Abstract
Millions globally suffer from visual impairment, complicating the management of eye diseases due to various ocular barriers. The eye's complex structure and the limitations of existing treatments have spurred interest in tissue engineering (TE) as a solution. This approach offers new functionalities and improves therapeutic outcomes over traditional drug delivery methods, creating opportunities for treating various eye disorders, from corneal injuries to retinal degeneration. In our review of recent articles concerning the use of scaffolds for eye repair, we categorized scaffolds employed in eye TE from recent studies into four types based on tissue characteristics: natural, synthetic, biohybrid, and decellularized tissue. Additionally, we gathered data on the cell types and animal models associated with each scaffold. This allowed us to gather valuable insights into the benefits and drawbacks of each material. Our research elucidates that, in comparison to conventional treatment modalities, scaffolds in TE emulate the extracellular matrix (ECM) of the eye and facilitate cell proliferation and tissue regeneration. These scaffolds can be precisely tailored to incorporate growth factors that augment the healing process while also providing considerable advantages such as bacterial inhibition, biocompatibility, and enhanced durability. However, they also have drawbacks, such as potential immune responses, poor tissue integration, complex and costly manufacturing, and inconsistent degradation rates that can affect their effectiveness. In this review, we provide an overview of the present condition of eye regenerative treatments, assess notable preclinical and clinical research endeavors, contemplate the obstacles encountered, and speculate on potential advancements in the upcoming decade.
Keywords: Tissue engineering, Regenerative medicine, Scaffold, Ophthalmology, Eye regeneration
1. Introduction
253 million individuals worldwide had vision impairment in 2017, of which 35 million were totally blind, according to the WHO. In fact, blindness and loss of vision rank among the most significant health issues that have an impact on patients' physical and mental well-being, particularly in older ages [1,2]. Unfortunately, damage to the eye often results in irreversible vision loss, with current treatments unable to fully restore lost vision [3]. At present, there is no established therapy for these degenerative conditions affecting the retina, cornea, and lens [4].
Ocular tissue engineering (TE) offers considerable benefits compared to existing standard treatments by emphasizing regenerative capabilities, customization, and sustainable solutions. It seeks to repair damaged ocular tissues, such as the cornea and retina, which may lead to the reversal of vision loss, while conventional treatments typically focus on alleviating symptoms without addressing the root causes [5]. By employing the patient's own cells, ocular TE minimizes the likelihood of rejection and complications associated with the use of foreign materials [6]. Moreover, it provides lasting solutions that enhance functional outcomes, improving visual acuity and overall quality of life, in contrast to standard treatments that often necessitate ongoing care [7]. Additionally, this approach offers innovative strategies for managing complex ocular diseases that are frequently inadequately treated by traditional methods [8]. In summary, TE in ophthalmology has shown great promise within the area of ocular regeneration [9].
Scientists have developed artificial corneas made from biocompatible materials that can be implanted in patients with corneal damage or disease [10,11]. One major aspect of TE in ophthalmology is the use of scaffolds, which play an essential role in supporting structural integrity and promoting cell growth and TE. Scaffolds are three-dimensional structures that imitate the extracellular matrix (ECM) of tissues and offer a structure for the attachment, growth, and differentiation of cells [12,13]. In ophthalmology, scaffolds can be used to repair damaged corneal tissue, restore vision, and treat various ocular diseases. Various scaffolds have been employed in ophthalmic TE, encompassing both natural materials like collagen, fibrin, and hyaluronic acid, and synthetic materials such as poly (lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) [10,14]. The scaffolds can be tailored to possess distinct characteristics, such as biocompatibility, biodegradability, and mechanical strength, in order to meet the requirements of various ocular tissues [15].
Scientists have also investigated the utilization of stem cells in conjunction with scaffolds for the purpose of ocular regeneration. Stem cells possess the capacity to undergo differentiation into distinct cell types that are present in the eye. This characteristic makes them a highly promising and viable option for the regeneration of impaired tissues [16]. By seeding stem cells onto scaffolds, researchers can create complex tissue structures that closely resemble native eye tissues [17]. In recent years, advancements in biomaterials science and TE techniques have enabled researchers to develop innovative scaffold-based therapies for various ocular conditions. For example, corneal scaffolds have been used to treat corneal ulcers, while retinal scaffolds have been developed for the treatment of retinal degenerative disorders, such as age-related macular degeneration [18,19]. Current management options for these diseases have limitations: 1. Laser surgery and vitrectomy, while utilized, are frequently damaging and do not adequately tackle the fundamental crucial Basis of these disorders [4,20]. 2. Photodynamic therapy (PDT) may result in problems such as bleeding in the retina and vitreous, as well as the rupture of cells in the retinal pigment epithelium (RPE) [21]. 3. Angiostatic steroids necessitate repeated intravitreal injections and are associated accompanied by a multitude of adverse reactions when used for an extended period of time [22]. 4. Anti-vascular endothelial growth factor (Anti-VEGF) agents, while effective for some, fail to consider the function of inflammation in the development of diseases, and a substantial portion of patients continue to be unresponsive to this treatment [4,21]. 5. Gene therapies such as Luxturna, while revolutionary, encounter obstacles in manufacturing, research methodology, evaluation of long-term safety, and marketability, in addition to having potential negative effects [23]. 6. Tissue transplantation faces issues such as donor shortages, high rejection rates, and post-operative complications like infections [4,[24], [25], [26]]. TE strategies offer potential solutions to overcome these limitations. This interdisciplinary field combines biology with material science and employs two main approaches: Cell-based tactics involve the manipulation of cells to establish a customized microenvironment prior to transplantation. On the other hand, scaffold-based strategies utilize an artificial extracellular matrix (ECM) that imitates the natural structures of the body [27].
Scaffold-based methods stand out for their adaptability. In scaffold-based TE, scaffolds are designed with specific physical and chemical properties to promote cell adhesion, differentiation, and growth [28]. Ensuring scaffold biocompatibility is crucial for supporting cellular proliferation. Biodegradable and bioactive scaffolds have continued to develop over the last two decades with the goal of avoiding secondary surgeries to remove implants, stimulating cellular activities and functions, and eventually facilitating tissue regeneration in situ with leveraging the natural regenerative abilities of body tissues [4,24,27,29]. Moving forward, the translation of scaffold-based therapies from bench to bedside will require rigorous preclinical testing and clinical trials to ensure their safety and efficacy. Collaborations between researchers, clinicians, regulatory agencies, and industry partners will be vital for bringing these innovative therapies to patients in need. This study aims to review recent developments in eye TE and reconstruction, focusing on new strategies, challenges, and prospects. In this review article, we conducted a comprehensive search of databases including PubMed, ScienceDirect, and Google Scholar, selecting around 126 original and review research papers published between 2000 and 2024 for inclusion in our analysis.
1.1. Tissue engineering and common eye diseases
The first to third most common eye diseases that may be addressed through TE include corneal diseases (prevalence rate approximately 1 in 500), age-related macular degeneration (AMD) (affecting about 10 % of individuals over 65), and diabetic retinopathy (prevalence rate around 30 % among diabetics), all of which present opportunities for innovative therapeutic interventions [[30], [31], [32], [33]].
TE presents significant potential for the treatment of various ophthalmopathies through the regeneration or repair of damaged ocular tissues. A key focus area is corneal diseases, where TE techniques can facilitate the regeneration of corneal stroma using biomaterials and stem cells to restore transparency and functionality [34]. In cases such as keratoconus, methods like collagen cross-linking and grafting are utilized to enhance corneal strength [35]. Furthermore, in the context of ocular surface disorders like limbal stem cell deficiency, the cultivation of limbal stem cells on appropriate scaffolds has been shown to restore the corneal epithelium and improve visual outcomes [36]. Engineered grafts also contribute to the healing of the corneal surface in instances of persistent epithelial defects [37].
In the field of retinal disorders, TE strategies are being investigated for conditions such as age-related macular degeneration (AMD). The development of retinal pigment epithelium (RPE) patches derived from stem cells offers a potential solution for replacing damaged cells [38]. Additionally, engineered retinal patches can aid in the reattachment and support of the retina in cases of retinal detachment [39]. These advancements are critical for restoring vision in patients with degenerative retinal diseases.
Moreover, TE is advancing in the treatment of glaucoma through the creation of implants designed to regulate intraocular pressure or regenerate damaged optic nerve fibers [40]. In the case of uveitis, the development of tissue-engineered drug-delivery systems allows for localized treatment aimed at reducing inflammation in the uvea [41]. Finally, TE approaches are also focused on regenerating the optic nerve using nerve grafts or scaffolds to support neuronal growth [42]. The field of eye TE has emerged as a significant area of clinical practice and research, offering innovative strategies that collectively promise to enhance the quality of life for patients with various ocular conditions [30,43].
1.2. Tissue engineering in regeneration
The main element for sustaining human life is the innate ability to repair itself naturally after physical damage [44]. Over the past three decades, there has been a notable development in the field of TE and regenerative medicine. This field primarily aims to regenerate impaired tissues, rather than resorting to their complete replacement. The approach involves the creation of biological substitutes that have the potential to restore and enhance tissue functionality [45]. The term 'tissue engineering' was initially introduced at a workshop organized by the National Science Foundation (NSF) at Granlibakken, California [22]. TE is classified into three categories, namely: 1. isolated cell implantation, which involves the transplantation of individual cells; 2. administration of growth agents to the cells to promote cellular proliferation; and 3. incorporation of cells onto or within various scaffolds designed to stimulate the production or secretion of ECM. The latter is the most frequently utilized TE technique, which involves the placement of viable cells onto synthetic or natural extracellular scaffolds to generate a substrate that can be implanted [46]. The surface of a scaffold serves as the primary locus for interaction with the surrounding milieu, thereby influencing the cell adhesion, cell proliferation, and cell differentiation [47]. The critical juncture lies in the chance to promote vascularization of the voluminous scaffolds, thereby facilitating the adequate supply of minerals, nutrients, oxygen, and growth factors necessary for tissue regeneration [48].
The scaffold has been created to specifically attract cells to the required volume for regeneration, enabling them to subsequently undergo cell division, and specialization, and finally form tissue within the scaffold. Over a period, the scaffold will deteriorate, resulting in the exclusive presence of the regenerated tissue [44,49]. Tissue-engineered nerve grafts (TENGs) have been identified as a viable alternative for autologous nerve grafts, which are considered as the most effective method for repairing peripheral nerves [50]. Henceforth, cell-based therapies are regarded as a preferred approach in tissue regeneration [51,52]. Restoring the functionality of impaired bodily tissues through the repair or reconstruction of faulty structures has long been a goal of medical science. TE has emerged as a field dedicated to tackling this very challenge [53].
1.3. Safety concerns for scaffold biomaterials
Safety considerations regarding scaffold biomaterials in TE and regenerative medicine encompass several critical dimensions. Primarily, the assurance of biocompatibility is fundamental to prevent adverse immune responses or toxicity [54]. Furthermore, the degradation products generated by scaffolds must be non-toxic and should not elicit inflammatory reactions [55]. The mechanical properties of scaffolds are also of paramount importance, as they must possess adequate strength to support tissue development without structural failure [56]. Ensuring sterility is critical to preventing post-implantation infections, while the maintenance of long-term stability is essential for mitigating the risk of chronic complications; furthermore, strict adherence to regulatory standards is imperative to guarantee safety and efficacy prior to clinical application [[57], [58], [59]]. Additionally, scaffolds should facilitate appropriate cellular interactions, including adhesion and differentiation, while avoiding any aberrant cellular behavior [60]. The origin of biomaterials presents substantial concerns regarding potential disease transmission and ethical implications, necessitates a comprehensive evaluation of their environmental impact to mitigate ecological risks, and underscores the importance of individualized assessments of scaffold safety due to variability in patient responses [[61], [62], [63]]. Addressing these safety considerations is imperative for the successful application of scaffold biomaterials in clinical practice, particularly in the realm of ocular diseases, where factors such as biocompatibility, sterility, and mechanical properties of scaffolds are critical for achieving favorable outcomes and minimizing complications associated with the regeneration and repair of ocular tissues.
1.4. Perspective for ocular tissue engineering
Ocular TE is increasingly recognized as a pivotal area within regenerative medicine, focusing on the restoration of vision and the repair of compromised ocular structures through the application of innovative biomimetic scaffolds and cellular therapies [64]. Recent scholarly investigations underscore the development of advanced biomaterials that not only exhibit optimal biocompatibility and biodegradability but also possess mechanical properties specifically designed to accommodate the delicate environment of the eye [65]. Current research endeavors emphasize the utilization of stem cell technologies, particularly induced pluripotent stem cells (iPSCs), in conjunction with growth factors to enhance tissue regeneration and functionality in ocular applications [66]. Furthermore, the incorporation of 3D bioprinting and nanotechnology is gaining traction, enabling the fabrication of complex, structured tissues that closely replicate the architecture of natural ocular tissues, thereby enhancing the precision of TE methodologies [67,68].
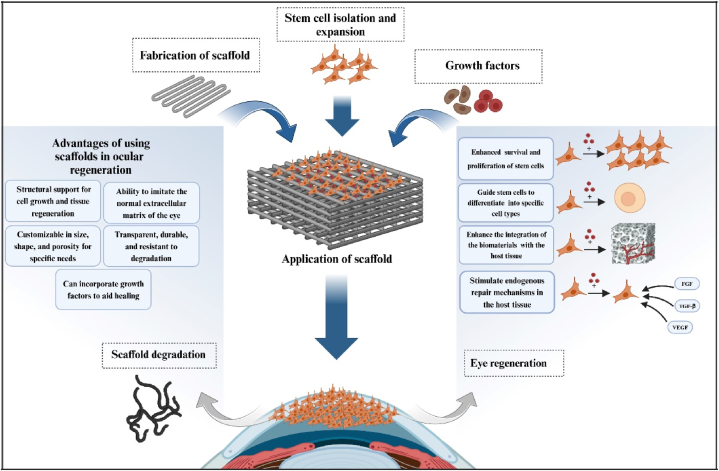
Recent literature also highlights the critical importance of addressing safety concerns, including the risks of immune rejection and infection, which are essential for the successful translation of these technologies into clinical practice [69]. Compliance with regulatory standards and the ethical implications associated with the use of biological materials remain significant considerations that necessitate careful navigation [70,71]. The successful implementation of these advanced techniques in ocular TE holds considerable promise for improving therapeutic options for a spectrum of ocular diseases, such as corneal injuries, retinal degeneration, and glaucoma, ultimately enhancing patient outcomes and quality of life. Emerging studies suggest that personalized approaches, including the development of patient-specific scaffolds and targeted delivery systems, may further revolutionize treatment paradigms within this field [72] (Fig. 1).
Fig. 1.
This image shows the important role of scaffolds in eye tissue engineering. Scaffolds provide structural support and facilitate cell adhesion and growth, in addition to adding growth factors to enhance cell activity and differentiation and promote eye regeneration. Together, they create an optimal environment for eye tissue repair.
2. Scaffolds used for eye tissue engineering
In this study, the scaffolds utilized for ocular TE have been classified into four categories according to tissue type: natural, synthetic, biohybrid, and decellularized tissue, with further details available in Table 1.
Table 1.
Cell types, model studies, and advantages/disadvantages of biomaterials used in ocular tissue engineering.
| Tissue | Material | Biomaterial | Cell types | Model study | Advantage | disadvantage | Ref. |
|---|---|---|---|---|---|---|---|
| Conjunctiva | Natural | autologous fibrin | CjECs | NZW strain rabbits | The availability, cost-effectiveness, and high tolerance to culture conditions, degrading swiftly without any detrimental impact on the survival of the cultivated limbal epithelial cells | not being contracted by stromal cells, in contrast to using collagen as substrate, | [73] |
| Biohybrid | (SF/PLCL) | rCjECs | mice | having outstanding biocompatibility, demonstrating exceptional manifestation of CjEC genes and decreasing manifestation of inflammatory mediators, the capability to create a well-organized conjunctival epithelium, which includes the presence of goblet cells | Not applicable | [96] | |
| (PLA/EFMs) surface coated by CNF and/or SP loaded with LF | CjECs | NZW (New Zealand white) rabbits | Effective suppression of bacterial growth and reduction of antibiotic usage after surgery | Not applicable | [93] | ||
| SF-rGo | CJMSCs | In vitro | offering advantageous mechanical and cytocompatibility characteristics, as well as a larger surface-to-area ratio compared to alternative manufacturing techniques by using SF, exhibiting exceptional electrical conductivity by use of rGO | Not applicable | [94] | ||
| (collagen/PLCL) | CjECs | in vitro | displaying advantageous mechanical properties, wettability, and the ability to promote cell proliferation/mimicking the ECM and supporting the growth and differentiation of goblet cells/not induce an upregulation of IL-4, IL-5, and IL-6 expression, unlike what is observed in TCPS (tricalcium phosphate scaffold) culture | Not applicable | [95] | ||
| Cornea | Natural | microgrooved collagen films | CEC | In vitro | displaying comparable optical clarity, swelling and biodegradability Compared to the natural cornea, which promoted epithelial cell migration, wound healing, and keratocyte fibrosis retardation | Not applicable | [74] |
| collagen-glycosaminoglycan | corneal keratocyte cell | In vitro | Characteristics include transparency, strength, elasticity, cell development, and resistance to collagenase destruction | Decreasing collagen synthesis | [75] | ||
| Biohybrid | PCL microfibrous scaffold infused with rat tail collagen type I | LSSCs (in vitro)-keratocytes (in vivo) | Rat | Enhancing the organization of collagen and reducing the presence of fibroblasts and myofibroblasts in injured corneas, promoting the ECM-related pathway and increasing the expression of various ECM-related genes in the injured group | Not applicable | [10] | |
| biodegradable silk fibroin-based scaffolds containing GDNF | – | mice | promoting epithelialization, keratocyte and epithelial cell proliferation, stromal nerve plexus development, and anti-apoptotic activity | Not applicable | [12] | ||
| (GelMA-HA) | Rabbit CS cell | In vitro | supplying CS cells with cues for spatial and directional organization and ECM remodeling | Not applicable | [101] | ||
| (PVA-COL) | Human and rabbit CEC | In vitro/rabbit | Making stratified epithelial histologically and functionally similar to healthy epithelial surface | Not expressing collagen type IV and VII even with soluble laminin and the protease inhibitor aprotinin, Failure to achieve stable epithelialization in vivo |
[19] | ||
| (AM)- (PVA-AM) | rabbit CEC | Japanese white rabbits | Being easy to handle and transplant to the cornea, Benefiting from AM tissue's inherent basement membrane and PVA's transparency and durability | Stabilizing the AM component of PVA-AM is still an issue remaining to be resolved | [97] | ||
| (GP/PVA/SF/n-HA) | HCFs cells | In vitro | Regularizing PVA/SF/n-HA composite hydrogel, enhancing heat stability, and reducing moisture | Not applicable | [98] | ||
| VH | – | rabbit | Biomechanical stability and optical transparency, preventing infections caused by S. aureus in implanted devices. in vivo and in vitro | Not applicable | [99] | ||
| Aligned (PVA-COL) | HKs and HCECs | In vitro | Similar mechanical strength to real corneal tissue, enhancing electrospun scaffold mechanical characteristics | Not applicable | [100] | ||
| sHAPN copolymers | rabbit cornea cells | In vitro/rabbit | Being a thermo-responsive carrier, enhancing the ocular bioavailability of multiple ophthalmic medications, delivering crucial therapeutic benefits such as anti-inflammatory properties and corneal protection | Not applicable | [103] | ||
| oHA | keratocytes | rabbit | enhancing gelatin microcarriers for greater oHA grafting by leveraging oxidation levels in aldehyde HA | Not applicable | [102] | ||
| Decellular | LCs | primary corneal endothelial cells | In vitro | Increasing the surface area of focal adhesions in cells cultured on coated liquid crystals by at least twofold compared to other settings | completed digestion after 13 h for LC and amniotic membrane, whereas the DM was digested after 17 h | [109] | |
| decellularized (SMILE) lenticule (SL), (AM), and collagen-coated plates | hADSCs | New Zealand male rabbits | can culture Keratocytes better | Not applicable | [108] | ||
| carbodiimide crosslinked RHC | – | Human | Being stable for four years without rejection episodes and without immunosuppression, correcting visual acuity of 20/54 and gaining more than 5 Snellen lines on an eye chart | Not applicable | [110] | ||
| Native porcine conjunctiva | CEC | rabbit | Better optical transmittance, tensile strength, stability, biocompatibility, and degradation resistance in vitro and in vivo, longer survive of donor cells | Not applicable | [111] | ||
| Porcine | rabbit corneal limbal epithelial cells | In vitro | Not applicable | The necessity of Future research to assess the endurance of the treated cornea and study in vitro cell recellularization and penetration in the corneal matrix | [112] | ||
| decellularized Human doner cornea | LEPC, LMSC and LM | In vitro | Using non-immunogenic tissue scaffolds for transplantation and having the ability to be repopulated by host cells either in situ or in vitro | Not applicable | [113] | ||
| (FD-APCS) | CEC | In vitro/NZW rabbits | Having no major differences from the APCS-transplanted or native cornea, providing a void area for cells and a collagen lamellae ultrastructure identical to native cornea stroma | Not applicable | [114] | ||
| Decellularized murine corneas | (hESC-CEC) | In vitro | Not applicable | Not applicable | [119] | ||
| decellularized human cornea | hCEC and hLEC | In vitro | Presenting characteristic indicators of (hCEC) and (hLEC) on their respective surfaces. | The vitality of Further research to evaluate if corneal structures are suitable for transplantation. | [120] | ||
| lacrimal gland | Synthetic | polyester membrane | pLGACs | In Vitro | Not applicable | Not applicable | [82] |
| PES | Lacrimal acinar epithelial cells of Sprague-Dawley rats | In Vitro | Excellent oxidative, thermal, and hydrolytic stability | Not being biodegradable | [81] | ||
| Decellular | SIS-Muc | Porcine LG epithelial cells | In Vitro | Promoting normal lacrimal fluid production in epithelial cells grown on SIS-Muc, mimicking natural LG acini polarization | Failure to observe polarization or acini-like features in synthetic tissue | [116] | |
| NZW rabbit lacrimal glands ECM | adult rabbit lacrimal gland progenitor cells | In vitro | keeping cells alive and secreting for four weeks | Further research is needed to optimize decellularization | [13] | ||
| DC-LG | LG epithelial cells | In vitro | A three-dimensional, supporting, and accessible matrix provides LG-specific ECM protein amounts, distribution, and composition | Requiring to Further evaluation of this LG construct by functional research in vivo | [115] | ||
| Lens | Biohybrid | biodegradable HA and nondegradable polymeric gel | – | Dutch Belt pigmented and NZW rabbits | Excellent cortical anatomy and lens clarity | Transparent regrowth in the lens and peripheral capsule bag, with opacified regrowth behind the polymeric scaffold | [104] |
| Retina | Natural | GCH | Human embryonic stem cells | mouse | promoting retinal cell differentiation over other anterior forebrain cells and inducing a modest immune response, allowing the implant to survive 12 weeks | basic retinal lamination and cytoplasmic transfer instead of photoreceptor layer implantation | [77] |
| Cask and Caskin1 | – | In vitro | Having Global synaptic function | Not applicable | [76] | ||
| RS1 | – | In vitro | stabilizing retinal integrity | Not applicable | [79] | ||
| Fibrin hydrogel | CJMSCs | In vitro | promoting cell growth and proliferation without harming cells Because of its flexibility and continual disintegration, fibrin hydrogel | Not applicable | [78] | ||
| Synthetic | PCL | Mouse and Human RPCs | In vitro/Adult Rho -/- or wildtype mice | the ability to engage with mRPCs and human RPCs and drive them toward a photoreceptor fate, allowing cell differentiation before transplantation | Not applicable | [17] | |
| PLLA and PLGA | RPCs | In vitro/rat | Being desired to simulate retinal polarization | Not applicable | [83] | ||
| PLGA | RGCs | rabbits and monkeys | been discovered in rabbits' intraocular environments after 3 months | The necessity of Future studies to adjust the molecular weight of PLGA substance and extending the observation duration to determine the scaffold's biodegradability in vivo | [85] | ||
| Laminin coated novel nanowire PCL | Mouse RPCs | Rho −/− mice | Showing Biocompatibility by cell attachment and sustained proliferation | Not applicable | [84] | ||
| polyethylene terephthalate or poly(L-lactide-co-ε-caprolactone) | hfRPE | In vitro/female Chinchilla Bastard rabbit | showing favorable subretinal biocompatibility | Not applicable | [86] | ||
| microfabricated poly(glycerol-sebacate) | RPCs | In vitro | having a 10-fold higher maximum elongation at failure than earlier RPC scaffolds, significantly improving mechanical characteristics and reducing scaffold thickness | Not applicable | [87] | ||
| PCL, PGS and POC | RPCs | In vitro | enhancing scaffold hydrophilicity and degradation, accelerating human retinal pigment epithelial cell proliferation, decreasing fiber diameter, and boosting tensile modulus | Not applicable | [18] | ||
| 3-D PCL cell encapsulation scaffold | Mouse RPC | In vitro | Enabling regulated, accurate, targeted administration of cells to the subretinal area, providing various benefits compared to earlier 2 and 2.5-D structures used for retinal progenitor cell transplantation/having the structure which is highly porous, facilitating diffusion and potential cell interactions from both the neural retina and the RPE | Not applicable | [88] | ||
| PLGA | hiPSC -derived retinal progenitor cells | In vitro | Modifications in the dimensions of the pores, the distance between the slices, the distance between the hatches, and the type of hatching. | Not applicable | [89] | ||
| PLGA | hiPSC retinal organoid derived RGCs | rhesus monkey | allowing transplanted tissues to survive 1 year without tumorigenesis with enough graft–host contact | Not applicable | [14] | ||
| PCL and PEG included taurine | CJMSCs | In vitro | assisting CJMSCs develop into photoreceptors by Taurine | Not applicable | [16] | ||
| Biohybrid | (RWSF/PCL/Gt) | RPE | rabbits | Having good in vitro and in vivo cytocompatibility for RPE implantation as a prosthetic Bruch's membrane | Not applicable | [15] | |
| (SF/PLCL) | RPCs | in vitro | greatly increasing RPC proliferation, including photoreceptors with high porosity and ECM topography | Not applicable | [105] | ||
| gelatin/chitosan | RPE | in vitro | imitating additional cellular matrix and Bruch's membrane nanofibrous structure, without cytotoxicity, and not modifying grown hRPE cells on gelatin/scaffold | needing further clinical trials to prove these scaffolds can treat retinal disorders | [106] | ||
| HAMP/PCL | RPE cells | In vitro | Optimizing porosity, degradation, and biocompatibility | The necessity to use more realistic RPE cultures obtained from primary or stem cell cultures in Future investigations | [107] | ||
| Optic Nerve | Natural | Netrin-1 gradient | RGCs | In vitro | increasing the amount of transplanted RGCs whose axons reach the optic nerve head | not noticing polarized cell directionality | [80] |
| Synthetic | PCL and PBG | RGCP | In vitro | successfully constructed, supporting cell survival and durable long neurite development along fibers | Not applicable | [91] | |
| PCL coated by tosylate + PEDOT | chick dorsal root ganglia and a mouse neuroblastoma cell line | In vitro | directing the nerve bundle | Not applicable | [90] | ||
| PPy-G | RGCs | In vitro | Increasing RGC density and directing neurite outgrowth and nanofiber direction | Not applicable | [92] | ||
| Decellular | porcine decellularized optic nerve | neurotrophin-3-overexpressing Schwann cells | In vitro/Rat | Increasing dorsal root ganglion neurite directional growth, myelin regeneration, neural stem cell adhesion, survival, and migration, and reducing inflammatory cells and chondroitin sulfate proteoglycan expression | The necessity of future studies to modify ECM proteins on the scaffold, assess animal behavior and electrophysiological function, and conduct large animal models for preclinical efficacy testing | [118] | |
| DON | DRG neurites | In vitro | causing lengthier extension, greater distances, and branching on the DON than the ON, selectively removing axon-inhibitory substances including myelin-associated glycoprotein and chondroitin sulfate proteoglycans by Decellularization | Not applicable | [117] |
BrM = Bruch's membrane/ON = optic nerve/RGCs = Retinal Ganglion Cells/WHO = World Health Organization/TE = tissue engineering/NSF = National Science Foundation/AMD = Age-related Macular Degeneration/VEGF= Vascular Endothelial Growth Factor/TENGs = Tissue-engineered nerve grafts/HAM = human amniotic membrane/RS1= Retinoschisin/GCH=Gelatin/Chondroitin sulfate/Hyaluronic Acid/hESC = human embryonic stem cell/ONL= Outer Nuclear Layer/PES= Polyethersulfone/PCL= Polycaprolactone/PLLA= Poly L-lactic Acid/PLGA= Poly Lactic-co-Glycolic Acid/PGS= Poly Glycerol Sebacate/PPy-G = polypyrrole functionalized graphene/PLA= Poly Lactic Acid/EFMs = Electrospun nanofibrous membranes/CNF= Cellulose Nanofibrils/SP= Silk Peptide/LF = levofloxacin/SF= Silk Fibroin/rGo = reduced Graphene oxide/PLCL = poly (L-lactic acid-co-3- caprolactone)/SF/rGO= Silk Fibroin/reduced Graphene oxide/ ECM = extracellular matrix/ Collagen/PLCL = Collagen/poly (L-lactic acid-co-3- caprolactone)/CjECs = Conjunctival Epithelial Cells/SF/PLCL = Silk Fibroin/poly (L-lactic acid-co-3- caprolactone)/GelMA-HA = hyaluronic acid-modified gelatin-methacrylate/PVA-COL= Collagen-Immobilized Poly (Vinyl Alcohol)/AM = Amniotic Membrane/PVA-AM = polyvinyl alcohol hydrogel/GP/PVA/SF/n-HA= Genipin-crosslinked polyvinyl alcohol/silk fibroin/nanohydroxyapatite Hydrogel/PCL/collagen = Polycaprolactone/collagen/HA= Hyaluronic Acid/RWSF= Regenerated wild Antheraea pernyi Silk Fibroin/Gt = Gelatin/HAMP= Human amniotic membrane powder/LCs = Lens Capsules/FD-APCS= Freezing-Dry Acellular Porcine Cornea Stroma/SMILE= Small incision lenticule extraction/SL = lenticule/RHC= Recombinant Human Collagen/NZW= New Zealand White/(SIS-Muc) = Conversely decellularized porcine small intestine submucos/DC-LG = Decellularized porcine LG matrix/DON = Decellularized Optic Nerve/PLA=Poly Lactic Acid/SP= Silk Peptide/CJMSCs= Conjunctiva Mesenchymal Stem Cells/PLCL= Poly L-lactic acid-co-3- Caprolactone/rCjECs = rabbit Conjunctival Epithelial Cells/LCs = Lens Capsules/hADSCs = human Adipose Mesenchymal Stem Cells/PCL= Poly ε-Caprolactone/LSSCs= Limbal Stromal Stem Cells/GDNF = Glial Cell-Derived Neurotrophic Factor/LEPC= Limbal Epithelial Progenitor Cells/LMSC= Limbal Mesenchymal Stromal Cells/LM = Limbal Melanocytes/FD-APCS= Freezing Dry Acellular Porcine Cornea Stroma/GelMA-HA= Hyaluronic Acid-modified Gelatin-Methacrylate/CS= Corneal Stromal/hESC-CEC = human Embryonic Stem Cells- Corneal Epithelial Cells/PVA-COL= Colagen-Immobilized Poly Vinyl Alcohol/CEC= Corneal Epithelial Cells/GP= Genipin/n-HA = nanohydroxyapatite/HCFs = Human Corneal Fibroblasts/VH= Vancomycin-loaded collagen Hydrogels/hCEC = human Corneal Endothelial Cells/hLEC = human Limbal Epithelial Cells/PVA= Aligned Polyvinyl Acetate/HKs = Human Keratocytes/LG = Lacrimal Gland/PLGACs= Purified rabbit Lacrimal Gland Acinar Cells/RPCs= Retinal Progenitor Cells/RS= Retinoschisin/PCL= Polycaprolactone/mRPCs = mouse Retinal Progenitor Cells/hiPSC = human-induced Pluripotent Stem Cell/RPE = Retinal Pigment Epithelial/PEG= Polyethylene Glycol/DRG = Dorsal Root Ganglion/PBG= Poly-gamma-Benzyl-L-Glutamate/RGCP= Retinal Ganglion Cell Progenitors/PEDOT= PSS (polystyrene sulfonate) in water and isopropanol/TCPS = tricalcium phosphate scaffold/LCs = Lens Capsules/DM = Descemet's membrane/DHC = Decellularized Human Cornea/FD-APCS= Freezing Dry Acellular Porcine Cornea Stroma/GelMA-HA= Hyaluronic Acid-modified Gelatin-Methacrylate/PVA= Polyvinyl Alcoholhydrogel/POC= Poly (1,8-Octanediol-co-Citrate)/LSSCs= Limbal Stromal Stem Cells/hfRPE = Human fetal Retinal Pigment Epithelium cells/IL-4, IL-5, and IL-6 = interleukin-4, interleukin-5, Interleukin-6/sHAPN= Sulfated Hyaluronic acid with amine-terminated poly(N-isopropylacrylamide)/oHA = Oxidized hyaluronan.
2.1. Natural biomaterials
2.1.1. Conjunctiva
Autologous fibrin has emerged as an effective conjunctival scaffold, particularly in studies utilizing the New Zealand white strain rabbit model. This approach involves harvesting fibrin from the patient, which is then utilized as a structural matrix for cultivating conjunctival tissue aimed at repairing conjunctival abnormalities. Research indicates that the efficacy of autologous fibrin in this context is comparable to that of human amniotic membrane (HAM), which has long been considered a gold standard in ocular surface reconstruction due to its anti-inflammatory properties and ability to promote epithelialization [73].
2.1.2. Cornea
In the field of corneal TE, innovative scaffold designs such as microgrooved collagen films and collagen glycosaminoglycan composites have been explored for their potential to mimic natural corneal properties. These scaffolds, tested in vitro, demonstrate several advantageous characteristics, including optical clarity, swelling behavior similar to that of natural cornea, and biodegradability. The microgrooved architecture of the collagen scaffolds is particularly beneficial, as it stimulates epithelial cell migration, accelerates wound healing processes, and reduces keratocyte fibrosis, thereby enhancing the overall regenerative capacity of the corneal tissue [74,75].
2.1.3. Retina
In the context of retinal scaffold development, a diverse array of biomaterials has been investigated, including Cask and Caskin1 proteins, the retinal protein retinoschisin (RS1), a Gelatin/Chondroitin sulfate/Hyaluronic Acid (GCH) composite, and fibrin hydrogel. Cask is essential for maintaining the integrity of all retinal synapses, facilitating synaptic signaling and structural stability. In contrast, Caskin1 appears to have specialized roles in particular retinal synapses, such as promoting neural pathway development and stabilizing synaptic connections during retinal maturation. The GCH scaffold has shown promise in delivering human embryonic stem cell-derived retinal progenitor cells (RPC) to the outer nuclear layer (ONL) of the retina in mouse models of retinal degeneration. Notably, some RPCs have been observed migrating into the inner retinal layers, indicating the scaffold's potential to not only support cell survival but also to facilitate integration within the retinal architecture. Furthermore, the fibrin hydrogel enhances the availability of oxygen and nutrients to transplanted cells, which is crucial for their viability and function post-transplantation [[76], [77], [78], [79]].
2.1.4. Optic nerve
recent studies have explored the application of a Netrin-1 gradient in optic nerve regeneration, yielding encouraging outcomes. The strategic use of a protein gradient on a radially electrospun scaffold has been shown to significantly enhance retinal ganglion cell (RGC) axon growth, guiding axonal extensions in alignment with the developmental pathways of the optic nerve head. This innovative approach holds substantial promise for advancing cell transplantation therapies aimed at treating glaucoma and other optic neuropathies, as it may improve the survival and functional integration of transplanted cells within the damaged neural environment [80].
2.2. Synthetic biomaterials
2.2.1. Lacrimal gland
The fabrication of scaffolds for the lacrimal gland often utilizes polyester membranes and polyethersulfone (PES) due to their favorable mechanical properties. PES is particularly noted for its exceptional stability under in vitro conditions, exhibiting significant resistance to oxidation, thermal degradation, and hydrolysis. These characteristics make PES a promising candidate for a variety of biomedical applications. However, it is important to highlight that PES is non-biodegradable, which may limit its long-term applicability in biological systems, especially in scenarios where gradual degradation is beneficial for tissue integration and healing processes [81,82].
2.2.2. Retina
In the realm of retinal scaffolds, an extensive range of materials has been employed, including Laminin-coated novel nanowire polycaprolactone (PCL), poly(e-caprolactone) (PeCL), poly (L-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), polyethylene terephthalate (PET), and poly(L-lactide-co-ε-caprolactone). The synthetic strategies for these materials frequently involve electrospinning to produce nanofibrous structures that enhance surface area and porosity, thereby facilitating cellular infiltration and nutrient exchange. Additional techniques such as 3D bioprinting allow for precise control over scaffold architecture, while solvent casting combined with freeze-drying generates porous structures that closely mimic the ECM. Other materials, including microfabricated poly(glycerol-sebacate), poly(glycerol sebacate) (PGS), poly(1,8-octanediol-co-citrate) (POC), 3-D polycaprolactone (3DPCL), and PEG-modified taurine, have also been explored for their potential as scaffolding materials in retinal TE [[16], [17], [18],[83], [84], [85], [86], [87], [88], [89]]. The use of Laminin-coated novel nanowire PCL in Rho−/− mice has shown promising biological compatibility, as evidenced by improved cell adhesion and sustained proliferation. These scaffolds have the potential to direct the differentiation of stem cells into photoreceptors, providing a viable platform for pre-transplantation cell differentiation. This capability is essential for preparing cells that are more likely to integrate successfully into host tissue following transplantation [84]. Furthermore, PLGA is particularly advantageous due to its ability to replicate the polarized characteristics of the retina, owing to its adjustable degradation rates and biocompatibility [83,85]. Studies indicate that PLGA scaffolds can support the survival and functionality of retinal cells, making them a promising option for retinal repair strategies. Additionally, PET has demonstrated favorable biocompatibility in studies involving female Chinchilla Bastard rabbits, particularly in the subretinal region, suggesting its viability for clinical applications in retinal surgery [86].
2.2.3. Optic nerve
In the context of optic nerve scaffolds, PCL, and poly-gamma-benzyl-L-glutamate (PBG) have been utilized, alongside PCL coated with tosylate and PEDOT (polystyrene sulfonate) in a water-isopropanol mixture, as well as polypyrrole functionalized graphene (PPy-G). The synthetic methodologies employed for these materials often include solvent casting, phase separation techniques, and layer-by-layer assembly, which facilitate the creation of porous structures that promote nerve regeneration. Additionally, chemical crosslinking methods can enhance the mechanical properties and stability of the scaffolds. These materials have been engineered to improve cell viability and encourage the growth of elongated neurites aligned with the fiber direction. Notably, PCL coated with tosylate and PEDOT has proven effective in guiding nerve bundles, highlighting its potential application in nerve regeneration. This guidance is crucial for restoring functional connectivity in damaged nerves, which could significantly enhance recovery outcomes for patients with optic nerve injuries [[90], [91], [92]].
2.3. Biohybrid scaffolds
2.3.1. Conjunctiva
The development of conjunctival biohybrid scaffolds has increasingly leveraged innovative biohybrid materials and methodologies to enhance biocompatibility, functionality, and overall efficacy in ocular applications. Central to this advancement are poly (lactic acid) (PLA) electrospun nanofibrous membranes (EFMs), often surface-coated with cellulose nanofibrils (CNF) and/or silk peptide (SP) loaded with levofloxacin (LF). This coating confers robust antibacterial properties, thereby significantly reducing the necessity for post-surgical antibiotic administration. These scaffolds have undergone rigorous testing in vivo, particularly in studies involving New Zealand white rabbits, which have demonstrated their effectiveness in promoting healing and preventing infections [93]. In addition to PLA-EFMs, scaffolds composed of silk fibroin (SF) and reduced graphene oxide (rGO) have emerged as promising alternatives. The incorporation of SF facilitates the formation of a larger surface area, effectively mimicking the natural ECM in multiple dimensions. This structural mimicry is crucial for enhancing cell adhesion, migration, and proliferation. Furthermore, rGO is characterized by its exceptional electrical conductivity, which can stimulate cellular responses and improve tissue integration [94]. Moreover, collagen combined with poly L-lactic acid-co-ε-caprolactone (PLCL) has been extensively studied for its favorable properties. Notably, in vitro analyses have shown that the presence of conjunctival epithelial cells on collagen/PLCL scaffolds does not induce an increase in the expression of pro-inflammatory cytokines such as IL-4, IL-5, and IL-6, indicating a supportive immunological profile [95]. Additionally, conjunctival epithelial cells cultivated on SF/PLCL hybrid scaffolds have demonstrated the ability to develop a stratified conjunctival epithelium, inclusive of goblet cells, when evaluated in murine models [96].
2.3.2. Cornea
Corneal biohybrid scaffolds are progressively incorporating innovative materials to improve tissue regeneration and functionality, particularly PeCL microfibrous scaffolds infused with rat tail collagen type I, which have shown considerable promise in facilitating cellular infiltration and promoting ECM deposition. Additionally, biodegradable silk fibroin-based scaffolds that contain glial cell-derived neurotrophic factor (GDNF) have been recognized for their ability to facilitate epithelialization and enhance the proliferative activity of epithelial cells in murine models. Other noteworthy materials include hyaluronic acid-modified gelatin-methacrylate (GelMA-HA) and collagen-immobilized polyvinyl alcohol (PVA-COL), both of which have proven effective in creating scaffolds that closely mimic the natural corneal environment. Furthermore, amniotic membrane-immobilized polyvinyl alcohol hydrogel (PVA-AM) combines the advantageous properties of natural basement membrane components found in amniotic tissue with the transparency and durability of artificial PVA, rendering it a valuable option for corneal applications.
The genipin-crosslinked polyvinyl alcohol/silk fibroin/nanohydroxyapatite hydrogel (GP/PVA/SF/n-HA) has also been investigated for its potential to enhance mechanical properties and biocompatibility, thereby facilitating corneal tissue integration. Experimental studies have shown that corneas subjected to injury and treated with PCL/collagen scaffolds exhibit a more uniform distribution of collagen fibers and a reduced presence of fibroblasts and myofibroblasts, indicating improved healing outcomes. Moreover, scaffolds that incorporate vancomycin, such as vancomycin-loaded collagen hydrogels (VH), have proven effective in inhibiting Staphylococcus aureus infections associated with implanted devices, as demonstrated by both in vitro and in vivo studies conducted on rabbits [10,12,19,[97], [98], [99], [100], [101]]. sHAPN copolymers, composed of sulfated HA and amine-terminated poly(N-isopropylacrylamide), enhance ocular bioavailability and provide anti-inflammatory effects. Similarly, Oxidized hyaluronan (oHA) promotes keratocyte growth in rabbits by leveraging oxidation levels in aldehyde HA, improving gelatin microcarriers for effective scaffolding in ocular health [102,103].
2.3.3. Lens
Nondegradable polymeric gel and biodegradable hyaluronic acid (HA) were utilized in lens scaffolds. The combination of HA with nondegradable polymeric gels has shown superior outcomes in terms of lens clarity, tested on Dutch Belt pigmented and New Zealand white rabbits [104].
2.3.4. Retina
Regenerated wild Antheraea pernyi silk fibroin (RWSF), PCL, and gelatin (RWSF/PCL/Gt) exhibit excellent cytocompatibility in laboratory settings and biocompatibility in vivo, positioning them as promising candidates for prosthetic Bruch's membrane development. Gelatin/chitosan scaffolds successfully mimic the composition and nanofibrous architecture of the ECM without adverse effects, while human amniotic membrane powder combined with PCL (HAMP/PCL) scaffolds demonstrates optimal porosity and biocompatibility, although further investigation is warranted [15,[105], [106], [107]].
2.4. Decellularized tissue
2.4.1. Cornea
The field of corneal TE has seen the exploration of various materials, including human crystalline lens capsules (LCs), freeze-dried acellular porcine corneal stroma (FD-APCS), decellularized human small incision lenticule extraction (SMILE) lenticule (SL), amniotic membrane (AM), collagen-coated plates, carbodiimide crosslinked recombinant human collagen (RHC), native porcine conjunctiva, porcine cornea, decellularized human donor cornea, decellularized murine corneas, and vancomycin-loaded hydrogels (VH). In addition to these natural and decellularized materials, synthetic approaches have also been developed to enhance corneal scaffold performance. For instance, electrospinning techniques have been employed to create nanofibrous scaffolds that mimic the ECM, promoting cell adhesion and proliferation. Scaffolds made from biodegradable polymers such as polycaprolactone (PCL) and gelatin have been designed to provide mechanical support while maintaining biocompatibility. Furthermore, silk fibroin scaffolds enriched with growth factors, such as glial cell-derived neurotrophic factor (GDNF), have demonstrated improved epithelialization capabilities. Research findings indicate that cells cultured on coated LCs exhibit a focal adhesion surface area that is at least double that of cells in control conditions. The SMILE technique has facilitated the efficient culture of keratocytes, with successful trials conducted on New Zealand male rabbits. In clinical settings, RHC has been the sole material applied, resulting in an average corrected visual acuity of 20/54 among patients over four years, with many showing improvements of more than five Snellen lines on visual acuity charts. Additionally, the use of decellularized human cornea (DHC) offers patients the potential benefit of utilizing non-immunogenic tissue scaffolds for transplantation. Finally, studies involving FD-APCS in New Zealand white rabbits have demonstrated that the collagen lamellae present in FD-APCS closely resemble those found in native corneal stroma, highlighting its potential as an effective scaffold material. The combination of synthetic and natural approaches presents a promising avenue for advancing corneal TE [[108], [109], [110], [111], [112], [113], [114]].
2.4.2. lacrimal gland
Synthetic approaches involving the use of lacrimal gland scaffolds have gained attention in recent studies. Notably, the ECM derived from New Zealand White (NZW) rabbit lacrimal glands, decellularized porcine jejunum (SIS-Muc), and decellularized porcine lacrimal gland matrix (DC-LG) have all been explored in vitro. Among these, the SIS-Muc demonstrated a similar pattern of epithelial cell polarization on its upper surface, akin to that observed in the epithelial acini of the natural lacrimal gland. These scaffolds offer promising avenues for TE and regenerative medicine in the context of lacrimal gland function restoration [13,115,116].
2.4.3. Optic nerve
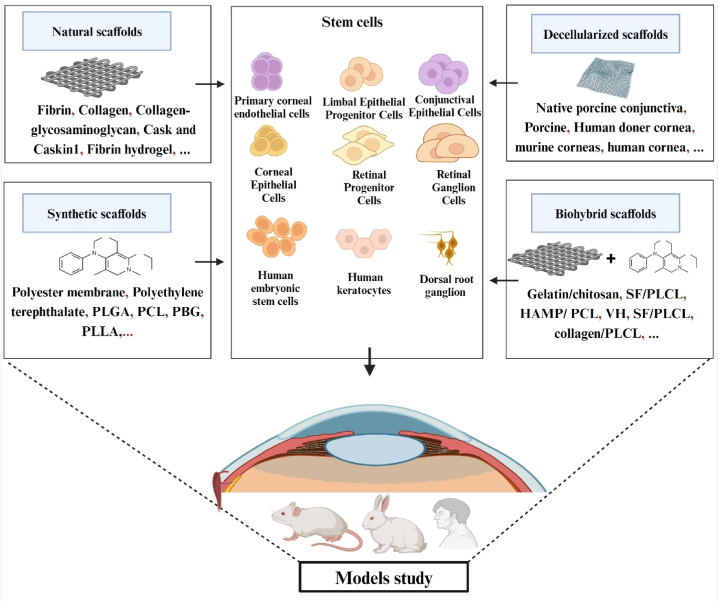
Advancements in synthetic approaches for nerve regeneration have facilitated the creation of optic nerve scaffolds derived from decellularized optic nerve (DON) and porcine decellularized optic nerve. Both types of scaffolds have undergone in vitro evaluation, while the porcine variant has also been utilized in an in vivo study involving rats. Remarkably, the decellularized optic nerve from embryonic pigs exhibited longer axonal extensions, increased distances, and enhanced branching compared to the conventional optic nerve (ON). The benefits of these scaffolds stem from the targeted elimination of axon-inhibitory factors, including myelin-associated glycoprotein and chondroitin sulfate proteoglycans, achieved through the decellularization process. This underscores the significant potential of these decellularized scaffolds in promoting nerve regeneration and improving functional outcomes [117,118] (Fig. 2).
Fig. 2.
Schematic illustration of depicts various scaffold types utilized in eye regeneration, including natural scaffolds, synthetic scaffolds, biohybrid scaffolds and decellularized scaffold Additionally, it highlights the different types of stem cells employed within these scaffolds.
3. Cell types used for eye tissue engineering
As we see in Table 1, a variety of cell have been utilized for eye TE. Conjunctiva mesenchymal stem cells (CJMSCs) and rabbit conjunctival epithelial cells (rCjECs) were the major cell types used in conjunctival scaffolds and they were both successfully raised [93,94,96]. Human adipose mesenchymal stem cells (hADSCs), limbal stromal stem cells (LSSCs), keratocytes, rabbit corneal limbal epithelial cells, limbal epithelial progenitor cells (LEPC), limbal mesenchymal stromal cells (LMSC), and limbal melanocytes (LM), Rabbit Corneal stromal cell, human embryonic stem cells-corneal epithelial cells (hESC-CEC), rabbit corneal epithelial cells, corneal epithelial cells (CEC), human corneal fibroblasts (HCFs) cells, human corneal endothelial cells (hCEC), human limbal epithelial cells (hLEC), Human keratocytes (HKs), human corneal epithelial cells (HCECs), and corneal keratocyte cell were the main cellular components employed in the construction of cornea scaffolds and every one of them was grown successfully [10,98,100,108,113,119,120].
Adult rabbit lacrimal gland (LG) progenitor cells, Lacrimal acinar epithelial cells of Sprague-Dawley rats, Porcine LG epithelial cells, Purified rabbit lacrimal gland acinar cells (pLGACs) and LG epithelial cells were found as cells seeded in lacrimal glands scaffolds [13,81,82,115,116].
Mouse retinal progenitor cells (mRPCs), retinal ganglion cells (RGCs), retinal pigment epithelium cells (RPE), human Fetal RPE (hfRPE), human embryonic stem cells, human-induced pluripotent stem cell (hiPSC), and CJMSCs are the main cells used effectively in retinal scaffolds [14,16,78,83,85,86]. In the construction of optic nerve scaffolds neurotrophin-3-overexpressing Schwann cells, dorsal root ganglion (DRG) neurites, RGCs, retinal ganglion cell progenitors (RGCP), chick dorsal root ganglia and a mouse neuroblastoma cell line are the main cellular components employed well [80,[90], [91], [92],117].
4. Advantages and disadvantages of eye tissue engineering scaffolds
TE and regenerative medicine methodologies have demonstrated promising results in the context of eye tissue regeneration [121]. To address the issue of insufficient supply, numerous efforts have been undertaken to develop a functional implant through bioengineering. This implant serves as a substitute for donor tissues in eye grafting procedures [122]. The survival and functionality of cells after transplantation can be influenced by the environmental conditions in which they grow and mature. Ocular regeneration using scaffolds has shown promise in treating various eye conditions. Typically, an optimal scaffold should possess biocompatibility, non-immunogenicity, and mechanical strength to withstand manipulation during the process of implantation [123]. The variability in the outcomes of patients suffering from blindness who undergo stem cell grafts for the purpose of restoring eye health and improving vision can be attributed to the utilization of various biological and synthetic scaffolds employed in the delivery of these cells to the ocular tissue [124]. Here are some advantages, disadvantages, and limitations of using scaffolds in ocular regeneration.
Advantages.
-
1.
Scaffolds serve as a framework that provide structural support for cells to proliferate and undergo differentiation, promoting tissue regeneration [15].
-
2.
They have the ability to imitate the normal ECM of the eye, hence improving the adhesion and growth of cells [96].
-
3.
Scaffolds can be tailored to specific requirements, such as size, shape, and porosity, to optimize tissue regeneration [120].
-
4.
Growth factors or medicines can be incorporated into them to augment healing and mitigate inflammation [104].
-
5.
Post-surgery bacterial inhibition, biocompatibility, stimulating the epithelialization process, Facilitating the movement of epithelial cells and expediting the process of wound healing [74].
-
6.
Transparency and durability, being resistant to degradation by collagenase, outstanding oxidative, thermal, and hydrolytic stability [116].
-
7.
Also mimicking the topographic features and the structure of the goal tissue and guiding specific cells in right direction are a part of prominent benefits of specific eye scaffolds [106].
Disadvantages.
-
1.
Scaffolds may trigger an immune response or cause inflammation in some patients [109].
-
2.
They may not integrate well with surrounding tissues, leading to complications such as scarring or rejection [19].
-
3.
The fabrication of scaffolds can be complex and costly, limiting their widespread use [106].
-
4.
Scaffolds may degrade too quickly or too slowly, affecting their effectiveness in promoting tissue regeneration [80].
In Table 1, we have compiled the advantages and disadvantages of noticeable recent studies in this field, based on their respective materials.
5. Clinical trials using tissue engineering scaffolds
Here are two clinical trials in the field of eye scaffolds. The first one (EudraCT no. 2006-006585-42) has used carbodiimide crosslinked RHC implants as corneal scaffold to tackle the global scarcity of available corneas for donation [110]. Over a span of four years, the revived neo-corneas exhibited stable integration within the ocular environment, devoid of any instances of rejection. Notably, the recipients of these neo-corneas were spared the requirement of enduring the protracted immunosuppressive regimen typically mandated for individuals receiving donor corneas. No recruitment of inflammatory dendritic cells into the implant region was detected [110]. However, in the case of recipients of donor corneas, even with the administration of immunosuppressive agents, migration of dendritic cells into the central cornea was detected, which coincided with the occurrence of a rejection episode. Regeneration, as demonstrated by the ongoing repopulation of nerve and stromal cells, transpired over the course of a four-year period, resulting in the approximation of the micro-architecture akin to that of normal, healthy corneas [110]. Patients who underwent implantation procedures exhibited an average corrected visual acuity of 20/54 over a span of 4 years. Furthermore, these individuals experienced a notable improvement in their visual capabilities, as evidenced by a gain of more than 5 Snellen lines on an eye chart. Enhancement of visual acuity may be achieved through the utilization of more resilient materials that exhibit superior capacity for shape preservation [110]. The subsequent clinical trial conducted in this particular domain pertained to the corneal scaffold (EudraCT number: 2010-024290-40) [125]. The trial employed a nano-structured fibrin agarose corneal substitute, which integrated allogeneic cells, effectively emulating the mechanical, optical, and biological characteristics of the anterior human native cornea. This ongoing clinical trial, conducted in ten hospitals in Spain, is a controlled, randomized, open-label study encompassing both phase I and phase II [125]. Its primary objective is to assess the safety and feasibility of a bioengineered human corneal substitute in adults afflicted with severe trophic corneal ulcers that have proven resistant to conventional treatment, or those who have developed complications because of prior ulcers. Additionally, the trial aims to gather clinical evidence regarding the efficacy of this novel intervention [125]. The assessment of adverse events, implant condition, symptoms of infection, and induced formation of new blood vessels are important factors in determining the safety and practicality of the bioengineered graft. These factors are considered the main outcomes in this study. The measurement of study endpoints is conducted over a span of 24 months, encompassing a total of 27 post-implant assessment visits. These visits occur at decreasing intervals throughout the follow-up period [125]. Ultimately, the utilization of scaffolds in TE methods shows significant potential for the regeneration of ocular tissues. By harnessing the power of biomaterials science and stem cell technology, researchers are paving the way for new treatments that could revolutionize the field of ophthalmology and improve outcomes for patients with various eye conditions [120].
6. Limitation and challenges
Overall, the future of ocular regeneration looks promising, with ongoing research efforts aimed at developing new treatments for a wide range of eye conditions [81]. While much work still needs to be done before these therapies become widely available, advancements in regenerative medicine offer hope for improved outcomes for patients with vision loss and other ocular disorders [126].In summary, although scaffold-based ocular regeneration shows promise in enhancing patient outcomes, additional study is required to overcome obstacles and maximize scaffold utilization in this domain [88,127]. Some limitations in the therapeutic use of scaffolds in ocular diseases are described below.
-
1.
The ongoing research is focused on examining the durability and effectiveness of scaffold-based ocular regeneration therapies over an extended period of time [112].
-
2.
Scaffolds may not be suitable for all types of ocular injuries or diseases [97].
-
3.
The optimal design and composition of scaffolds for ocular regeneration have not been fully established [75].
-
4.
Clinical trials are needed to evaluate the effectiveness of scaffold-based therapies in treating
various eye conditions [85].
7. Conclusion
In our analysis of recent publications on scaffolds for ocular TE, we categorized the scaffolds utilized in eye TE into four types—natural, synthetic, biohybrid, and decellularized tissue—based on their tissue characteristics, while also compiling data on the associated cell types and animal models to gain valuable insights into the advantages and disadvantages of each material. Our findings indicate that scaffolds in TE not only mimic the ECM of the eye but also promote cell proliferation and tissue regeneration, offering significant benefits such as bacterial inhibition, biocompatibility, and enhanced durability. However, these scaffolds present challenges, including potential immune responses, inadequate tissue integration, complex and costly manufacturing processes, and inconsistent degradation rates that may affect their efficacy. This review comprehensively examines the current landscape of eye regenerative therapies, evaluates key preclinical and clinical research initiatives, addresses the challenges faced, and considers potential advancements over the next decade.
CRediT authorship contribution statement
Zeinab Mousavi: Writing – original draft, Investigation, Conceptualization. Masood Bagheri: Writing – original draft, Validation, Investigation. Gelavizh Rostaminasab: Writing – original draft, Investigation. Abdolhamid Mikaeili: Writing – original draft, Software. Ali R. Djalilian: Writing – review & editing, Writing – original draft. Leila Rezakhani: Writing – review & editing, Supervision, Project administration.
Data availability statement
All data are fully available without restriction.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used QuillBot and Monica to improve the readability and language of the manuscript. After using these services, the authors reviewed and edited the content as needed and take full responsibility for the content of the published article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran. This study was carried out under the approval code IR.KUMS.REC.1403.255 at Kermanshah University of Medical Sciences, Kermanshah, Iran.
Abbreviation
- BrM
Bruch's membrane
- ON
Optic nerve
- RGCs
Retinal Ganglion Cells
- WHO
World Health Organization
- TE
Tissue engineering
- NSF
National Science Foundation
- AMD
Age-related Macular Degeneration
- VEGF
Vascular Endothelial Growth Factor
- TENGs
Tissue-engineered nerve grafts
- HAM
Human amniotic membrane
- RS1
Retinoschisin
- GCH
Gelatin/ Chondroitin sulfate/ Hyaluronic Acid
- hESC
Human embryonic stem cell
- ONL
Outer Nuclear Layer
- PES
Polyethersulfone
- PCL
Polycaprolactone
- PLLA
Poly L-lactic Acid
- PLGA
Poly Lactic-co-Glycolic Acid
- PGS
Poly Glycerol Sebacate
- PPy-G
polypyrrole functionalized graphene
- PLA
Poly Lactic Acid
- EFMs
Electrospun nanofibrous membranes
- CNF
Cellulose Nanofibrils
- SP
Silk Peptide
- LF
Levofloxacin
- SF
Silk Fibroin
- rGo
Reduced Graphene oxide
- PLCL
Poly (L-lactic acid-co-3- caprolactone)
- SF/rGO
Silk Fibroin/ reduced Graphene oxide
- ECM
Extracellular matrix
- Collagen/PLCL
Collagen/poly (L-lactic acid-co-3- caprolactone)
- IL-4, IL-5, and IL-6
Interleukin-4, interleukin-5, Interleukin-6
- CjECs
Conjunctival Epithelial Cells
- SF/PLCL
Silk Fibroin /poly (L-lactic acid-co-3- caprolactone)
- GelMA-HA
Hyaluronic acid-modified gelatin-methacrylate
- PVA-COL
Collagen-Immobilized Poly (Vinyl Alcohol)
- AM
Amniotic Membrane
- PVA-AM
Polyvinyl alcohol hydrogel
- GP/PVA/SF/n-HA
Genipin-crosslinked polyvinyl alcohol/silk fibroin/nanohydroxyapatite Hydrogel
- PCL/collagen
Polycaprolactone/collagen
- HA
Hyaluronic Acid
- RWSF
Regenerated wild Antheraea pernyi Silk Fibroin
- Gt
Gelatin
- HAMP
Human amniotic membrane powder
- LCs
Lens Capsules
- FD-APCS
Freezing-Dry Acellular Porcine Cornea Stroma
- SMILE
Small incision lenticule extraction
- SL
lenticule
- RHC
Recombinant Human Collagen
- NZW
New Zealand White
- (SIS-Muc)
Conversely decellularized porcine small intestine submucosa
- DC-LG
Decellularized porcine LG matrix
- DON
Decellularized Optic Nerve
- PLA
Poly Lactic Acid
- SP
Silk Peptide
- CJMSCs
Conjunctiva Mesenchymal Stem Cells
- PLCL
Poly L-lactic acid-co-3- Caprolactone
- rCjECs
Rabbit Conjunctival Epithelial Cells
- LCs
Lens Capsules
- hADSCs
Human Adipose Mesenchymal Stem Cells
- PCL
Poly ε-Caprolactone
- LSSCs
Limbal Stromal Stem Cells
- GDNF
Glial Cell-Derived Neurotrophic Factor
- LEPC
Limbal Epithelial Progenitor Cells
- LMSC
Limbal Mesenchymal Stromal Cells
- LM
Limbal Melanocytes
- FD-APCS
Freezing Dry Acellular Porcine Cornea Stroma
- GelMA-HA
Hyaluronic Acid-modified Gelatin-Methacrylate
- CS
Corneal Stromal
- PVA-COL
Colagen-Immobilized Poly Vinyl Alcohol
- CEC
Corneal Epithelial Cells
- GP
Genipin
- n-HA
Nanohydroxyapatite
- HCFs
Human Corneal Fibroblasts
- VH
Vancomycin-loaded collagen Hydrogels
- hCEC
Human Corneal Endothelial Cells
- hLEC
Human Limbal Epithelial Cells
- PVA
Aligned Polyvinyl Acetate
- HKs
Human Keratocytes
- LG
Lacrimal Gland
- PLGACs
Purified rabbit Lacrimal Gland Acinar Cells
- RPCs
Retinal Progenitor Cells
- RS
Retinoschisin
- PCL
Polycaprolactone
- mRPCs
Mouse Retinal Progenitor Cells
- hiPSC
Human-induced Pluripotent Stem Cell
- RPE
Retinal Pigment Epithelial
- PEG
Polyethylene Glycol
- DRG
Dorsal Root Ganglion
- PBG
Poly-gamma-Benzyl-L-Glutamate
- RGCP
Retinal Ganglion Cell Progenitors
- PEDOT
PSS (polystyrene sulfonate) in water and isopropanol
- TCPS
Tricalcium phosphate scaffold
- LCs
Lens Capsules
- DM
Descemet's membrane
- DHC
Decellularized Human Cornea
- FD-APCS
Freezing Dry Acellular Porcine Cornea Stroma
- GelMA-HA
Hyaluronic Acid-modified Gelatin-Methacrylate
- PVA
Polyvinyl Alcoholhydrogel
- POC
Poly (1,8-Octanediol-co-Citrate)
- LSSCs
Limbal Stromal Stem Cells
- hESC-CEC
human Embryonic Stem Cells- Corneal Epithelial Cells
- hfRPE
Human fetal Retinal Pigment Epithelium cells
- sHAPN
Sulfated Hyaluronic acid with amine-terminated poly(N-isopropylacrylamide)
- oHA
Oxidized hyaluronan
References
- 1.Abedin Zadeh M., et al. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov. Today. 2019;24(8):1669–1678. doi: 10.1016/j.drudis.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Visalli F., et al. Innovative bioscaffolds in stem cell and regenerative therapies for corneal pathologies. Bioengineering. 2024;11(9):859. doi: 10.3390/bioengineering11090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sensoy E., Citirik M. A comparative study on the knowledge levels of artificial intelligence programs in diagnosing ophthalmic pathologies and intraocular tumors evaluated their superiority and potential utility. Int. Ophthalmol. 2023;43(12):4905–4909. doi: 10.1007/s10792-023-02893-x. [DOI] [PubMed] [Google Scholar]
- 4.Sahle F.F., et al. Nanotechnology in regenerative ophthalmology. Adv. Drug Deliv. Rev. 2019;148:290–307. doi: 10.1016/j.addr.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva J.C., Ferreira F.C. MDPI; 2024. Advanced Polymeric Scaffolds for Stem Cell Engineering and Regenerative Medicine; p. 2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radu M., et al. Exploring stem-cell-based therapies for retinal regeneration. Life. 2024;14(6):668. doi: 10.3390/life14060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi V.P., et al. Newer approaches to dry eye therapy: nanotechnology, regenerative medicine, and tissue engineering. Indian J. Ophthalmol. 2023;71(4):1292–1303. doi: 10.4103/IJO.IJO_2806_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santa Cruz-Pavlovich F.J., et al. Beyond vision: an overview of regenerative medicine and its current applications in ophthalmological care. Cells. 2024;13(2):179. doi: 10.3390/cells13020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakpal D., Gharat S., Momin M. Recent advancements in polymeric nanofibers for ophthalmic drug delivery and ophthalmic tissue engineering. Biomater. Adv. 2022;141 doi: 10.1016/j.bioadv.2022.213124. [DOI] [PubMed] [Google Scholar]
- 10.Xu W., et al. PCL scaffold combined with rat tail collagen type I to reduce keratocyte differentiation and prevent corneal stroma fibrosis after injury. Exp. Eye Res. 2022;217 doi: 10.1016/j.exer.2022.108936. [DOI] [PubMed] [Google Scholar]
- 11.Oliver J.D., Vyas K.S. In: Handbook of Tissue Engineering Scaffolds: Volume Two. Mozafari M., Sefat F., Atala A., editors. Woodhead Publishing; 2019. 62 - scaffolds for tissue engineering in optic nerve regeneration; pp. 711–720. [Google Scholar]
- 12.Gavrilova N.A., et al. The effect of biodegradable silk fibroin-based scaffolds containing glial cell line-derived neurotrophic factor (GDNF) on the corneal regeneration process. Int. J. Biol. Macromol. 2021;185:264–276. doi: 10.1016/j.ijbiomac.2021.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Lin Hui, G.S, He Hong, Botsford Benjamin, Mackenzie Li, Elisseeff Jennifer H., Yiu Samuel C. Three-dimensional culture of functional adult rabbit lacrimal gland epithelial cells on decellularized scaffold. Tissue Eng. 2016;22(1–2):65–74. doi: 10.1089/ten.TEA.2015.0286. [DOI] [PubMed] [Google Scholar]
- 14.Luo Z., et al. Biodegradable scaffolds facilitate epiretinal transplantation of hiPSC-Derived retinal neurons in nonhuman primates. Acta Biomater. 2021;134:289–301. doi: 10.1016/j.actbio.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Xiang P., et al. A novel Bruch's membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials. 2014;35(37):9777–9788. doi: 10.1016/j.biomaterials.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Nadri S., Nasehi F., Barati G. Effect of parameters on the quality of core-shell fibrous scaffold for retinal differentiation of conjunctiva mesenchymal stem cells. J. Biomed. Mater. Res. 2017;105(1):189–197. doi: 10.1002/jbm.a.35897. [DOI] [PubMed] [Google Scholar]
- 17.Jing Yao C.W.K., Baranov Petr Y., Regatieri Caio V., Redenti Stephen, Tucker Budd A., Mighty Jason, Tao Sarah L., Young Michael J. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng. 2015;21(7–8):1247–1260. doi: 10.1089/ten.tea.2013.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatemeh J., et al. Development of an electrospun Scaffold for retinal tissue engineering. Polymer science. Ser. Bibliogr. 2020;62(3):290–298. [Google Scholar]
- 19.Miyashita H., et al. Collagen-immobilized poly (vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epithelium. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76B(1):56–63. doi: 10.1002/jbm.b.30332. [DOI] [PubMed] [Google Scholar]
- 20.Karl M., Reh T. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol. Med. 2010;16(4):193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho C.P., Lai T.Y. Current management strategy of polypoidal choroidal vasculopathy. Indian J. Ophthalmol. 2018;66(12):1727. doi: 10.4103/ijo.IJO_975_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarao V., et al. Intravitreal steroids for the treatment of retinal diseases. Sci. World J. 2014;2014 doi: 10.1155/2014/989501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon K., et al. Gene therapies in ophthalmic. Nat. Rev. Drug Discov. 2019;18:415. doi: 10.1038/d41573-018-00016-1. [DOI] [PubMed] [Google Scholar]
- 24.Zarbin M.A., et al. Regenerative nanomedicine and the treatment of degenerative retinal diseases. Wiley interdisciplinary reviews: nanomedicine and nanobiotechnology. 2012;4(1):113–137. doi: 10.1002/wnan.167. [DOI] [PubMed] [Google Scholar]
- 25.Mclaughlin S., et al. Nano-engineered biomaterials for tissue regeneration: what has been achieved so far? Frontiers in Materials. 2016;3:27. [Google Scholar]
- 26.Ellis‐Behnke R., Jonas J.B. Redefining tissue engineering for nanomedicine in ophthalmology. Acta Ophthalmol. 2011;89(2):e108–e114. doi: 10.1111/j.1755-3768.2010.01982.x. [DOI] [PubMed] [Google Scholar]
- 27.Karamichos D. MDPI; 2015. Ocular Tissue Engineering: Current and Future Directions; pp. 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alizadeh M., et al. The effect of Scrophularia striata on cell attachment and biocompatibility of decellularized bovine pericardia. Cell Tissue Bank. 2022;23(2):261–269. doi: 10.1007/s10561-021-09939-3. [DOI] [PubMed] [Google Scholar]
- 29.Peng M., et al. Reconfigurable scaffolds for adaptive tissue regeneration. Nanoscale. 2023;15(13):6105–6120. doi: 10.1039/d3nr00281k. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoldi E.L., et al. 3D printing of biocompatible scaffolds for eye tissue engineering. Procedia CIRP. 2022;110:213–218. [Google Scholar]
- 31.Stapleton F., et al. "The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye WorkShop. Ocul. Surf. 2007;19:1–20. doi: 10.1016/s1542-0124(12)70082-4. 2021. [DOI] [PubMed] [Google Scholar]
- 32.Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA Ophthalmology. 2021;139(3):313–320. [Google Scholar]
- 33.Barioulet L., et al. Meteorological factors and rhegmatogenous retinal detachment in metropolitan France. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-69591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boroumand S., et al. The landscape of clinical trials in corneal regeneration: a systematic review of tissue engineering approaches in corneal disease. J. Biomed. Mater. Res. B Appl. Biomater. 2024;112(8) doi: 10.1002/jbm.b.35449. [DOI] [PubMed] [Google Scholar]
- 35.Alqudah N. Keratoconus: imaging modalities and management. Medical Hypothesis. Discovery and Innovation in Ophthalmology. 2024;13(1):44. doi: 10.51329/mehdiophthal1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dua H.S., et al. Limbal stem cell deficiency and its treatment. Br. J. Ophthalmol. 2017 doi: 10.1136/bjo.83.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Girolamo N. Biologicals and biomaterials for corneal regeneration and vision restoration in limbal stem cell deficiency. Adv. Mater. 2024 doi: 10.1002/adma.202401763. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A., Jaganathan B.G. Stem cell therapy for retinal degeneration: the evidence to date. Biol. Targets & Ther. 2021:299–306. doi: 10.2147/BTT.S290331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng C., et al. Advances in biomaterials as a retinal patch for the repair of rhegmatogenous retinal detachment. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.997243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhawale K.K., Tidake P., Dhawale K.K. A comprehensive review of recent advances in minimally invasive glaucoma surgery: current trends and future directions. Cureus. 2024;16(7) doi: 10.7759/cureus.65236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y., et al. Innovative nanotechnology in drug delivery systems for advanced treatment of posterior segment ocular diseases. Adv. Sci. 2024 doi: 10.1002/advs.202403399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaheen N., et al. 2024. Neurostimulation in Neuro-Ophthalmology: Mechanisms and Therapeutic Potential. [Google Scholar]
- 43.Hsueh Y.-J., et al. The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int. J. Mol. Sci. 2022;23(3):1255. doi: 10.3390/ijms23031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulghani S., Mitchell G.R. Biomaterials for in situ tissue regeneration: a review. Biomolecules. 2019;9(11) doi: 10.3390/biom9110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khazaei M.R., et al. The decellularized calf testis: introducing suitable scaffolds for spermatogenesis studies. International Journal of Fertility & Sterility. 2024;18(1):32. doi: 10.22074/IJFS.2023.1989173.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mi H.-Y., et al. Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: effects of fabrication approaches and hydroxyapatite size. J. Mater. Sci. 2013;49 [Google Scholar]
- 47.Suamte L., Tirkey A., Babu P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Materials in Medicine. 2023;4:243–256. [Google Scholar]
- 48.Diomede F., et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020;21(9) doi: 10.3390/ijms21093242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khazaei M., Alizadeh M., Rezakhani L. Resveratrol‐loaded decellularized ovine pericardium: ECM introduced for tissue engineering. Biotechnol. Appl. Biochem. 2024;71(2):387–401. doi: 10.1002/bab.2547. [DOI] [PubMed] [Google Scholar]
- 50.Mahdian M., et al. Nerve regeneration using decellularized tissues: challenges and opportunities. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1295563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hazrati R., Davaran S., Omidi Y. Bioactive functional scaffolds for stem cells delivery in wound healing and skin regeneration. React. Funct. Polym. 2022;174 [Google Scholar]
- 52.Khazaei M.R., et al. Decellularized kidney capsule as a three-dimensional scaffold for tissue regeneration. Cell Tissue Bank. 2024:1–14. doi: 10.1007/s10561-024-10136-1. [DOI] [PubMed] [Google Scholar]
- 53.Montaseri Z., et al. Composite silk fibroin hydrogel scaffolds for cartilage tissue regeneration. J. Drug Deliv. Sci. Technol. 2023;79 [Google Scholar]
- 54.Williams D.F. On the nature of biomaterials. Biomaterials. 2008;29(13):1980–1986. [Google Scholar]
- 55.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 56.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14(3):88–95. [Google Scholar]
- 57.Kuo C.K., Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001;54(1):112–121. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 58.Levenberg S., Langer R. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2004;22(5):863–866. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 59.(FDA), U.S.F.a.D.A. 2016. Guidance for Industry: Considerations for the Design of Safety Studies for Gene Therapy Products. [Google Scholar]
- 60.Suhag D., Kaushik S., Taxak V.B. vol. 1. Springer; 2024. Fundamentals of biomaterials; pp. 25–54. (Handbook of Biomaterials for Medical Applications). Fundamentals. [Google Scholar]
- 61.Kahn B.B., Flier J.S. Obesity and insulin resistance. The Journal of clinical investigation. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garkal A., et al. Handbook of Biodegradable Polymers. Jenny Stanford Publishing; 2024. Biodegradable polymers in tissue engineering and regenerative medicine; pp. 449–524. [Google Scholar]
- 63.Puccetti M., et al. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024 doi: 10.1016/j.phrs.2024.107086. [DOI] [PubMed] [Google Scholar]
- 64.Mishra D., et al. Ocular application of electrospun materials for drug delivery and cellular therapies. Drug Discov. Today. 2023;28(9) doi: 10.1016/j.drudis.2023.103676. [DOI] [PubMed] [Google Scholar]
- 65.Ferraz M.P. Biomaterials for ophthalmic applications. Appl. Sci. 2022;12(12):5886. [Google Scholar]
- 66.Balkan B.C., Erbaş O. Recent advances in stem cell-based therapeutics in ophthalmology. Demiroglu Science University Florence Nightingale Journal of Medicine. 2023;9(3):175–182. [Google Scholar]
- 67.Gómez-Fernández H., et al. Comprehensive review of the state-of-the-art in corneal 3D bioprinting, including regulatory aspects. Int. J. Pharm. 2024 doi: 10.1016/j.ijpharm.2024.124510. [DOI] [PubMed] [Google Scholar]
- 68.Mehta N.J., Mehta S.N. Nanotechnology in retinal disease: current concepts and future directions. J. Ocul. Pharmacol. Therapeut. 2024;40(1):3–12. doi: 10.1089/jop.2023.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bg P.K., et al. 3D printing in personalized medicines: a focus on applications of the technology. Mater. Today Commun. 2023;35 [Google Scholar]
- 70.Sanduja S., et al. Regulatory Aspects of Gene Therapy and Cell Therapy Products. A Global Perspective; 2023. United States food and drug administration regulation of human cells, tissues, and gene therapies; pp. 71–89. [DOI] [PubMed] [Google Scholar]
- 71.Mannino G., et al. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cell. 2021;13(6):632. doi: 10.4252/wjsc.v13.i6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Personalized medicine in ocular tissue engineering: tailoring scaffolds for individual patients. Journal of Tissue Engineering and Regenerative Medicine. 2023;17(3):282–295. [Google Scholar]
- 73.Safinaz M.K., et al. The use of autologous fibrin as a scaffold for cultivating autologous conjunctiva in the treatment of conjunctival defect. Cell Tissue Bank. 2014;15(4):619–626. doi: 10.1007/s10561-014-9436-y. [DOI] [PubMed] [Google Scholar]
- 74.Xiongabc S., et al. vol. 2019. The Royal Society of Chemistry; 2019. (Microgrooved Collagen-Based Corneal Scaffold for Promoting Collective Cell Migration and Antifibrosis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doillon C.J., et al. A collagen-based scaffold for a tissue engineered human cornea: physical and physiological properties. Int. J. Artif. Organs. 2003;26(8):764–773. doi: 10.1177/039139880302600810. [DOI] [PubMed] [Google Scholar]
- 76.Anjum R., Ayoubian H., Schmitz F. Differential synaptic distribution of the scaffold proteins Cask and Caskin1 in the bovine retina. Mol. Cell. Neurosci. 2014;62:19–29. doi: 10.1016/j.mcn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Singh D., et al. A biodegradable scaffold enhances differentiation of embryonic stem cells into a thick sheet of retinal cells. Biomaterials. 2018;154:158–168. doi: 10.1016/j.biomaterials.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 78.Soleimannejad M., et al. Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif. Cell Nanomed. Biotechnol. 2018;46(4):805–814. doi: 10.1080/21691401.2017.1345922. [DOI] [PubMed] [Google Scholar]
- 79.Heymann J.B., et al. Cryo-EM of retinoschisin branched networks suggests an intercellular adhesive scaffold in the retina. JCB (J. Cell Biol.) 2019;218(3):1027–1038. doi: 10.1083/jcb.201806148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kador K.E., et al. Retinal ganglion cell polarization using immobilized guidance cues on a tissue-engineered scaffold. Acta Biomater. 2014;10(12):4939–4946. doi: 10.1016/j.actbio.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long L., et al. Polyethersulfone dead-end tube as a scaffold for artificial lacrimal glands in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2006;78B(2):409–416. doi: 10.1002/jbm.b.30502. [DOI] [PubMed] [Google Scholar]
- 82.Selvam S., et al. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. American Journal of Physiology-Cell Physiology. 2007;293(4):C1412–C1419. doi: 10.1152/ajpcell.00200.2007. [DOI] [PubMed] [Google Scholar]
- 83.Lavik E.B., et al. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26(16):3187–3196. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Redenti S., et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. Journal of Ocular Biology, Diseases, and Informatics. 2008;1(1):19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li K., et al. HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta Biomater. 2017;54:117–127. doi: 10.1016/j.actbio.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 86.Liu Z., et al. Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials. 2014;35(9):2837–2850. doi: 10.1016/j.biomaterials.2013.12.069. [DOI] [PubMed] [Google Scholar]
- 87.Neeley W.L., et al. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29(4):418–426. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sodha S., et al. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011;22(4–6):443–456. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 89.Worthington K.S., et al. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017;55:385–395. doi: 10.1016/j.actbio.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edin J., Scalet N., Griffith M. AB020. 3D scaffolds for optic nerve regeneration. Annals of Eye Science. 2018;3(3):AB020. [Google Scholar]
- 91.Chang K., et al. Promoting optic nerve regeneration with a novel biocompatible fibrous scaffold. Investigative Ophthalmology & Visual Science. 2017;58(8) 2592-2592. [Google Scholar]
- 92.Yan L., et al. Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces. 2016;8(11):6834–6840. doi: 10.1021/acsami.5b12843. [DOI] [PubMed] [Google Scholar]
- 93.Yan D., et al. Insight into levofloxacin loaded biocompatible electrospun scaffolds for their potential as conjunctival substitutes. Carbohydrate Polymers. 2021;269 doi: 10.1016/j.carbpol.2021.118341. [DOI] [PubMed] [Google Scholar]
- 94.Rahmani A., et al. Conductive electrospun scaffolds with electrical stimulation for neural differentiation of conjunctiva mesenchymal stem cells. Artif. Organs. 2019;43(8):780–790. doi: 10.1111/aor.13425. [DOI] [PubMed] [Google Scholar]
- 95.Yao Q., et al. Electrospun collagen/poly(L-lactic acid-co-ε-caprolactone) scaffolds for conjunctival tissue engineering. Exp. Ther. Med. 2017;14(5):4141–4147. doi: 10.3892/etm.2017.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qinke Yao ab Y.H.a., Yuab Fei, Zhangab Weijie, Fu Yao. Royal Society of Chemistery; 2018. A Novel Application of Electrospun Silk fibroin/poly(L-Lactic Acid-Co-ε-Caprolactone) Scaffolds for Conjunctiva Reconstruction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uchino Y., et al. Amniotic membrane immobilized poly(vinyl alcohol) hybrid polymer as an artificial cornea scaffold that supports a stratified and differentiated corneal epithelium. J. Biomed. Mater. Res. B Appl. Biomater. 2007;81B(1):201–206. doi: 10.1002/jbm.b.30654. [DOI] [PubMed] [Google Scholar]
- 98.Zhou H., et al. Genipin-crosslinked polyvinyl alcohol/silk fibroin/nano-hydroxyapatite hydrogel for fabrication of artificial cornea scaffolds—a novel approach to corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2019;30(17):1604–1619. doi: 10.1080/09205063.2019.1652418. [DOI] [PubMed] [Google Scholar]
- 99.Riau A.K., et al. Collagen-based artificial corneal scaffold with anti-infective capability for prevention of perioperative bacterial infections. ACS Biomater. Sci. Eng. 2015;1(12):1324–1334. doi: 10.1021/acsbiomaterials.5b00396. [DOI] [PubMed] [Google Scholar]
- 100.Wu Z., et al. Engineering of corneal tissue through an aligned PVA/collagen composite nanofibrous electrospun scaffold. Nanomaterials. 2018;8(2):124. doi: 10.3390/nano8020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang R., et al. Remodelling 3D printed GelMA-HA corneal scaffolds by cornea stromal cells. Colloid and Interface Science Communications. 2022;49 [Google Scholar]
- 102.Nguyen D.D., et al. Oxidation-mediated scaffold engineering of hyaluronic acid-based microcarriers enhances corneal stromal regeneration. Carbohydrate Polymers. 2022;292 doi: 10.1016/j.carbpol.2022.119668. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen D.D., Luo L.-J., Lai J.-Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Materials Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gwon A., Gruber L. Engineering the crystalline lens with a biodegradable or non-degradable scaffold. Exp. Eye Res. 2010;91(2):220–228. doi: 10.1016/j.exer.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Zhang D., et al. Electrospun SF/PLCL nanofibrous membrane: a potential scaffold for retinal progenitor cell proliferation and differentiation. Sci. Rep. 2015;5(1) doi: 10.1038/srep14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noorani B., et al. Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells. International Journal of Polymeric Materials and Polymeric Biomaterials. 2018;67(12):754–763. [Google Scholar]
- 107.Majidnia E., et al. Development of an electrospun poly(ε-caprolactone)/collagen-based human amniotic membrane powder scaffold for culturing retinal pigment epithelial cells. Sci. Rep. 2022;12(1):6469. doi: 10.1038/s41598-022-09957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghiasi M., et al. Combination of natural scaffolds and conditional medium to induce the differentiation of adipose-derived mesenchymal stem cells into keratocyte-like cells and its safety evaluation in the animal cornea. Tissue Cell. 2023 doi: 10.1016/j.tice.2023.102117. [DOI] [PubMed] [Google Scholar]
- 109.Van den Bogerd B., Ní Dhubhghaill S., Zakaria N. Characterizing human decellularized crystalline lens capsules as a scaffold for corneal endothelial tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(4):e2020–e2028. doi: 10.1002/term.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fagerholm P., et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35(8):2420–2427. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]
- 111.Zhao H., et al. Xenogeneic acellular conjunctiva matrix as a scaffold of tissue-engineered corneal epithelium. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du L., et al. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br. J. Ophthalmol. 2011;95(3):410–414. doi: 10.1136/bjo.2008.142539. [DOI] [PubMed] [Google Scholar]
- 113.Polisetti N., et al. A decellularized human corneal scaffold for anterior corneal surface reconstruction. Sci. Rep. 2021;11(1):2992. doi: 10.1038/s41598-021-82678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiao J., et al. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials. 2011;32(29):6962–6971. doi: 10.1016/j.biomaterials.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 115.Kristina Spaniol M.M., Roth Mathias, Greve Burkhard, Mertsch Sonja, Geerling Gerd, Schrader Stefan. Engineering of a secretory active three-dimensional lacrimal gland construct on the Basis of decellularized lacrimal gland tissue. Tissue Eng. 2015;21(19–20):2605–2617. doi: 10.1089/ten.TEA.2014.0694. [DOI] [PubMed] [Google Scholar]
- 116.Massie I., et al. Evaluation of decellularized porcine jejunum as a matrix for lacrimal gland reconstruction in vitro for treatment of dry eye syndrome. Investigative Ophthalmology & Visual Science. 2017;58(12):5564–5574. doi: 10.1167/iovs.16-20759. [DOI] [PubMed] [Google Scholar]
- 117.Sun J.-H., et al. Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120289. [DOI] [PubMed] [Google Scholar]
- 118.Bai Y.R., et al. Decellularized optic nerve functional scaffold transplant facilitates directional axon regeneration and remyelination in the injured white matter of the rat spinal cord. Neural Regen Res. 2021;16(11):2276–2283. doi: 10.4103/1673-5374.310696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang J., et al. Universal corneal epithelial-like cells derived from human embryonic stem cells for cellularization of a corneal scaffold. Translational Vision Science & Technology. 2018;7(5) doi: 10.1167/tvst.7.5.23. 23-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Z., et al. Bioengineered multilayered human corneas from discarded human corneal tissue. Biomedical Materials. 2015;10(3) doi: 10.1088/1748-6041/10/3/035012. [DOI] [PubMed] [Google Scholar]
- 121.Mahdavi S.S., et al. Bioengineering approaches for corneal regenerative medicine. Tissue Eng Regen Med. 2020;17(5):567–593. doi: 10.1007/s13770-020-00262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soleimani M., et al. Application of biomaterials and nanotechnology in corneal tissue engineering. J. Int. Med. Res. 2023;51(7) doi: 10.1177/03000605231190473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajendran Nair D.S., et al. Tissue engineering strategies for retina regeneration. Appl. Sci. 2021;11(5):2154. doi: 10.3390/app11052154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nguyen K.N., et al. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018;65:21–35. doi: 10.1016/j.actbio.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 125.Miguel González-Andrades, R.M., María del Carmen González-Gallardo, Santiago Medialdea, Salvador Arias-Santiago, Juliana Martínez-Atienza, Antonio Ruiz-García, Lorena Pérez-Fajardo, Antonio Lizana-Moreno, Ingrid Garzón, Antonio Campos, Miguel Alaminos, Gloria Carmona, Natividad Cuende, A study protocol for a multicentre randomised clinical trial evaluating the safety and feasibility of a bioengineered human allogeneic nanostructured anterior cornea in patients with advanced corneal trophic ulcers refractory to conventional treatment. BMJ Open, Dec2012. 7(9). [DOI] [PMC free article] [PubMed]
- 126.Hynes S.R., Lavik E.B. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefe's Archive for Clinical and Experimental Ophthalmology. 2010;248(6):763–778. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 127.Treharne A.J., et al. The chemistry of retinal transplantation: the influence of polymer scaffold properties on retinal cell adhesion and control. Br. J. Ophthalmol. 2011;95(6):768–773. doi: 10.1136/bjo.2010.184002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction.