart from this, butyrate acts as a major source of energy for colonocytes, which are essential for modulating intestinal inflammation, promoting genomic stability, and maintaining mucosal integrity. For instance, the ability of butyrate to assist in the removal of dysfunctional cells and synchronize apoptosis and differentiation of colonocytes emphasizes its potentiality in preventing colon cancer [43].
3.3. Phenolic compounds
Studies have proved that kaempferol, a flavonoid present in pulses like chickpeas, has a positive effect on reducing the chances of inflammatory bowel disease (IBD) by inhibiting the nuclear factor-kappa B (NK- κB) signaling pathway and boosting the secretion of interleukin-8 (IL-8) [44]. Besides, it is also evident that cooked chickpeas contain phenolic compounds like formononetin, biochanin A, daidzein, quercetin, etc. These compounds mainly possess anti-inflammatory and antioxidative action and lessen the membrane permeability, which enhances gut health and diminishes pathologies like colitis [8]. Besides, Chlorogenic acid in legumes exhibits potent antimicrobial properties, permeabilizing and binding bacterial cell membranes while also regulating gut microbiota by promoting the growth of specific beneficial bacteria [45]. Another key compound in chickpeas, anthocyanins, has the ability to improve the growth of Bifidobacterium spp. which commits mucosal layer thickening by stimulating mucosa secretion. It also decreases the permeability of the gut membrane by the distribution and localization of restored tight junction protein (ZO-1 and Occludin) [[18], [46]]. An experiment showed that Naringin, another key compound of chickpeas, can improve the integrity of intestinal barriers from disruption induced by TNF-α (Lin et al., 2019).). Studies have also shown that by the inhibition of the NF-κB signaling pathway, caffeic acid can decrease the production of pro-inflammatory cytokines, comprising tumor necrosis factor-alpha, interleukin-6 (IL-6), and infiltration of F4/80 macrophages, CD177+ neutrophils and CD3 + T cells [45,47].
4. Health benefits of chickpea
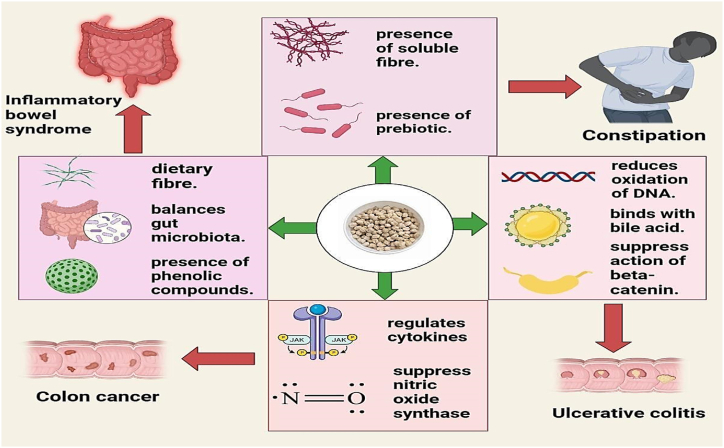
Being an abundant source of several nutrients, chickpea is recognized as an excellent food for the prevention of numerous chronic gut disorders such as Inflammatory Bowel Syndrome (IBS), diabetes, colorectal cancer, etc., which majorly occur due to gut microbiota dysbiosis [8]. The inclusion of chickpeas into a regular diet not only helps to meet the daily nutritional requirement but also fulfills several nutrients required for human gut health as illustrated in Fig. 2.Moreover, most of the acute and chronic intestinal disorders often disrupt the natural integrity of the intestinal barrier [46].Chickpea can be used as an effective alternative for carcinogenesis. For instance, sprouted chickpea is represented as chemo-preventive and has a potential effect on colorectal cancer treatment due to the presence of isoflavonoids and dietary selenium. This leads to apoptosis due to overexpression of cell surface death receptors and thus reduces tumor cell growth [48]. Besides, it is found that daily consumption of chickpea (200 gm/day) reduces the risk of colorectal cancer due to the production of SCFA such as butyrate, which induces apoptosis and inhibits cell growth [49]. Similarly, several meta-analyses have stated that daily intake of ⅔ cup of chickpea can effectively maintain the health of an individual (Kadyan et al., 2022). Besides, the gut bacteria perform fermentation on the prebiotic carbohydrates of chickpeas, which prevents the occurrence of colon cancer [50]. This might be due to the ability of dietary fiber to bind with secondary bile acids (BA's), which controls the level of BA's. Also, this structural connection may influence host physiology by avoiding the build-up of toxic BA's responsible for polyps and colon cancer [15].Similarly, chickpea protein by modulating immune response and produce butyrate that induces apoptosis in cancer cells (Wang et al., 2021). Moreover, studies indicated that daily supplementation of cooked chickpeas exhibited anti-inflammatory and antioxidant properties, during carcinogenesis by decreasing DNA oxidation, and expression of inflammatory enzymes COX-2, iNOS, and β-catenin, an oncogenic protein [51]. Furthermore, raffinose oligosaccharides derived from chickpeas have prebiotic that can promote probiotic strains and contribute antioxidant activity which helps in managing colon cancer [52].
Fig. 2.
Potential health benefits of chickpea on gut health (Chickpeas promote gut health, guarding against colon cancer through isoflavonoids and selenium. Dietary fiber prevents constipation, acts as a prebiotic, and eases IBS and ulcerative colitis symptoms by reducing inflammation.).
Chickpeas, like other legumes, are rich in dietary fibre, and contribute a positive effect on diseases like constipation. Besides, consuming legumes like chickpeas has a positive effect on reducing constipation by regulating transit time, stool size, and microbial effects [53]. Studies have also shown that pulses and foods rich in pulse fibre improve laxation by increasing the weight of feces, though the frequency of the stools depends on age and intake [54]. With a consequent release of SCFA, legumes like chickpeas, rich in fermented soluble fiber, act as prebiotics, contributing to an increase in biomass and fecal mass [40]. Moreover, chickpeas can be clinically utilized in the treatment of constipation and other gut-related diseases due to the presence of dietary fiber in it [55]. Similarly, the dietary fiber content in chickpeas possesses the ability to retain water which hydrates the stool and causes an irritation in intestinal mucosa, leading to the release of water and mucus that improves laxation [56].
Inflammatory Bowel Syndrome (IBS) is a common gastrointestinal disorder that is caused by abdominal pain and a disruption in the rhythm of bowel movements, usually lasting three months.It is found that IBS is caused due to stress, imbalance between microbiota and neurotransmitters, infection, inflammation, etc., and the treatment includes dietary supplements like foods rich in dietary fiber [57]. Studies found that the phenolic compounds present in chickpeas possess anti-inflammatory properties that can reduce the symptoms of several gastrointestinal diseases including IBS mediated through functional gut microbiota [18]. Similarly, studies have stated that dietary fiber content in chickpeas has a potential effect on bowel regulation, changing intestinal permeability and managing microbiota composition, which in total positively manages the etiology of IBS [58].
Ulcerative colitis occurs typically in the colon and rectum and mainly manifests as abdominal pain, bloody diarrhea with or without mucus, and weight loss [59]. Studies reported a strong protective effect of chickpeas on colitis owing to their ability to regulate inflammation-mediated cytokines and signaling pathways [60]. Research has shown that chickpea has a potential effect on colitis induced by DSS, related to their ability to deactivate STAT3 and NK-κ β pathways. This leads to the inhibition of inflammatory mediators, contributing to positive effects on colitis treatment [59]. Also, in a model for colon cancer associated with colitis, it has been observed that the intake of chickpeas can impede the development of colon cancer. Notably, among the ways in which chickpea consumption offers protection, includes the suppression of nitric oxide synthase and COX-2, both of which play a role in inflammatory processes [51].
5. Key factors influencing functionality of chickpea on gut health
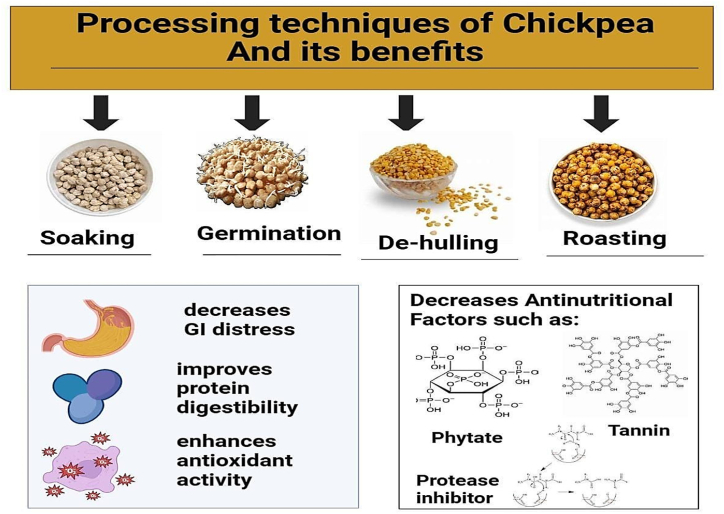
Chickpea contains various bioactive compounds and dietary fibers which play beneficial roles in human health. Processing techniques like soaking, roasting, germination, etc., improve the nutritional profile and quality of chickpeas. Mainly, this section discusses the impact of different processing techniques on the bioavailability of chickpea components illustrated in Fig. 3. Soaking chickpeas before consumption is considered beneficial due to the lack of the essential alpha-galactosidase enzyme in the gut. Inadequate hydrolysis of alpha galacto-oligosaccharides in chickpeas, such as verbascose, raffinose, and stachyose, can lead to gastrointestinal issues [61]. Soaking serves as a remedy for these problems and a pre-treatment, facilitating starch gelatinization and protein denaturation, resulting in a softer texture for the legumes [15]. Moreover, soaking the chickpeas decreases trypsin inhibitors and tannins because of the water-soluble phenolic compounds [62]. It leaches out phytates and significantly reduces oxalates and lectins [[63], [64]]. These can be attributed to the increased activity of endogenous enzymes in chickpeas during soaking [65]. Similarly, soaked chickpeas have decreased levels of alpha-galactosidase, proteolytic enzyme inhibitors, and total sugars [38]. In addition, soaking causes increased water absorption and starch gelatinization in chickpeas, altering them for safe consumption [66]. Dehulling, a process of removing the seed husk of the grains, helps to eliminate the bitter taste of legumes, reducing the tannin content of grain [67,68]. Besides, it influences the total composition of chickpeas, particularly affecting the fiber and increasing the protein content [69]. In addition, dehulling also improves the color, texture, and aroma of legumes, and reduces excess fiber content responsible for bloating [64]. Moreover, dehulling enhances starch and protein digestibility as well as zinc and calcium bioavailability (Oghabaei and Prakash,2020). Similarly, researchers observed that chickpeas, when properly dehulled, can be digested easily and utilized potentially by the gut [70].
Fig. 3.
Processing techniques like soaking, dehulling, germination, and roasting enhance chickpea bioavailability, digestibility, and overall appeal by reducing anti-nutritional factors, improving palatability, and boosting nutrient content.
Chickpea quality can be improved by germination, owing to the improvement in bioavailability of nutrients and phytochemicals, and the degradation of antinutrients [71]. During the germination process, the synthesis of absorbable polypeptides and essential amino acids is increased due to enzymatic activity [64]. Similarly, proteolysis during germination leads to an increase in free amino acids and non-protein nitrogen [12]. The rise in amino acid profile during germination can be due to the breakdown of ‘globulin’, an abundant chickpea protein. Besides, a reduction in the fat content of chickpeas during germination can be attributed to their utilization as a source of energy for initiating the sprouting process [65]. Studies conducted on germinated chickpeas showed that protein content in treated samples is higher than that of untreated samples. This can be due to the enhancement of protein quality during germination by enzymatic breakdown of it into simpler compounds through protease activity [72]. According to research, germination results in increased levels of phytochemicals, thus improving the antioxidant capacity [67]. Similarly, the increase in antioxidant activity in germinated chickpeas can also be due to enhanced vitamin C content during sprouting (Masood et al., 2014). Also, germination leads to the modification of antioxidant activity in chickpeas, due to the degeneration of carbohydrates in the seed [73]. Similarly, germination transforms chickpeas into a source of bioactive compounds, by increasing antioxidant activity, and elevating phenolic compounds and gamma-aminobutyric acid (GABA) levels [74]. This increase in antioxidant activity during germination is associated with the rise in free phenolic compounds, resulting in the reduction of the bound form [75]. Moreover, germination elevated the dietary fiber content in chickpeas, transforming it into a functional food that can help maintain gut microbiota and decrease transit time [76]. Antinutritional factors in chickpeas like phytates and saponins are reduced by the germination process [64]. Activation of phytase enzymes during sprouting is reported as the major cause of the reduction of phytic acid in legumes [62]. Similarly, chickpeas utilize phytic acid as a source of phosphorus and cations for germination, which also leads to diminished levels of phytic acid [12]. Moreover, the decrease in phytic acid during germination increases the bioavailability of other minerals whereas, diminished saponin content reduces hemolytic properties and off-flavor of chickpea when consumed [72]. Furthermore, germination leads to reduction in alpha-amylase inhibitors, chymotrypsin inhibitors, and trypsin inhibitors (TI) [68].
Roasting helps improve the color, flavor, and shelf life of legumes as well as enhances protein digestibility and denaturation [77]. Moreover, it leads to the transformation of both macronutrients and micronutrients into a more palatable form and improves its overall acceptability by improving flavor, texture, and taste [78]. Similarly roasting chickpeas helps in eliminating volatiles and other compounds like pyrazines and alkylated pyrazines, which impart pleasant flavor as well as mask's off flavors [79]. Furthermore, heat treatments like roasting increased protein digestibility through protein denaturation, and deactivation of anti-digestive factors can be achieved [80]. Similarly, roasted chickpeas also possessed high antioxidant activity, improved levels of essential amino acids, reduced water activity, and hence increased shelf life [81]. Besides, due to increased starch gelatinization, roasting leads to an increase in carbohydrate levels in chickpeas [82]. Additionally, roasting inactivates the enzymes responsible for deterioration during the storage period, thus making chickpeas more suitable for consumption [83]. Unlike other processing techniques, roasting is considered the most effective method since it causes less contamination and consumes less time in chickpea processing [81]. Moreover, anti-nutritional factors, e.g.tannin and phytate content of chickpeas have a significant decrease. Studies have shown that roasted chickpeas possess less glycemic index and high resistant starch content [84].
6. Value-added products of chickpeas for promoting gut health
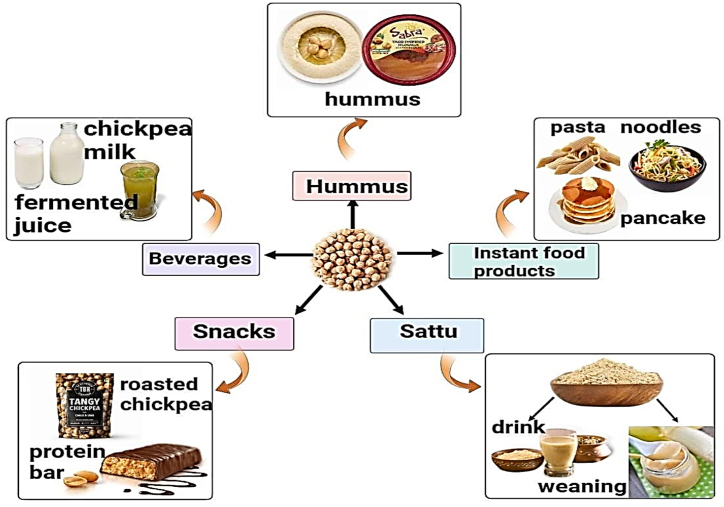
Chickpeas are considered a potential functional food for gut health due to its enrichment in various nutrients and bioactive compounds. Several studies have been carried out for the incorporation of chickpeas in the development of beverages, instant food products, snacks, etc, either by substituting any other grain or as the base ingredient as illustrated in Fig. 4. Similarly, Table .3, explains the effect of chickpea based functional foods on gut health.
Fig. 4.
Chickpea based functional foods like hummus, sattu, snacks, beverages, and instant items offer affordable and nutritious choices for gut health. Being a rich source of protein, fiber, and essential nutrients, they are suitable for different diets and health needs.
Table 3.
The effect of chickpea based functional foods on gut health.
| Chickpea based functional foods | Description | Major nutrients | Effects on gut health | References |
|---|---|---|---|---|
| Chickpea beverages | Fermented beverages and milk | Protein, Carbohydrates, B complex vitamin, Probiotics. | Supports digestion and gut microbiome balance. | [[82], [85]]. |
| Chickpea based instant food products | Bread, pasta, soup mix, pancake mix | Protein, Fiber | Provides protein and prebiotic fiber for flourishing gut microbiota. | [42,86,87]. |
| Chickpea based snacks | Protein bars, crackers, biscuits. | Protein, essential amino acids, dietary fiber | Propionate and butyrate production balances colonocytes and maintains mucosal integrity. | [40,[88], [89]]. |
| Chickpea based dips | Hummus | Protein, fiber | SCFA decreases carcinogenesis in the gut as well as increases the abundance of Bacillus and Bacteroids. | [[41], [90]]. |
| Chickpea based prebiotic foods | Yoghurt, Tempeh. |
Prebiotics, protein, dietary fiber. | Promotes gut bacterial growth and aids digestion. | [91,92]. |
6.1. Chickpea based beverages
Fermentation improves nutritional profile and bioactive compounds in food while also preserving it by using microorganisms as well as enzymes to break down complex substances [50]. It is suggested that microbial fermentation can be an effective method to
improve the nutritional profile of chickpea by enhancing the bioavailability of its nutrients (Zhu et al., 2023). Moreover, the microbial variant also plays a critical role in framing the nutritional potentiality of the fermented product [93]. Importance of fermented probiotic rich beverages demonstrates the fact that fermentation is accepted worldwide, as it is a cost effective and easy method for improving nutritional and sensory qualities of food products [50]. Fermented chickpea slurries are used to develop different types of beverages which can be used as an alternative for many other industrial beverages. In a study, three strains including Streptococcus thermophilus (ST), a co-culture of ST with Lactobacillus plantarum (STLP), and Lactococcus lactis (STLL) were used to prepare the fermented chickpea-based beverage. The product possesses excellent sensory properties, functional properties, and high protein content and can be consumed as a nutrient-rich, plant-based beverage [94]. Moreover, chickpea-based beverages produced using lactic acid bacteria (LAB) fermentation have shown potential improvement in aroma, vitamin B6 (Pyridoxine) content, total flavonoids, antioxidant, and lactic acid content [95]. These changes not only lead to an enhanced organoleptic property but also suggest potential gut health benefits, as LAB in fermented products are known to promote a healthy gut microbiota. Furthermore, researchers have developed a potential alternative for dairy milk, from chickpea and coconut, which is nutritionally stable with a good amount of carbohydrates, protein, fat, and calcium, compared to cow's milk. Chickpea-based beverages are considered better than peanuts and soya drinks as there are no officially recognized allergens in chickpeas, making it a favorable choice for gut health-conscious consumers [85]. Similarly, chickpea based beverages are developed as an effective alternative to soy based beverages, as it is comparatively less in fat and highly fermentable [96].
6.2. Sattu or roasted Chickpea flour
Sattu is a traditional Indian food product, made of roasted and powdered chickpeas, consumed in the form of drink, ladoo and litti chokha [97]. Due to its shelf stability, high protein content, taste, and easy availability, sattu is considered a supplement in rural India [3]. Additionally, the low glycemic index of sattu allows it to be preferred by diabetic individuals. Moreover, in order to prevent malnutrition, it is served as a nutrient rich drink to infants during their translational age [6]. It is generally consumed in the form of a slurry with milk, which has shown beneficial effects on gastrointestinal discomfort [98]. Similarly, sattu is chosen for its low glycemic index and high fiber, which aids in regulated bowel movement [99]. Due to its cooling effect on the body, sattu is mainly consumed as a drink during the summer season in the Northern parts of India [100]. According to Ayurveda, sattu is recommended to be consumed with sugar and ghee for better results during summer [101]. It is also used in developing stirred bio yogurt, as a prebiotic which is observed to be beneficial for gut health [98]. Besides, sattu can be used in the form of Mantha, a traditional instant energy drink, which relieves fatigue and thirst [101].
6.3. Chickpea based snacks
In the global food sector, snack industries are the fastest growing sector and people nowadays are consuming snacks regularly. Chickpeas, in roasted form, coated with jaggery or spices are largely consumed as a healthier snack in India [102]. In reference to gut health, chickpeas could be an effective alternative to commercially available snacks due to its high nutrient content, source of prebiotics, gluten free alternative, low cost, and environment-friendly approach [103]. Generally, snacks contain an anti-nutritional factor called ‘acrylamide’, which can be reduced by incorporating chickpea flour into it [104]. Nevertheless, chickpea based extruded product is considered crispy and dense in nutrients with low lipid levels, and high protein and mineral content, making it an acceptable ready to eat snack in the market [102]. Crackers developed with chickpea flour are also observed to be a rich source of protein, dietary fiber, and minerals. Moreover, they exhibit good starch digestibility, making them a nutritious choice for promoting gut health [88]. Besides, germinated chickpeas are utilized as fat replacers along with wheat flour in biscuits with nutraceutical properties [79]. Similarly, chickpea-based high protein bars are also developed by researchers, with good overall nutrition and palatability [86]. Moreover, cracker-type, gluten-free snacks for children, made using chickpea flour possessed a balanced amino acid content with low glycemic index [89].
6.4. Chickpea based instant food products
Wheat flour is replaced with chickpea flour, in gluten free pasta, which is more acceptable in terms of taste, texture, high protein and fiber content along with less fat profile [105]. Instant soup mix formulated from chickpeas can be consumed as a functional food by the elderly, which can be palatable and will meet the nutritional requirements [87]. Researchers have developed instant beverage powder by sequential alcalase and alpha-amylase catalysis of chickpeas, which can be beneficial for vegan consumers, due to the solubility and nutritional quality [106]. Furthermore, studies have partially replaced conventional wheat flour with fermented chickpea flour, aiming to develop a functional bread [107]. Moreover, gluten-free bread developed using roasted chickpea flour possessed soft crumbs, and improved nutritional and technological characteristics [108]. Similarly, the incorporation of chickpea flour into bread resulted in appetite suppression, reduced diastolic blood pressure, and improvements in glycaemia, insulinaemia, and antioxidant status of blood [109]. Gluten free instant sweet pancake mixes are developed successfully by replacing cereal flours with chickpea flour, which can be accepted by a broad range of customers. The product possessed high protein content, low carbohydrate and salt levels, in comparison to the conventional cereal-based pancake mix [110].
6.5. Hummus
Hummus is a product made out of chickpea paste along with the addition of spices and oil and is usually used as a dip or a spread [49]. It is a traditional dish originating from Middle East and Mediterranean countries. The overall chickpea consumption in the world is clearly associated with hummus, which is made by blending cooked and mashed chickpeas with lime juice, tahini, spices, and olive oil [111]. Due to its good nutritional profile, including high amounts of protein, dietary fiber, folate, and unsaturated fats, hummus has been seen as a healthier food in recent years [112]. Similarly, the store bought hummus is also a source of unsaturated oils, dietary fiber, folate, calcium, and magnesium [100]. Studies showed that the moderate consumption of hummus is exhibited to maintain gastrointestinal health by slowing down carbohydrate absorption and delayed gastric emptying [49]. Moreover, even a short term consumption of a mediterranean diet including hummus exhibited good glycemic control [113]. However, the formulation of commercial hummus is constantly altered and tailored aiming to develop a tastier and healthier functional food [114]. Furthermore, probiotic hummus is also available in the market, developed by the addition of selected probiotic culture to freshly prepared conventional hummus [115].
6.6. Chickpea based prebiotic products
Chickpea, with its significant level of prebiotics, serves as an ideal food for enhancing prebiotic consumption in individuals, due to its numerous physiological benefits in gut health [116]. Studies conducted on the effect of chickpeas as a prebiotic source concluded that the levels of Bacteroidetes and Actinobacteria were higher whereas the level of pathogenic bacteria ‘Firmicutes’ was lower in the host [50]. On the increasing demand for plant based prebiotics, chickpea based prebiotic food products are available in the market. Besides, commercial prebiotic products like Bimuno and Benefiber, derived from fructooligosaccharides and inulin promote only gut bacteria, whereas chickpea based prebiotics offer additional benefits like high protein and fiber content [116]. Indeed, commercial prebiotic products are less shelf stable due to presence of live cultures whereas chickpea based prebiotics have longer shelf life when dried or canned (Sidhu et al., 2020). Bio-yoghurt developed by utilizing yoghurt starter with other probiotic strains exhibited prebiotic characteristics due to the incorporation of chickpea as a prebiotic [117]. Similarly, yoghurt integrated with chickpea flour is a promised source of prebiotics, protein and fiber [91]. Tempeh is a fermented food product from chickpeas, which has prebiotic and probiotic properties [92]. Moreover, among non-dairy matrices, chickpea is the most prominently used legume, for the preparation of fermented drinks as it is prebiotic in nature [118]. Furthermore, researchers have also developed prebiotic snack bars using chickpeas rather than conventional dairy based bars or supplements [119]. Beside, the addition of chickpea mucilage to kefir improves its prebiotic quality, potentially enriching its beneficial effects on gut health [120]. Similarly, researchers have developed prebiotic gluten-free cookies by incorporating chickpea flour [121].
7. Conclusion
Chickpea, which can be easily included in the daily diet, is considered a good source of all the nutrients for preventing malnutrition and promoting health. As pulses are rich in protein, they are seen as a staple food in the vegetarian diet. Besides, the bioactive compounds present in chickpeas have potential benefits for gut health. It is also evident that dietary fiber and protein content in chickpeas will prevent the occurrence of cancer, reduce inflammation, and improve digestion. Furthermore, the application of different processing techniques like soaking, dehulling, roasting, and germination improves the nutritional profile of chickpeas along with the significant reduction in anti-nutritional factors like tannin, phytic acid, and protease inhibitors. The incorporation of chickpea-based functional foods in daily diet will reduce the risk of digestive tract diseases like IBS, colon cancer, and ulcerative colitis as well as nourish the beneficial gut microbiota. Besides, the review identifies contributions that can guide future research, encouraging further exploration of chickpeas’ role in gut health and the development of interventions. Moreover, future studies should focus more on clinical and subclinical trials to confirm the mechanism in which chickpea constituents influence gut health. Indeed, there is a need to explore the synergistic effect of chickpea with other other components and prebiotics to identify its effective combination for gut health.
CRediT authorship contribution statement
Aswani Ajay: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Supriya Singh Gaur: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Rafeeya Shams: Writing – original draft, Visualization, Validation, Resources, Project administration, Methodology, Investigation. Kshirod Kumar Dash: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Shaikh Ayaz Mukarram: Visualization, Validation, Software, Resources. Béla Kovács: Visualization, Validation, Software, Resources.
Ethical declaration
Not applicable.
Data availability
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
Research involving human and animal Rights
This article does not contain any studies with human or animal subjects.
Funding
Project No. TKP2021-NKTA-32 was implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme, and supported by the University of Debrecen Program for Scientific Publication.
Declaration of Competing interest
There is no conflict of interest between the authors.
Contributor Information
Supriya Singh Gaur, Email: supriya.27320@lpu.co.in.
Kshirod Kumar Dash, Email: kshirod@tezu.ernet.in.
Béla Kovács, Email: kovacsb@agr.unideb.hu.
Abbreviations
- BA
Bile Acid
- CD3+
Cluster of Differentiation 3
- COX-2
Cyclooxygenase-2
- DSS
Dextran Sodium Sulphate
- IBD
Inflammatory Bowel Disease
- IBS
Inflammatory Bowel Syndrome
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- LAB
Lactic Acid Bacteria
- MUFA
Mono Unsaturated Fatty Acid
- NK-κB
Nuclear Factor Kappa–B
- NK-κβ
Nuclear Factor- Kappa Beta
- PUFA
Polyunsaturated Fatty Acid
- RTCPM
Ready To Cook Porridge Mix
- SCFA
Short Chain Fatty Acid
- ST
Streptococcus Thermophilus
- STAT3
Signal Transducer and Activation Transcription 3
- STLL
Streptococcus Thermophilus with Lactobacillus Lactis
- STLP
Streptococcus Thermophilus with Lactobacillus Plantarum
- TI
Trypsin Inhibitors
- TNF-α
Tumor Necrosis Factor-α
- ZO-1
Zonula Occluden-1
References
- 1.Sajja S.B., Samineni S., Gaur P.M. Botany of chickpea. The chickpea genome. 2017:13–24. doi: 10.1007/978-3-319-66117-9_3. [DOI] [Google Scholar]
- 2.Merga B., Haji J. Economic importance of chickpea: production, value, and world trade. Cogent Food Agric. 2019;5 doi: 10.1080/23311932.2019.1615718. [DOI] [Google Scholar]
- 3.Sharma A., Mazumdar B., Keshav A. Formulation, standardization and characterization of novel sattu beverage enriched with beetroot juice. J. Food Sci. Technol. 2020;57:1936–1943. doi: 10.1007/s13197-019-04229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-El Dadon S., Abbo S., Reifen R. Leveraging traditional crops for better nutrition and health-The case of chickpea. Trends Food Sci. 2017;64:39–47. doi: 10.1016/j.tifs.2017.04.002. [DOI] [Google Scholar]
- 5.Núñez-Gómez V., Periago M.J., Navarro-González I., Campos-Cava M.P., Baenas N., González-Barrio R. Influence of raspberry and its dietary fractions on the in vitro activity of the colonic microbiota from normal and overweight subjects. Plant Foods Human Nutr. 2021;76:494–500. doi: 10.1007/s11130-021-00923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur R., Prasad K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)-A review. Trends Food Sci. Technol. 2021;109:448–463. doi: 10.1016/j.tifs.2021.01.044. [DOI] [Google Scholar]
- 7.Hamed H.A., Kobacy W., Mahmoud E.A., El-Geddawy M.M. Looking for a novel vegan protein supplement from faba bean, lupine, and soybean: a dietary and industrial standpoint. Plant Foods Hum. Nutr. 2024;79:90–97. doi: 10.1007/s11130-023-01125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monk J.M., Lepp D., Wu W., Graf D., McGillis L.H., Hussain A.…Power K.A. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct.Foods. 2017;38:663–674. doi: 10.1016/j.jff.2017.02.002. [DOI] [Google Scholar]
- 9.Guo W., Zhang Z., Li L., Liang X., Wu Y., Wang X.…Yuan C.S. Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol. Res. 2022;182 doi: 10.1016/j.phrs.2022.106355. [DOI] [PubMed] [Google Scholar]
- 10.Milán-Noris A.K., Gutiérrez-Uribe J.A., Santacruz A., Serna-Saldívar S.O., Martínez-Villaluenga C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.) Food Chem. 2018;268:66–76. doi: 10.1016/j.foodchem.2018.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R.K., Gupta K., Sharma A., Das M., Ansari I.A., Dwivedi P.D. Health risks and benefits of chickpea (Cicer arietinum) consumption. J. Agric. Food Chem. 2017;65:6–22. doi: 10.1021/acs.jafc.6b02629. [DOI] [PubMed] [Google Scholar]
- 12.Dida Bulbula D., Urga K. Study on the effect of traditional processing methods on nutritional composition and anti nutritional factors in chickpea (Cicer arietinum) Cogent Food Agric. 2018;4 doi: 10.1080/23311932.2017.1422370. [DOI] [Google Scholar]
- 13.Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.J., Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beam A., Clinger E., Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13:2795. doi: 10.3390/nu13082795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell host microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Gallausiaux C., Marinelli L., Blottière H.M., Larraufie P., Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80(1):37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 17.Ashaolu T.J. Immune boosting functional foods and their mechanisms: a critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110625. [DOI] [PubMed] [Google Scholar]
- 18.Aldars-Garcia L., Chaparro M., Gisbert J.P. Systematic review: the gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms. 2021;9:977. doi: 10.3390/microorganisms9050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinod B.R., Asrey R., Rudra S.G., Urhe S.B., Mishra S. Chickpea as a promising ingredient substitute in gluten-free bread making: an overview of technological and nutritional benefits. Food Chem. Advances. 2023;3 doi: 10.1016/j.focha.2023.100473. [DOI] [Google Scholar]
- 20.Ipekesen S., Basdemir F., Tunc M., Bicer B.T. Minerals, vitamins, protein and amino acids in wild Cicer species and pure line chickpea genotypes selected from a local population. J. Elem. 2022;27 [Google Scholar]
- 21.Abedinia A., Alimohammadi F., Teymori F., Razgardani N., Saeidi Asl M.R., Ariffin F., Roslan J. Characterization and cell viability of probiotic/prebiotics film based on duck feet gelatin: a novel poultry gelatin as a suitable matrix for probiotics. Foods. 2021;10(8):1761. doi: 10.3390/foods10081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B., Singh J.P., Shevkani K., Singh N., Kaur A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017;54:858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boukid F. Chickpea (Cicer arietinum L.) protein as a prospective plant‐based ingredient: a review. Int. J. Food Sci. Technol. 2021;56:5435–5444. doi: 10.1111/ijfs.15046. [DOI] [Google Scholar]
- 24.Tan X., Zhang S., Malde A.K., Tan X., Gilbert R.G. Effects of chickpea protein fractions on α-amylase activity in digestion. Food Hydrocolloids. 2022;133 doi: 10.1016/j.foodhyd.2022.108005. [DOI] [Google Scholar]
- 25.Khan M.A., Ammar M.H., Migdadi H.M., El-Harty E.H., Osman M.A., Farooq M., Alghamdi S.S. Comparative nutritional profiles of various faba bean and chickpea genotypes. Int. J. Agric. Bio. 2015;17:449–457. [Google Scholar]
- 26.Demirkesen-Bicak H., Tacer-Caba Z., Nilufer-Erdil D. Pullulanase treatments to increase resistant starch content of black chickpea (Cicer arietinum L.) starch and the effects on starch properties. Int. J. Biol. Macromol. 2018;111:505–513. doi: 10.1016/j.ijbiomac.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Brummer Y., Kaviani M., Tosh S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015;67:117–125. doi: 10.1016/j.foodres.2014.11.009. [DOI] [Google Scholar]
- 28.Singh L., Srivastava N., Kumar K. Nutritional evaluation of some kabuli and desi varieties of chickpea (Cicer arietinum L.) Indian J. Agric. Biochem. 2018;31:195–197. doi: 10.5958/0974-4479.2018.00032.1. [DOI] [Google Scholar]
- 29.Ghribi A.M., Sila A., Gafsi I.M., Blecker C., Danthine S., Attia H., Besbes S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015;75:276–282. doi: 10.1016/j.ijbiomac.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Xiao S., Li Z., Zhou K., Fu Y. Chemical composition of kabuli and desi chickpea (Cicer arietinum L.) cultivars grown in Xinjiang, China. Food Sci. Nutr. 2023;11:236–248. doi: 10.1002/fsn3.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khetarpaul N. Total mineral content present (calcium, magnesium, potassium, phosphorus, iron, zinc, manganese, copper, and boron and nickel) in leaves of Desi and Kabuli chickpea varieties (on dry matter basis) Int. J. Commun. Syst. 2018;6:633–635. [Google Scholar]
- 32.Madurapperumage A., Tang L., Thavarajah P., Bridges W., Shipe E., Vandemark G., Thavarajah D. Chickpea (Cicer arietinum L.) as a source of essential fatty acids–a biofortification approach. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.734980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marioli Nobile C.G., Carreras J., Grosso R., Inga C.M., Silva M.P., Aguilar R., Martinez M.J. Scientific Research Publishing; 2013. Proximate Composition and Seed Lipid Components of “Kabuli”-type Chickpea (Cicer Arietinum L.) from Argentina. [Google Scholar]
- 34.Shabbir H., Shabbir I., Aslam M., Sarwar M.F., Sarwar M.H., Sarwar M. Fundamental aspects of vitamin B complex in human nourishment and fitness. Am. J. Food Sci. Health. 2020;6:109–118. [Google Scholar]
- 35.Kujawska M., Ewertowska M., Ignatowicz E., Adamska T., Szaefer H., Zielińska-Dawidziak M.…Jodynis-Liebert J. Evaluation of safety of iron-fortified soybean sprouts, a potential component of functional food, in rat. Plant Foods Hum. Nutr. 2016;71:13–18. doi: 10.1007/s11130-016-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamid A., Kalsoom S. Comparative analysis of nutritional composition and effect of dietary fiber extracts of chickpea and Bengal gram on blood glucose and cholesterol levels of male induced diabetic and hypercholesterolemic rats. Pak. J. Zool. 2017;49:487–492. doi: 10.17582/journal.pjz/2017.49.2.487.492. [DOI] [Google Scholar]
- 37.Niño-Medina G., Muy-Rangel D., Urías-Orona V. Chickpea (Cicer arietinum) and soybean (Glycine max) hulls: byproducts with potential use as a source of high value-added food products. Waste Biomass Valorization. 2017;8:1199–1203. doi: 10.1007/s12649-016-9700-4. [DOI] [Google Scholar]
- 38.Vasishtha H., Srivastava R.P. Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J. Food Sci. Technol. 2013;50:579–584. doi: 10.1007/s13197-011-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein N., Reifen R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022;5:167–178. doi: 10.1016/j.gaost.2022.06.002. [DOI] [Google Scholar]
- 40.Arroyo M.C., Laurie I., Rotsaert C., Marzorati M., Risso D., Karnik K. Age-dependent prebiotic effects of soluble corn fiber in M-SHIME® gut microbial ecosystems. Plant Foods Hum. Nutr. 2023;78:213–220. doi: 10.1007/s11130-023-01043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J., Zhang R., Muheyati D., Lv M.X., Aikebaier W., Peng B.X. The effect of chickpea dietary fiber on lipid metabolism and gut microbiota in high-fat diet-induced hyperlipidemia in rats. J. Med. Food. 2021;24:124–134. doi: 10.1089/jmf.2020.4800. [DOI] [PubMed] [Google Scholar]
- 42.Kårlund A., Gómez-Gallego C., Turpeinen A.M., Palo-Oja O.M., El-Nezami H., Kolehmainen M. Protein supplements and their relation with nutrition, microbiota composition and health: is more protein always better for sportspeople? Nutrients. 2019;11:829. doi: 10.3390/nu11040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu B., Das P., Lv X., Aa J., Wang k., Duan L., Gilbert J.A., Ni Y., Wu X. The effect of ‘healthy’ fecal microbiota transplanatation against the detireotion of depression of fawn- hooded rats. J.A.S.M. 2022;7:3. doi: 10.1128/msystems.00218-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coman V., Vodnar D.C. Hydroxycinnamic acids and human health: recent advances. J. Sci. Food Agric. 2020;100:483–499. doi: 10.1002/jsfa.10010. [DOI] [PubMed] [Google Scholar]
- 46.Huang B., Gui M., An H., Shen J., Ye F., Ni Z., Lin J. Babao Dan alleviates gut immune and microbiota disorders while impacting the TLR4/MyD88/NF-кB pathway to attenuate 5-Fluorouracil-induced intestinal injury. Biomed. Pharmacother. 2023;166 doi: 10.1016/j.biopha.2023.115387. [DOI] [PubMed] [Google Scholar]
- 47.Dugum M., Barco K., Garg S. Managing irritable bowel syndrome: the low-FODMAP diet. Clev. Clin. J. Med. 2016;83:655–662. doi: 10.3949/ccjm.83a.14159. [DOI] [PubMed] [Google Scholar]
- 48.Guardado-Félix D., Antunes-Ricardo M., Rocha-Pizaña M.R., Martínez-Torres A.C., Gutiérrez-Uribe J.A., Saldivar S.O.S. Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J. Funct.Foods. 2019;53:76–84. doi: 10.1016/j.jff.2018.07.003. [DOI] [Google Scholar]
- 49.Wallace T.C., Murray R., Zelman K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients. 2016;8:766. doi: 10.3390/nu8120766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson N., Johnson C.R., Thavarajah P., Kumar S., Thavarajah D. The roles and potential of lentil prebiotic carbohydrates in human and plant health. Plants, People, Planet. 2020;2:310–319. doi: 10.1002/ppp3.10103. [DOI] [Google Scholar]
- 51.Chino X.M.S., Martínez C.J., Garzón V.R.V., González I.Á., Treviño S.V., Bujaidar E.M.…Hoyos R.B. Cooked chickpea consumption inhibits colon carcinogenesis in mice induced with azoxymethane and dextran sulfate sodium. J. Am. Coll. Nutr. 2017;36:391–398. doi: 10.1080/07315724.2017.1297744. [DOI] [PubMed] [Google Scholar]
- 52.Pandae N., Krangkrathok W., Sawangwan T., Ngernyuang N., Chantorn S. Bioactivity and prebiotic properties of raffinose oligosaccharides derived from different chickpeas for alternative functional food application. Bioactive Carb. Dietary Fibre. 2024;31 doi: 10.1016/j.bcdf.2024.100412. [DOI] [Google Scholar]
- 53.Gill S.K., Rossi M., Bajka B., Whelan K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 54.Dahl W.J., Alvarez M.M. Whole pulses and pulse fiber: modulating gastrointestinal function and the microbiome. Health benefits of pulses. 2019:91–108. doi: 10.1007/978-3-030-12763-3_7. [DOI] [Google Scholar]
- 55.Rao S.S.C., Yu S., Fedewa A. Systematic review: dietary fibre and FODMAP‐restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 56.Bellini M., Tonarelli S., Barracca F., Rettura F., Pancetti A., Ceccarelli L.…Rossi A. Chronic constipation: is a nutritional approach reasonable? Nutrients. 2021;13:3386. doi: 10.3390/nu13103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Wang Z., Wang G., Song X., Qian Y., Liao Z., Xia Y. Understanding the connection between gut homeostasis and psychological stress. J. Nutr. 2023;153(4):924–939. doi: 10.1016/j.tjnut.2023.01.026. [DOI] [PubMed] [Google Scholar]
- 58.Liu X., Wu Y., Li F., Zhang D. Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis. Nutr. Res. 2015;35:753–758. doi: 10.1016/j.nutres.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Kim M., Chung K.S., Hwang S.J., Yoon Y.S., Jang Y.P., Lee J.K., Lee K.T. Protective effect of Cicer arietinum L.(Chickpea) ethanol extract in the dextran sulfate sodium-induced mouse model of ulcerative colitis. Nutrients. 2020;12:456. doi: 10.3390/nu12020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen P., Zhou X., Zhang L., Shan M., Bao B., Cao Y.…Ding A. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. J. Ethnopharmacol. 2015;162:207–214. doi: 10.1016/j.jep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 61.Njoumi S., Josephe Amiot M., Rochette I., Bellagha S., Mouquet-Rivier C. Soaking and cooking modify the alpha-galacto-oligosaccharide and dietary fibre content in five Mediterranean legumes. Int. J. Food Sci. Nutr. 2019;70:551–561. doi: 10.1080/09637486.2018.1544229. [DOI] [PubMed] [Google Scholar]
- 62.Singh P.K., Shrivastava N., Sharma B., Bhagyawant S.S. Effect of domestic processes on chickpea seeds for antinutritional contents and their divergence. Am. J. Food Sci. Technol. 2015;3:111–117. doi: 10.12691/ajfst-3-4-3. [DOI] [Google Scholar]
- 63.Shi L., Arntfield S.D., Nickerson M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018;107:660–668. doi: 10.1016/j.foodres.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 64.Yegrem L. Nutritional composition, antinutritional factors, and utilization trends of Ethiopian chickpea (Cicer arietinum L.) Int. J. Food Sci. 2021;2021 doi: 10.1155/2021/5570753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desalegn B.B. Effect of soaking and germination on proximate composition, mineral bioavailability and functional properties of chickpea flour. Food Pub. Health. 2015;5:108–113. doi: 10.5923/j.fph.20150504.02. [DOI] [Google Scholar]
- 66.Costa R., Fusco F., Gandara J.F. Mass transfer dynamics in soaking of chickpea. J. Food Eng. 2018;227:42–50. doi: 10.1016/j.jfoodeng.2018.02.004. [DOI] [Google Scholar]
- 67.Pal R.S., Bhartiya A., Yadav P., Kant L., Mishra K.K., Aditya J.P., Pattanayak A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris) J. Food Sci. Technol. 2017;54:909–920. doi: 10.1007/s13197-016-2351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patterson C.A., Curran J., Der T. Effect of processing on antinutrient compounds in pulses. Cereal Chem. 2017;94(1):2–10. doi: 10.1094/CCHEM-05-16-0144-FI. Cogent Food Agric., 5:1615718, 2019. [DOI] [Google Scholar]
- 69.Vasishtha H., Srivastava R.P., Verma P. Effect of dehusking and cooking on protein and dietary fiber of different genotypes of desi, kabuli and green type chickpeas (Cicer arietinum) J. Food Sci. Technol. 2014;51:4090–4095. doi: 10.1007/s13197-012-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghermezgoli K.M., Ghassemzadeh H.R., Moghaddam M. Optimization of Kabuli chickpea dehulling process. J. Bio. Env. Sci. 2017;10:115–125. [Google Scholar]
- 71.Oghbaei M., Prakash J. Effect of dehulling and cooking on nutritional quality of chickpea (Cicer arietinum L.) germinated in mineral fortified soak water. J. Food Compos. Anal. 2020;94 doi: 10.1016/j.jfca.2020.103619. [DOI] [Google Scholar]
- 72.Devisetti R., Prakash J. Comparative assessment of organic and non-organic–chickpea and cowpea, nutritional composition and antinutrients upon germination. World J. Adv. Res. Rev. 2020;8:262–270. doi: 10.30574/wjarr.2020.8.2.0432. [DOI] [Google Scholar]
- 73.Domínguez-Arispuro D.M., Cuevas-Rodríguez E.O., Milán-Carrillo J., León-López L., Gutiérrez-Dorado R., Reyes-Moreno C. Optimal germination condition impacts on the antioxidant activity and phenolic acids profile in pigmented desi chickpea (Cicer arietinum L.) seeds. J. Food Sci. Technol. 2018;55:638–647. doi: 10.1007/s13197-017-2973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Idate A., Shah R., Gaikwad V., Kumathekar S., Temgire S. A comprehensive review on antinutritional factors of chickpea (Cicer arietinum L.) J. Pharm. Innov. 2021;10:816–823. doi: 10.22271/tpi.2021.v10.i5k.6306. [DOI] [Google Scholar]
- 75.Ferreira C.D., Bubolz V.K., da Silva J., Dittgen C.L., Ziegler V., de Oliveira Raphaelli C., de Oliveira M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT. 2019;111:363–369. doi: 10.1016/j.lwt.2019.05.049. [DOI] [Google Scholar]
- 76.Sofi S.A., Rafiq S., Singh J., Mir S.A., Sharma S., Bakshi P., Dar B.N. Impact of germination on structural, physicochemical, techno-functional, and digestion properties of desi chickpea (Cicer arietinum L.) flour. Food Chem. 2023;405 doi: 10.1016/j.foodchem.2022.135011. [DOI] [PubMed] [Google Scholar]
- 77.Kumar Y., Sharanagat V.S., Singh L., Mani S. Effect of germination and roasting on the proximate composition, total phenolics, and functional properties of black chickpea (Cicer arietinum) Legume Sci. 2020;2:e20. doi: 10.1002/leg3.20. [DOI] [Google Scholar]
- 78.Qureshi I., Bashir K., Jan S., Tarafdar A., Habib M., Jan K. Effect of sand roasting on physicochemical, thermal, functional, antiutritional, and sensory properties of sattu, a nourishing form of chickpea. J. Food Qual. 2023;2023 doi: 10.1155/2023/5564365. [DOI] [Google Scholar]
- 79.Saeed S.M.G., Ali S.A., Naz J., Mirza M., Elkhadragy M.F., Yehia H.M., Giuffrè A.M. Techno-functional, antioxidants, microstructural, and sensory characteristics of biscuits as affected by fat replacer using roasted and germinated chickpea (Cicer arietinum L.) Int. J. Food Prop. 2023;26:2055–2077. doi: 10.1080/10942912.2023.2242602. [DOI] [Google Scholar]
- 80.Yadav L., Bhatnagar V. Effect of soaking and roasting on nutritional and anti-nutritional components of chickpea (anti-nutritional components of chickpea (Pratap-14) Bioscan. 2017;12:771–774. [Google Scholar]
- 81.Jogihalli P., Singh L., Sharanagat V.S. Effect of microwave roasting parameters on functional and antioxidant properties of chickpea (Cicer arietinum) LWT-Food Sci. Technol. 2017;79:223–233. doi: 10.1016/j.lwt.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 82.Raza H., Zaaboul F., Shoaib M., Zhang L. An overview of physicochemical composition and methods used for chickpeas processing. Int. J. Agric. Innovations Res. 2019;7:495–500. [Google Scholar]
- 83.Zaaboul F., Raza H., Cao C., Yuanfa L. The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil bodies. Food Chem. 2019;280:270–277. doi: 10.1016/j.foodchem.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 84.Simsek S., Herken E.N., Ovando‐Martinez M. Chemical composition, nutritional value and in vitro starch digestibility of roasted chickpeas. J. Sci. Food Agric. 2016;96:2896–2905. doi: 10.1002/jsfa.7461. [DOI] [PubMed] [Google Scholar]
- 85.Cabanillas B., Jappe U., Novak N. Allergy to peanut, soybean, and other legumes: recent advances in allergen characterization, stability to processing and IgE cross‐reactivity. Mol. Nutr. Food Res. 2018;6 doi: 10.1002/mnfr.201700446. [DOI] [PubMed] [Google Scholar]
- 86.Gupta S., Liu C., Sathe S.K. Quality of a chickpea‐based high protein snack. J. Food Sci. 2019;84:1621–1630. doi: 10.1111/1750-3841.14636. [DOI] [PubMed] [Google Scholar]
- 87.Mohamed R.S., Abozed S.S., El-Damhougy S., Salama M.F., Hussein M.M. Efficiency of newly formulated functional instant soup mixtures as dietary supplements for elderly. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun W., Tribuzi G., Bornhorst G.M. Particle size and water content impact breakdown and starch digestibility of chickpea snacks during in vitro gastrointestinal digestion. Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113201. [DOI] [PubMed] [Google Scholar]
- 89.Martín-Esparza M.E., Raigón M.D., García-Martínez M.D., Albors A. Toward the development of potentially healthy low-energy-density snacks for children based on pseudocereal and pulse flours. Foods. 2023;12:2873. doi: 10.3390/foods12152873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Planes-Muñoz D., Frontela-Saseta C., Ros-Berruezo G., López-Nicolás R. Effect of gazpacho, hummus and ajoblanco on satiety and appetite in adult humans: a randomised crossover study. Foods. 2021;10:606. doi: 10.3390/foods10030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patil N.D., Bains A., Sridhar K., Rashid S., Kaur S., Ali N.…Sharma M. Effect of sustainable pretreatments on the nutritional and functionality of chickpea protein: implication for innovative food product development. J. Food Biochem. 2024;2024 doi: 10.1155/2024/5173736. [DOI] [Google Scholar]
- 92.Teoh S.Q., Chin N.L., Chong C.W., Ripen A.M., Firdaus M.S.H.B.M., Lim J.J.L. A review on health benefits and processing of tempeh with outlines on its functional microbes. Future Foods. 2024;100330 doi: 10.1016/j.fufo.2024.100330. [DOI] [Google Scholar]
- 93.Wu J., He T., Wang Z., Mao J., Sha R. The dynamic analysis of non-targeted metabolomics and antioxidant activity of Dendrobium officinale Kimura et Migo by the synergistic fermentation of bacteria and enzymes. LWT. 2024;203 doi: 10.1016/j.lwt.2024.116354. [DOI] [Google Scholar]
- 94.Mefleh M., Faccia M., Natrella G., De Angelis D., Pasqualone A., Caponio F., Summo C. Development and chemical-sensory characterization of chickpeas-based beverages fermented with selected starters. Foods. 2022;11:3578. doi: 10.3390/foods11223578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang P., Tang F., Cai W., Zhao X., Shan C. Evaluating the effect of lactic acid bacteria fermentation on quality, aroma, and metabolites of chickpea milk. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1069714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang S., Chelikani V., Serventi L. Evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. Lwt. 2018;97:570–572. doi: 10.1016/j.lwt.2018.07.067. [DOI] [Google Scholar]
- 97.Nath N., Singh S.S., Kushawaha A. Development and quality assessment of cookies prepared by using “Wheat flour & Sattu”. Pharma Innov. 2017;6:36–39. [Google Scholar]
- 98.Arooj A., Faiz S., Shah J.A., Ramzan A., Ihsan M., Saleem M. Technological, processing and nutritional aspects of chickpea (Cicer arietinum) Saudi J. Pathol. Microbiol. 2021;6:150–155. doi: 10.36348/sjpm.2021.v06i04.006. [DOI] [Google Scholar]
- 99.Shakeb M., Dharanya E.K., Pandey S. Standardization and quality profile of sattu mix. J. Food Sci. Technol. 2022:1–15. doi: 10.1007/s13197-021-05277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Munir A., Ali J., Naeem M. vol. 61. 2020. Improvement of fruit and vegetable quality through postharvest biology. (1st International Conference on Sustainable Agriculture: Food Security under Changing Climate Scenarios). [Google Scholar]
- 101.Singh P., Vyas H., Vasava A., Vasani D., Patil S. Standardization and sensory evaluation of Sattu-Mantha: a traditional energy drink. Int. J. Ayurvedic Med. 2021;12:583–587. [Google Scholar]
- 102.Kaur R., Prasad K. Process optimization for the development of traditionally roasted chickpea flour for meal replacement beverages. Food Chem. Adv. 2023;3 doi: 10.1016/j.focha.2023.100452. [DOI] [Google Scholar]
- 103.Escobedo A., Mojica L. Pulse‐based snacks as functional foods: processing challenges and biological potential. Compr. Rev. Food Sci. Food Saf. 2021;20:4678–4702. doi: 10.1111/1541-4337.12809. [DOI] [PubMed] [Google Scholar]
- 104.Rachwa-Rosiak D., Nebesny E., Budryn G. Chickpeas—composition, nutritional value, health benefits, application to bread and snacks: a review. Crit. Rev. Food Sci. Nutr. 2015;55:1137–1145. doi: 10.1080/10408398.2012.687418. [DOI] [PubMed] [Google Scholar]
- 105.Cristina Oliveira de Lima V., Piuvezam G., Leal Lima Maciel B., Heloneida de Araújo Morais A. Trypsin inhibitors: promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J. Enzyme Inhib. Med. Chem. 2019;34:405–419. doi: 10.1080/14756366.2018.1542387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silvestre‐De‐León R., Espinosa‐Ramírez J., Pérez‐Carrillo E., Serna‐Saldívar S.O. Extruded chickpea flour sequentially treated with alcalase and α‐amylase produces dry instant beverage powders with enhanced yield and nutritional properties. Int. J. Food Sci. Technol. 2021;56:5178–5189. doi: 10.1111/ijfs.15199. [DOI] [Google Scholar]
- 107.Shrivastava C., Chakraborty S. Bread from wheat flour partially replaced by fermented chickpea flour: optimizing the formulation and fuzzy analysis of sensory data. Lwt. 2018;90:215–223. doi: 10.1016/j.lwt.2017.12.019. [DOI] [Google Scholar]
- 108.Kahraman G., Harsa S., Casiraghi M.C., Lucisano M., Cappa C. Impact of raw, roasted and dehulled chickpea flours on technological and nutritional characteristics of gluten-free bread. Foods. 2022;11:199. doi: 10.3390/foods11020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amoah I., Cairncross C., Osei E.O., Yeboah J.A., Cobbinah J.C., Rush E. Bioactive properties of bread formulated with plant-based functional ingredients before consumption and possible links with health outcomes after consumption-a review. Plant Foods Hum. Nutr. 2022;77:329–339. doi: 10.1007/s11130-022-00993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santos C.S., Vasconcelos M.W. Nutritional and sensorial analysis of a lentil flour-based sweet pancake premix. Open Res. 2023;3:20. doi: 10.12688/openreseurope.15254.1. Europe. [DOI] [Google Scholar]
- 111.Olaimat A.N., Al-Holy M.A., Ghoush M.A., Al-Nabulsi A.A., Holley R.A. Control of Salmonella enterica and Listeria monocytogenes in hummus using allyl isothiocyanate. Int. J. Food Microbiol. 2018;278:73–80. doi: 10.1016/j.ijfoodmicro.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 112.Alvarez M.D., Fuentes R., Olivares M.D., Canet W. Effects of high hydrostatic pressure on rheological and thermal properties of chickpea (Cicer arietinum L.) flour slurry and heat-induced paste. Innovative Food Sci. Emerging Technol. 2014;21:12–23. doi: 10.1016/j.ifset.2013.11.005. [DOI] [Google Scholar]
- 113.Reister E.J., Belote L.N., Leidy H.J. The benefits of including hummus and hummus ingredients into the American diet to promote diet quality and health: a comprehensive review. Nutrients. 2020;12:3678. doi: 10.3390/nu12123678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alvarez M.D., Fuentes R., Guerrero G., Canet W. Characterization of commercial Spanish hummus formulation: nutritional composition, rheology, and structure. Int. J. Food Prop. 2017;20:845–863. doi: 10.1080/10942912.2016.1186692. [DOI] [Google Scholar]
- 115.Al-Awwad N.J., Yamani M.I., Takruri H.R. Development of probiotic hummus. J. Saudi Soc. Food Nutr. 2014;9(1):1–10. [Google Scholar]
- 116.Siva N., Johnson C.R., Richard V., Jesch E.D., Whiteside W., Abood A.A.…Thavarajah D. Lentil (Lens culinaris Medikus) diet affects the gut microbiome and obesity markers in rat. J. Agric. Food Chem. 2018;66:8805–8813. doi: 10.1021/acs.jafc.8b03254. [DOI] [PubMed] [Google Scholar]
- 117.Hussein H., Awad S., El-Sayed I., Ibrahim A. Impact of chickpea as prebiotic, antioxidant and thickener agent of stirred bio-yoghurt. Ann. Agric. Sci. 2020;65:49–58. doi: 10.1016/j.aoas.2020.03.001. [DOI] [Google Scholar]
- 118.Chaturvedi S., Chakraborty S. Review on potential non‐dairy synbiotic beverages: a preliminary approach using legumes. Int. J. Food Sci. Technol. 2021;56:2068–2077. doi: 10.1111/ijfs.14779. [DOI] [Google Scholar]
- 119.Rajagukguk Y.V., Arnold M., Sidor A., Kulczyński B., Brzozowska A., Schmidt M., Gramza-Michałowska A. Antioxidant activity, probiotic survivability, and sensory properties of a phenolic-rich pulse snack bar enriched with Lactiplantibacillus plantarum. Foods. 2022;11:309. doi: 10.3390/foods11030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ould Saadi L., Zaidi F., Sanz T., Haros C.M. Effect of faba bean and chickpea mucilage incorporation in the structure and functionality of kefir. Food Sci. Technol. Int. 2020;26:503–511. doi: 10.1177/1082013220908089. [DOI] [PubMed] [Google Scholar]
- 121.Gök İ., Çelik F.H. Use of some functional flours for development of prebiotic gluten-free cookies. Food Health Technol. Innovations. 2021;4:251–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.