Abstract
The fall armyworm, Spodoptera frugiperda (J.E. Smith), is a devastating pest that attacks a wide range of crops, including sugarcane, rice, and maize. The purpose of this study is to evaluate the toxicity potential of native plant extracts (Azadirachta indica, Eucalyptus globulus, Parthenium hysterophorus, Cannabis sativa, Citrullus colocynthis, Nicotiana tabacum) against S. frugiperda. Four different concentrations (50, 100, 200, and 400 ppm) of the ethanolic plant extracts was evaluated against S. frugiperda third-instar larvae to determine their median lethal concentration (LC50). After 72 h of exposure, the LC50 values of A. indica, E. globulus, P. hysterophorus, C. sativa, C. colocynthis, N. tabacum, and positive control (Spinetoram) were 186.104, 518.438, 320.027, 334.259, 252.651, 720.980 and 189.369 ppm respectively. The maximum percent mortality was caused by the highest concentration (400 ppm) of A. indica (64 ± 0.18), E. globulus (48 ± 0.22), P. hysterophorus (56 ± 0.18), C. sativa (56 ± 0.18), C. colocynthis (60 ± 0.00), and N. tabacum (40 ± 0.28), after 72 h of treatment while Spinetoram induced 100 ± 0.00 percent mortality of S. frugiperda and only 4 ± 0.18 percent mortality was recorded in a control group. Results showed that all plant extracts were found to be effective against S. frugiperda. The compounds from the two most effective ethanolic plant extracts were identified by using Gas chromatography-mass spectrometry analysis (GC-MS). The key compounds identified in neem leaf extract and kortuma fruit extract are predominantly biologically active molecules. Many of them were volatile compounds that belonged to different chemical categories, such as fatty acids, hydrocarbons, esters, terpenoids, phenolic compounds, and amines. Terpenes exhibited a wide range of different biological activities, such as serving as insecticides and antifeedant. The presence of various functional groups in the plant extract was determined by conducting a Fourier Transform Infrared Spectroscopy (FTIR). Farmers should employ these kinds of environmental friendly insecticides to lessen the impact of fall armyworm because these products are cheaper to use and better for the economy and the environment.
Keywords: Spodoptera frugiperda, Plant extracts, Toxicity, Gas chromatography-mass spectrometry, Lethal concentrations
Highlights
-
•
Toxicological assessments of native plant extracts against the maize pest.
-
•
Characterization of most effective ethanolic plant extracts.
-
•
Sustainable and eco-friendly management of fall armyworm.
1. Introduction
Maize (Zea mays L.) from Poaceae family was cultivated in South America before venturing throughout the world [1,2]. Afterwards it spread to China, Asia, Europe, Russia, Canada, USA, Mexico, and Pacific Islands. Maize ranks 3rd most economically valuable cereal crop worldwide, following wheat and rice, because of its versatile applications in human and animal nutrition, biofuel production, and construction materials [[3], [4], [5], [6]]. Diseases, insect pests, vegetation's, birds, nematodes, and environmental factors have the potential to substantially reduce the crop yield up to 75 % [7].
Spodoptera frugiperda (Lepidoptera: Noctuidae), also known as the fall armyworm (FAW), is a notorious pest that is native to North America. FAW feeds on more than 350 different plant species, with maize being the main food source [8,9]. S. frugiperda was reported first time during March 2019 from a number of locations in Sindh Province, Pakistan, with the exception of upper Sindh, Larkana, Jacobabad, and Shikarpur [10]. S. frugiperda predominately cause damage by feeding on both vegetative and reproductive parts of host plant. Developing effective control methods for insect infestations is elusive without an in-depth understanding of their life cycles. The habitat of the fall armyworm influences its life cycle. Approximately 30 days are required in summer season at 28 °C temperature for the completion of FAW life cycle [11]. However, in the spring and winter, this time can extend to 60–90 days. Globally, chemical, biological, and botanical strategies for controlling insect pests have been implemented. Synthetic insecticides have emerged as a highly efficient and economically convenient method to immediately combat FAW [12,13]. However, they have adverse impacts on the biological control agents, environment, and contribute to the development of insect pest resistance. S. frugiperda has become resistant to 41 different molecules, which makes it more challenging to get rid of them [14,15].
In order to mitigate pollution to the environment and combat insecticide resistance, it is necessary to develop a sustainable and resilient method of pest management [16]. Pesticides derived from plants are regarded as ecologically sustainable due to their low toxicity towards non target organisms and biodegradability [[17], [18], [19]]. Therefore, these plant based insecticides may be included in the integrated pest management module [20]. Numerous studies have been performed to identify plant sources of safe insecticides, in response to the increased advocacy for their use. Tetranortriterpenoid compound also known as azadirachtin, which is similar to an asteroid. It is extracted from the Azadirachta indica and has been recognized as a major insect repellant in vegetables [21,22]. Furthermore, the significance of neem in pharmaceuticals, agriculture [23], toiletries, and medicine, has significantly increased due to its abundant bioactive compounds [21]. Research has demonstrated that neem possesses a range of beneficial characteristics, including insecticidal, antifeedant, antiimplantation, antifungal, antiallergenic, antidermatic, antiviral, anti-inflammatory, larvicidal, antipyorrhoeic, and nematicidal effects [21,24].
Citrullus colocynthis has been documented for its insecticidal properties against several pests that infest stored grains and other field crops. Furthermore, it possesses an inherent capacity to repel pest infestation [25,26]. This plant exhibited repellent, antifeedant, and infertile properties against many pests [[27], [28], [29]]. An Australian hardwood tree species, Eucalyptus globulus has accomplished worldwide distribution due to its advantageous features such as the beneficial aspects of its leaves and versatility for pulpwood production [30]. The essential oil of Eucalyptus is recognized for its insecticidal properties [31]. Trogoderma granarium (Everts, 1898) (Coleoptera: Dermestidae), among numerous insect species that infest stored products, is susceptible to the toxicity of E. globulus oil in many research studies [32,33]. Volatile compounds such as limonene, α-pinene, lanalool, and 1,8-cineole are present in them, which enables them to kill insects [34].
The antifeedant activity was observed in aqueous crude extracts of tobacco (Nicotiana tabacum) leaves against FAW larvae [35,36]. Significantly, the larvae exhibited the highest mortality when exposed to the extracts at a concentration of 50 % [37]. Recently, Cannabis sativa has received interest as a conceivable alternative for application as a pesticide [38]. In addition to the widely recognized cannabinoids, Tetrahydrocannabinol (THC) and cannabidiol (CBD), the plant produces around 500 compounds, which include other cannabinoids, terpenoids, and flavonoids [39]. Currently, the specific compounds and processes through which C. sativa achieves its insecticidal effects are still unclear. Nonetheless, interactions between specific compounds as well as the pharmacological activities of terpenes and cannabinoids may be involved [40]. The purpose of this study was extraction, characterization and evaluation of insecticidal potential of native plant extracts against the third instar larvae of S. frugiperda.
2. Materials and methods
2.1. Description of the study area
The research experiments were conducted in bioassay laboratory of the Entomological Research Institute (Latitude, 31.404975°; Longitude, 73.0505453°), Ayub Agricultural Research Institute (AARI), Faisalabad. The institute is located in the northeastern plains of Punjab, between longitude 73°74 East and latitude 30°31.5 North, at an elevation of 184 m (604 feet) above sea level (Fig. 1).
Fig. 1.
Map of study area.
2.2. Preparation of plant extracts
Six native plant species were collected from various regions of Faisalabad, Punjab, Pakistan, as shown in Table 1, brought to the laboratory and washed extensively with tap water. The leaves/fruit were shade dried (30 ± 5 °C; 10 ± 2 RH) and coarse grinded by using an electric grinder (GRT 1500B). Soxhlet apparatus (J.P. Selecta, s.a; Serial #. 0481090) was used to prepare plant extracts, and ethanol (Sigma, 99.9 %) served as extraction solvent. The thimble was filled with a fine powder of each plant extract, weighing 50 g. A 250 mL volume of ethanol was used as a solvent. The source of heat was a heating mantle that was operating at a temperature of 60 °C. The aforementioned process was repeated multiple times over 13–15 h to complete the extraction. Rotary evaporator (Scilogex RE 100-S) equipped with a vacuum pump was used to purify the ethanolic plant extracts. After rotary evaporation, obtained yield of A. indica, E. globulus, P. hysterophorus, C. sativa, C. colocynthis and N. tabacum was 30 g, 33 g, 28g, 25g, 21g and 20g respectively. The plant extracts were kept at 4 °C in hermetic dark glass containers of 50 mL capacity until their use in the toxicity bioassays.
Table 1.
Comprehensive overview of the plant samples collected from Faisalabad.
| Sr. No. | Scientific Name | Vernacular Name | Diagrams | Family | Parts used | Coordinates |
|---|---|---|---|---|---|---|
| 1 | Azadirachta indica | Neem |  |
Meliaceae | Leaves | 31.40429° N, 73.05077° E |
| 2 | Eucalyptus globulus | Sufaida |  |
Mayrtaceae | Leaves | 31.40170° N, 73.05208° E |
| 3 | Parthenium hysterophorus | Gajar booti |  |
Asteraceae | Leaves | 31.40210° N, 73.04936° E |
| 4 | Cannabis sativa | Bhang |  |
Cannabaceae | Leaves | 30.99106° N, 72.87367° E |
| 5 | Citrullus colocynthis | Kortuma |  |
Cucurbitaceae | Fruit | 31.40166° N, 73.04918° E |
| 6 | Nicotiana tabacum | Tobacco |  |
Solanaceae | Leaves | 30.99155° N, 72.87339° E |
2.3. Collection and rearing of S. frugiperda
S. frugiperda larvae were collected from infested fields of maize in the Faisalabad region (31.40008° N, 73.04712° E) of Punjab, Pakistan. The larval population was brought to a laboratory for rearing. Individual S. frugiperda larva was reared in 90 mm × 15 mm plastic petri dishes. The larvae were fed with fresh maize leaves and succulent plant stem every day. Diet was refilled and feces were removed from Petri dishes on daily basis. Following pupation, pupae were kept in small plastic cages for adult emergence. The adult females and males at sex ratio of 1:1 was kept in cages in order to facilitate mating and the laying of eggs. The honey solution (10 %) was provided to adults as soaked cotton plugs. The muslin stripes were hung in cages for egg laying. The insect culture was maintained at a temperature of 26 ± 2 °C and relative humidity of 65 ± 5 % [41].
2.4. Bioassays of plant extracts against S. frugiperda in laboratory
The standard leaf-dip method was used to test ethanolic plant extracts against S. frugiperda 3rd instar larvae. After collection, fresh maize leaves were washed with tap water, and dried at 27 °C. Leaf discs of sized 10 cm were made and then dipped in aqueous solution of plant extracts for 30 s. Filter paper was used to drain off the extra liquid from leaf discs. Moreover, they were placed in petri plates with a 2 % agar media to keep them fresh. Five third instar S. frugiperda larvae that have been starved for 4 h was released into each petri plate. These plates were placed at 26 ± 2 °C, 65 ± 5 % Relative humidity (RH), and 16:8h (Light: Dark) photoperiod under controlled conditions. Each treatment having five replicates, ethanol-soaked leaves served as the control, while Spinetoram (Radiant 120SC; Cortiva Agri-Sciences) served as positive control. The larval mortality was assessed after treatment of 24, 48, and 72 h. If larvae cannot turn itself when placed on its dorsal surface, it was considered dead. In addition, four concentrations (50, 100, 200 and 400 ppm) of each ethanolic plant extract was prepared with distilled water and tested against S. frugiperda third instar larvae to determine their median lethal concentration (LC50).
2.5. Characterization of plant extracts
The compounds present in the ethanolic plant extracts were identified by using gas chromatography-mass spectrometry. Ethanolic plant extracts were analyzed through GC-MS (Agilent, 7890B). Data base from the National Institute of Standards and Technology (NIST) was used to interpret the mass spectrum of GC-MS. The presence of different functional groups in the plant extracts were determined by Fourier Transform Infrared Spectroscopy (Drawell-FTIR-530A). Scanning was carried out on plant samples between 4000 and 500 cm−1 at a specified cm−1 spectral resolution. The previous protocol for GC-MS and FTIR analyses was followed [21].
2.6. Statistical analysis
Data regarding insect mortality was recorded after 24, 48 and 72 h of exposure. Factorial analysis of variance (ANOVA) was used to analyze the mortality rate of larvae while Abbott formula [42] was used to correct the mortality rates. Obtained means of the treatment groups were compared by Tukey's post hoc test with 95 % of significance level. The lethal concentration (LC50) values, as well as the 95 percent fiducial limits (FL), slope, standard error, and chi-square value, were calculated for each plant extract and positive control by using the polo plus software [43].
3. Results
3.1. Toxicity bioassays of plant extracts against the third instar larvae of S. frugiperda
The laboratory conditions were employed to determine the effects of four different aqueous concentrations (50, 100, 200, and 400 ppm) of six plant extracts on the third instar larvae of fall armyworm. Each concentration was tested against twenty-five third instar larvae in five replicates. Third instar fall armyworm larvae were used because this stage of the lifecycle causes the most serious harm to plants and cereals worldwide [21]. All plant extracts showed detrimental effects against the fall armyworm, and these effects became more pronounced with increasing concentrations and exposure periods.
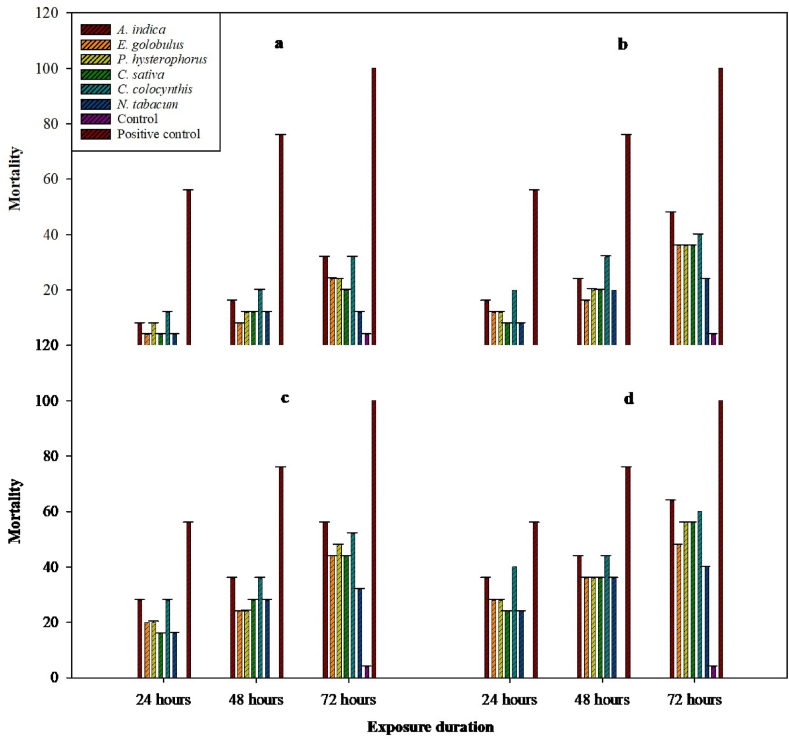
After 24 h maximum mortality was caused by C. colocynthis (40 %) followed by A. indica (36 %). The maximum mortality was caused in the positive control (56 %) while there was 0 % mortality in control (Fig. 2(d)). The LC50 values along with lower and upper fiducial limits of all plant extracts and positive control (Spinetoram) are described in Table 2. The LC50 value was the lowest for A. indica followed by C. colocynthis, E. globulus, C. sativa, N. tabacum, and P. hysterophorus.
Fig. 2.
Percent mortality of S. frugiperda 3rd instar larvae caused by plant extracts at 50 ppm (a), 100 ppm (b), 200 ppm (c), and 400 ppm (d) concentrations after 24, 48, and 72 h of exposure period.
Table 2.
LC50 values of plant extracts against third instar larvae of S. frugiperda after 24, 48, and 72 h of exposure period.
| Plant extracts | Na | Hours | LC50 (mgL−1)b | 95 % F.L.c |
Calculated values by Probit Analysis |
|||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Slope ± SEd | X2e | Dff | ||||
| Azadirachta indica | 25 | 24h | 720.564 | 300.006 | 1730.671 | 1.180 ± 0.19 | 0.964 | 2 |
| 25 | 48h | 534.730 | 195.865 | 1459.862 | 0.956 ± 0.22 | 0.989 | 2 | |
| 25 | 72h | 186.104 | 67.636 | 512.074 | 0.900 ± 0.22 | 0.958 | 2 | |
| Eucalyptus globulus | 25 | 24h | 1011.981 | 419.037 | 2443.951 | 1.275 ± 0.19 | 0.915 | 2 |
| 25 | 48h | 815.120 | 326.484 | 2035.077 | 1.139 ± 0.20 | 0.990 | 2 | |
| 25 | 72h | 518.438 | 151.450 | 1774.697 | 0.759 ± 0.27 | 0.942 | 2 | |
| Parthenium hysterophorus | 25 | 24h | 1686.344 | 525.532 | 5411.192 | 0.930 ± 0.26 | 0.994 | 2 |
| 25 | 48h | 1113.803 | 344.620 | 3599.781 | 0.859 ± 0.26 | 0.968 | 2 | |
| 25 | 72h | 320.027 | 125.290 | 817.445 | 0.994 ± 0.21 | 0.979 | 2 | |
| Cannabis sativa | 25 | 24h | 1517.811 | 556.952 | 4136.358 | 1.177 ± 0.22 | 0.990 | 2 |
| 25 | 48h | 938.740 | 308.906 | 2852.760 | 0.900 ± 0.25 | 0.989 | 2 | |
| 25 | 72h | 334.759 | 143.237 | 782.366 | 1.112 ± 0.19 | 0.927 | 2 | |
| Citrullus colocynthis | 25 | 24h | 725.278 | 269.043 | 1955.177 | 1.004 ± 0.22 | 0.996 | 2 |
| 25 | 48h | 838.410 | 185.443 | 3790.548 | 0.623 ± 0.33 | 0.926 | 2 | |
| 25 | 72h | 252.651 | 84.399 | 756.317 | 0.834 ± 0.24 | 0.995 | 2 | |
| Nicotiana tabacum | 25 | 24h | 1517.811 | 556.952 | 4136.358 | 1.177 ± 0.22 | 0.990 | 2 |
| 25 | 48h | 938.740 | 308.906 | 2852.760 | 0.900 ± 0.25 | 0.989 | 2 | |
| 25 | 72h | 720.980 | 291.735 | 1781.795 | 1.129 ± 0.20 | 0.887 | 2 | |
| Positive control | 25 | 24h | 1422.880 | 486.344 | 4162.871 | 0.761 ± 0.21 | 0.998 | 3 |
| 25 | 48h | 363.092 | 91.209 | 1445.432 | 0.593 ± 0.31 | 0.998 | 3 | |
| 25 | 72h | 189.369 | 97.183 | 369.002 | 1.556 ± 0.15 | 0.986 | 3 | |
: Number of FAW larvae used in experiment.
: LC50 values of each plant extract.
: 95 % Fiducial limits.
: Slope and standard error.
:Chi-square value.
: Degree of freedom.
After 48 h the LC50 values of plant extracts and positive control (Spinetoram) against the S. frugiperda along with lower and upper fiducial limits are described in Table 2. Among plant extracts the LC50 value was the lowest for A. indica followed by E. globulus, C. colocynthis, C. sativa, N. tabacum, and P. hysterophorus. Following an interval of 48 h, the control treatment did not exhibit any mortality, however the positive control treatment caused the highest mortality (Fig. 2).
The LC50 values of plant extracts and the positive control (Spinetoram) against S. frugiperda after a 72-h exposure period are detailed in Table 2. The LC50 value was the lowest for A. indica among the plant extracts, followed by C. colocynthis, P. hysterophorus, C. sativa, E. globulus, and N. tabacum.
3.2. Percent mortality of plant extracts against the S. frugiperda larvae
Fig. 2(a- d) Illustrates the percent mortality of four tested concentrations of all plant extracts (50, 100, 200, and 400 ppm) against S. frugiperda third instar larvae. The maximum percent mortality was caused by the highest concentration (400 ppm) of A. indica (64 ± 0.18), E. globulus (48 ± 0.22), P. hysterophorus (56 ± 0.18), C. sativa (56 ± 0.18), C. colocynthis (60 ± 0.00), and N. tabacum (40 ± 0.28), after 72 h of treatment while positive control (spinetoram) induced 100 ± 0.00 percent mortality of S. frugiperda. A 50 ppm concentration of A. indica, E. globulus, P. hysterophorus, C. sativa, C. colocynthis, and N. tabacum caused 32 ± 0.22, 24 ± 0.33, 24 ± 0.18, 20 ± 0.28, 32 ± 0.22 and 12 ± 0.22 percent mortality of S. frugiperda third instar larvae respectively, after 72 h while positive control caused 100 ± 0.00 percent mortality and only 4 ± 0.18 percent mortality was recorded in a control.
3.3. Gas chromatography mass spectrometry (GC-MS) analysis
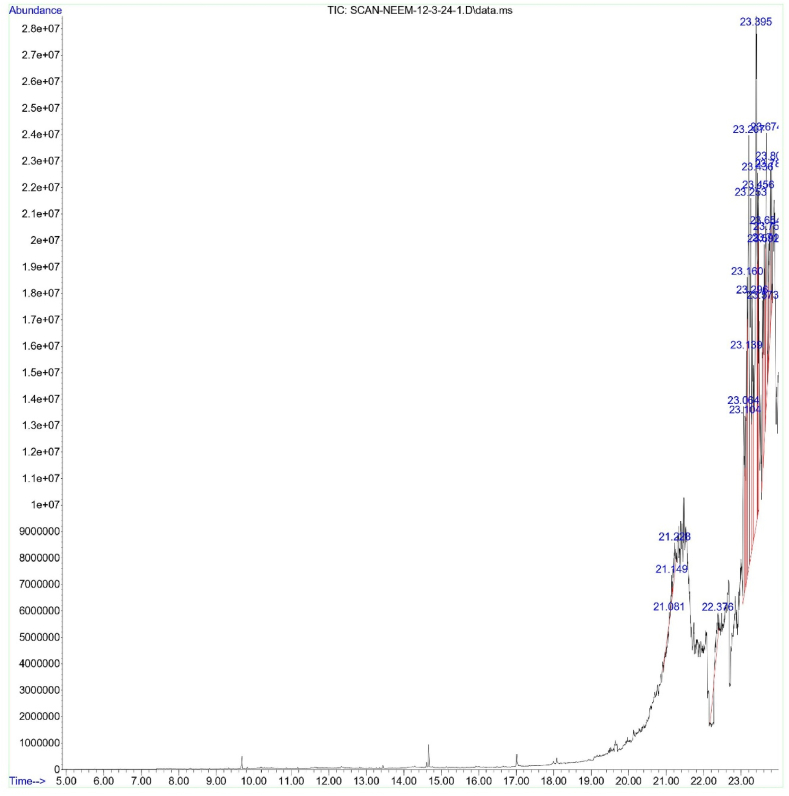
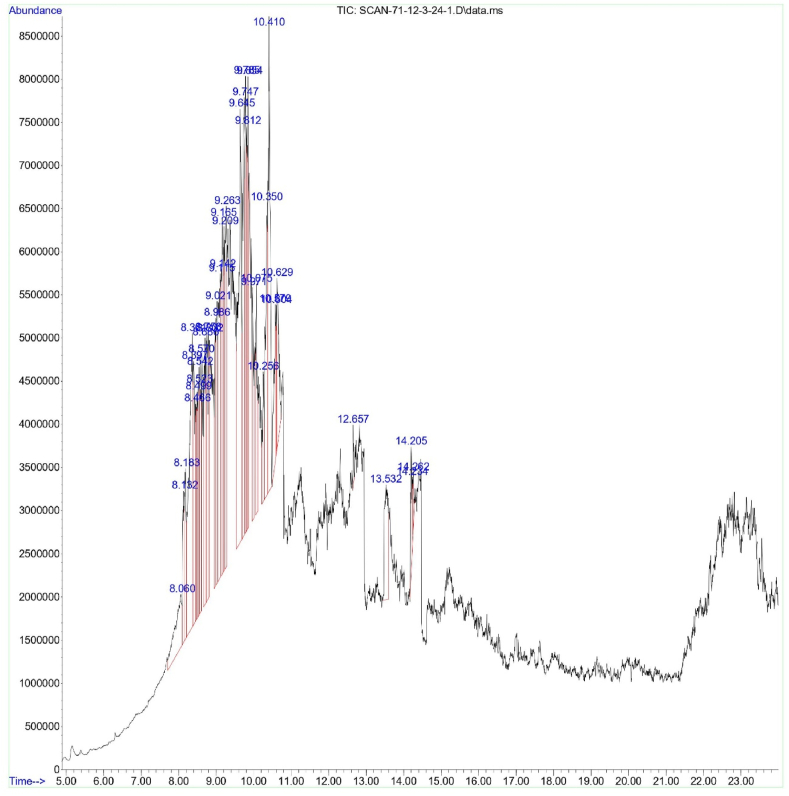
GC-MS of two most effective plant extracts (Neem leaf extract and kortuma fruit extract) was carried out to detect the compounds (Fig. 3, Fig. 4). The majority of the compounds detected were volatile compounds that belonged to different chemical categories, such as fatty acids, hydrocarbons, esters, terpenoids, phenolic compounds, and amines. The key compounds identified in neem leaf extract and kortuma fruit extract are predominantly biologically active molecules. Terpenes exhibited a wide range of different biological activities, such as serving as insecticides and antifeedants, and also possessing cytotoxic properties. The principle active compounds, retention time (R.T), peak area (%), molecular formula (M.F), and molecular weight (g/mol) are described in Table 3, Table 4.
Fig. 3.
Chromatogram of A. indica leaf extract.
Fig. 4.
Chromatogram of C. colocynthis fruit extract.
Table 3.
Gas chromatography mass spectrometry results of A. indica leaf extract.
| Sr. No. | Compound name | Retention time | Area % | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|---|
| 1 | 2,2-dicholoroethanol | 21.081 | 0.30 | C2H4Cl2O | 114.959 |
| 2 | 3-Hydroxystanozolol, di(trimethylsilyl) ether | 21.149 | 0.32 | C27H48N2O2Si2 | 488.8532 |
| 3 | 5,5-bis(trimethylstannyl)-2,2-Bithienyl | 21.228 | 1.21 | C14H22S2Sn2 | 493.921 |
| 4 | Benzamide, 2-bromo-N-(2-butyl)-N-hexadecyl | 23.064 | 7.76 | C23H38BrNO | 424.46 |
| 5 | 5,5-bis(trimethylstannyl)-2,2-Bithienyl | 23.104 | 2.93 | C14H22S2Sn2 | 491.87 |
| 6 | 5-Bromo-4, 4-dichloroimidazole | 23.139 | 3.89 | C3HBCl2N2 | 215.86 |
| 7 | 2,2′,3′,4′,5,5′,6-Heptachloro-3-methylsulfonylbiphenyl | 23.160 | 6.58 | C13H5Cl7O2S | 473.41 |
| 8 | 4-Hydroxy-2,2′,3′,5′,6′-pentachlorobiphenyl, pentafluoropropionate | 23.206 | 18.38 | C15H4Cl5F5O2 | 488.45 |
| 9 | Quassin | 23.254 | 10.94 | C22H28O6 | 388.460 |
| 10 | Roxburghine | 23.297 | 6.30 | C31H32N4O2 | 492.6 |
| 11 | 1,6-Methano-1H-indene, 2,3,3a,4,5,8-hexachloro-3a,6,7,7a-tetrahydro- | 23.395 | 20.46 | C10H6Cl6 | 338.873 |
| 12 | 7-Chloro-N-[[4′-chloro-5-[[diethylamino]methyl]-6-ethoxy]-1,1′-biphenyl-3-]-quinoline-4-amine | 23.436 | 4.78 | C28H29Cl2N3O | 494.5 |
| 13 | 2,4,5-Trichlorobenzoic acid | 23.456 | 4.42 | C7H3Cl3O2 | 225.5 |
| 14 | Pentabromomethyl benzene | 23.575 | 2.77 | C7H3Br5 | 486.62 |
| 15 | Beta-Chlordane | 23.591 | 5.87 | C10H6Cl8 | 409.8 |
| 16 | 3,4,4-Trichloro-3-butenal | 23.655 | 3.19 | C4H3Cl3O | 173.42 |
| 17 | Tetrachloroethylene | 23.677 | 5.33 | C2Cl4 | 165.8 |
| 18 | [1,2]Azaborino [1,2-a] [1,2] azaborine | 23.784 | 2.42 | C8H8BN | 128.967 |
| 19 | Beta-Chlordane | 23.805 | 1.53 | C10H6Cl8 | 409.8 |
Table 4.
Gas chromatography mass spectrometry results of C. colocynthis fruit extract.
| Sr. No. | Compound name | Retention time | Area % | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|---|
| 1 | Glycerin | 8.060 | 2.37 | C3H8O3 | 92.094 |
| 2 | 3-Ethoxy-1, 2-propanediol | 8.132 | 1.39 | C5H12O3 | 120.15 |
| 3 | Glycerin | 8.363 | 6.91 | C3H8O3 | 92.094 |
| 4 | Chlorbromuron | 8.465 | 1.48 | C9H10BrClN2O2 | 293.54 |
| 5 | Metobromuron | 8.524 | 1.12 | C9H11BrN2O2 | 259.10 |
| 6 | Monolinuron | 8.540 | 1.35 | C9H11ClN2O2 | 214.65 |
| 7 | Decanoic acid, propyl ester | 8.572 | 2.26 | C13H26O2 | 214.344 |
| 8 | 2,3,4,5,6-Pentabromobenzyl alcohol, 2-methylpropyl ether | 8.684 | 3.13 | C11H11Br5O | 558.724 |
| 9 | 4-Chloro-2-hydrazinyl-1, 3-benzothiazole | 8.984 | 2.64 | C7H6CIN3S | 199.66 |
| 10 | N,N'-(Oxydi-4,1-phenylene)bis(2,2,2-trichloroacetamide) | 9.021 | 2.52 | C16H10Cl6N2O3 | 491.0 |
| 11 | 2,2-dicholoroethanol | 9.112 | 4.93 | C2H4Cl2O | 114.959 |
| 12 | Tris(4-bromophenyl)amine | 9.209 | 2.59 | C18H12Br3N | 482.0 |
| 13 | Rhodium 1,5-cyclooctadiene chloride dimer | 9.263 | 3.82 | C16H24Cl2Rh2 | 493.079 |
| 14 | 5,5-bis(trimethylstannyl)-2,2-Bithienyl | 9.647 | 21.59 | C14H22S2Sn2 | 491.87 |
| 15 | 6-(3,5-Dichlorobenzoyl)-7-methyl-6,7-dihydro-5H-pyrrolo [3,4-d]pyrimidin e-2,4-diamine, 2TMS derivative | 9.749 | 18.79 | C20H29Cl2N5OSi2 | 482.5 |
| 16 | Benzamide, 2,6-difluoro-3-methyl-N-benzyl-N-hexadecyl | 9.787 | 8.78 | C24H39F2NO | 395.5694 |
| 17 | 3-Bromo-7,7′-dimethyl-4,4′-dihydroxy-1,1′-binaphthalene-5,5′,8,8′-tetrone | 9.856 | 7.17 | C22H13BrO6 | 453.24 |
| 18 | Rhodium, 1,5-cyclooctadiene chloride dimer | 10.350 | 13.03 | C16H24Cl2Rh2 | 493.079 |
| 19 | 8,2′-Thioanhydroadenosine, 3TMS derivative | 10.410 | 24.61 | C19H35N5O3SSi3 | 497.8 |
| 20 | 2,2-dichloroethanol | 10.578 | 1.87 | C2H4Cl2O | 114.96 |
| 21 | 2, 3, 4, 5, 6-Pentabromobenzyl alcohol, isopropyl ether | 12.657 | 0.26 | C10H9Br5O | 544.698 |
| 22 | Diselenide, bis[perfluoroisopropyl] | 13.532 | 2.70 | C6F14Se2 | 495.96 |
| 23 | 1,3,4,5,6-pentaphenyl, 2(1H)-Pyridinone | 14.205 | 1.91 | C35H25NO | 475.58 |
| 24 | Beta-Chlordane | 14.236 | 0.39 | C10H6Cl8 | 409.8 |
| 25 | Rhodium, 1,5-cyclooctadiene chloride dimer | 14.262 | 0.25 | C16H24Cl2Rh2 | 493.079 |
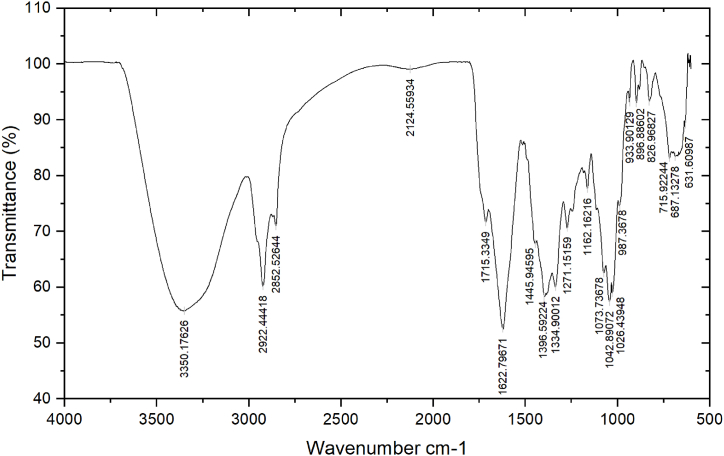
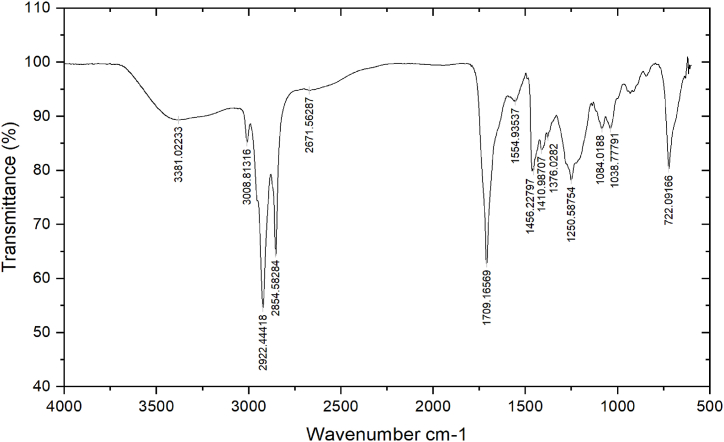
3.4. Analysis of plant extracts through FTIR spectroscopy
The presence of functional groups in the ethanolic extracts of neem leaf (Fig. 5) and kortuma fruit (Fig. 6) was determined using FTIR spectroscopy. Table 5, Table 6 illustrate the numerous functional groups that are identified in the two extracts, respectively.
Fig. 5.
FTIR spectrum of the ethanolic leaf extract of A. indica.
Fig. 6.
FT-IR spectrum of the ethanolic fruit extract of C. colocynthis.
Table 5.
Fourier Transform Infrared spectroscopy (FTIR) results indicating the presence of various functional groups in the A. indica leaf extract.
| Peaks | Wavenumber cm−1 | Transmittance % | Functional group | Bond |
|---|---|---|---|---|
| 1 | 3350.18 | 55.8 | Amine | = N-H (stretch) |
| 2 | 2922.44 | 60.22 | Alkanes | C-H (stretch) |
| 3 | 2852.53 | 71.03 | Alkane | C-H (stretch) |
| 4 | 2124.56 | 99.07 | Alkyne | C ≡ C |
| 5 | 1715.33 | 71.68 | Carbonyl | C=O (stretch) |
| 6 | 1622.79 | 52.57 | Amine | N-H (bend) |
| 7 | 1445.94 | 67.79 | Carbonyl | C=O (bend) |
| 8 | 1396.59 | 58.26 | Alkane | C-H (bend) |
| 9 | 1334.90 | 60.25 | Alkane | C-H (bend) |
| 10 | 1271.15 | 70.72 | Carboxylic acid | -O-H (bend) |
| 11 | 1162.16 | 77.71 | Alcohol | C-O (stretch) |
| 12 | 1073.74 | 62.55 | Ester | C-O (stretch) |
| 13 | 1042.89 | 57.49 | Primary amine | C-N (stretch) |
| 14 | 1026.44 | 59.05 | Primary amine | C-N (stretch) |
| 15 | 987.37 | 74.63 | Alkene | C-H bend) |
| 16 | 933.90 | 93.45 | Alkene | C-H (bend) |
| 17 | 896.89 | 93.15 | Arenes | C-H (bend) |
| 18 | 826.97 | 93.36 | Amines | N-H (wagging) |
| 19 | 715.92 | 83.5 | Alkane | C-H (bend) |
| 20 | 687.13 | 83.42 | Alkyne | C-H (deformed) |
| 21 | 631.61 | 89.47 | Alcohol | O-H (bend) |
Table 6.
Fourier Transform Infrared spectroscopy (FTIR) results indicating the presence of various functional groups in the C. colocynthis fruit extract.
| Peaks | Wavenumber cm−1 | Transmittance % | Functional group | Bond |
|---|---|---|---|---|
| 1 | 3381.02 | 89.33 | Amine | = N-H |
| 2 | 3008.81 | 85.21 | Alkene | = C-H |
| 3 | 2922.44 | 54.59 | Alkanes | C-H |
| 4 | 2854.58 | 64.42 | Alkane | C-H Stretch |
| 5 | 2671.56 | 94.8 | Alkene | C=C |
| 6 | 1709.16 | 62.84 | Carbonyl | C=O |
| 7 | 1554.93 | 92.82 | Aromatic | C=C |
| 8 | 1456.23 | 79.84 | Alkane | C-H bend |
| 9 | 1410.99 | 83.78 | Alkane | C-H bend |
| 10 | 1376.02 | 86.24 | Alkane | C-H bend |
| 11 | 1250.59 | 78.28 | Acyl | C-O stretch |
| 12 | 1084.02 | 87.79 | Alcohol | C-O stretch |
| 13 | 1038.78 | 87.79 | Ester | C-O |
| 14 | 722.09 | 80.31 | Alkane | C-H bend |
4. Discussion
Plant extracts are used in pest management due to their noteworthy effectiveness against various stages of the insect pest life cycle. Botanical insecticides provide effective and eco-friendly solution to control FAW [44]. Current study demonstrated the potential of plant extracts against FAW. The results of present research demonstrated that higher concentrations and prolonged exposure period of plant extracts showed higher mortality of fall armyworm. Plant based insecticides can be a sustainable alternative to control fall armyworm. Neem leaf and seed extract was found be highly toxic against fall armyworm at different concentrations [21]. Azadirachtin induced 96.7 % mortality among 2nd instar larvae of FAW after exposure of 120 h [43]. Neem leaf extract was found to be more toxic against fall armyworm larvae at 100 ppm. It indicated that mortality rate of fall armyworms was directly correlated with the increased concentration of plants extract and the duration of exposure [45]. Our present study demonstrates that A. indica is very effective botanical extract against FAW as it induced 64 % mortality of FAW 3rd instar larvae after 24 h of plant exposure.
The implementation of biopesticides provides an unconventional method for promoting the development of an ecosystem while preventing the detrimental impact on beneficial natural insect fauna and non-target pests. Hassam et al. [46] reported the toxicity levels of C. colocynthis seed extract against the populations of Erias vitella under the laboratory conditions. Results showed that LC30 and LC80 concentrations (ppm) of the methanol-based C. colocynthis seed extract was effective against E. vitella first instar larvae. The treated samples showed prolonged larval and pupal stages compared to the untreated ones. Our results demonstrated that C. colocynthis was very effective as compared to the other plant extracts against the third instar larvae of fall armyworm while maximum mortality was caused in the positive control. The current results indicate that botanical insecticides derived from C. colocynthis extracts have advantages in terms of promoting ecosystem sustainability and can be included into insect management programs, ensuring environmental safety. The highest concentration of C. colocynthis (400 ppm) caused 60 % mortality of FAW larvae after 72 h of exposure duration. Similar to our study, Ahmed et al. [27] reported that 20 mg/mL of C. colocynthis induced 76.67 % mortality among cabbage aphid after the 48 h of treatment. The mortality rate is almost similar, there is little difference that may be due to the concentration of plant extract, and laboratory conditions.
Sakadzo et al. [37] assessed the biological activity of N. tabacum leaf extract against fall armyworm at Great Zimbabwe University under laboratory conditions. The results demonstrated that pure aqueous leaf extracts of tobacco showed antifeedant activity ultimately resulted in FAW larval mortalities considerably higher at the maximum dose of 50 % (p < 0.05) compared to the minimum dosage of 25 % and the negative control after a duration of 20 h. Sakadzo et al. [37] reported that N. tabacum can serve as a viable substitute for synthetic insecticides in controlling the FAW. The LC50 values of N. tabacum were 1517.811, 938.740, and 720.980 ppm after 24, 48, and 72 h, respectively. Our results are more similar to another previous study conducted by Kardinan and Maris [47] as 40 % mortality of FAW was by N. tabacum in both previous and present study. Based upon this context, it can be concluded that tobacco leaf extract can serve as an alternative agent against the synthetic insecticides to control the fall armyworm.
The methanolic extracts of P. hysterophorus and A. adenophora were found to be effective against fifth-instar larvae of S. frugiperda after 72 h, with LC50 values of 5.92 % and 7.82 %, respectively. The efficacy of P. hysterophorus antifeedant action against the fall armyworm was found to be better than that of A. adenophora [48]. The present study is also relied on P. hysterophorus leaf extract to assess its efficacy against the third instar larvae of S. frugiperda. The results demonstrated that it can be an effective alternative to synthetic insecticides in controlling the fall armyworm. The LC50 value of P. hysterophorus was 1686.344 ppm after 24 h, followed by 1113.803 ppm after 48 h, and 320.027 ppm after 72 h.
Arey [49] assessed the toxicity of C. sativa extract against the fall armyworm larvae. A high level of mortality was recorded because of ingesting leaves treated with C. sativa leaf extract. The results of current study demonstrated the efficacy of an ethanolic leaf extract of C. sativa against third instar larvae of fall armyworm. The extract showed a LC50 value of 1517.811, 938.740, and 334.759 ppm after 24, 48, and 72 h, respectively. Up to our knowledge, no study has reported the percent mortality of C. sativa against the fall armyworm. Findings of Prvulovic et al. [50] demonstrated that the highest tested concentration (2 %) caused 42. 5 % mortality of Plodia interpunctella larvae after the 72 h of exposure period. Their results are in line with our study. According to our study, 44 % mortality was induced after exposure of 200 ppm concentration, while 56 % is induced due to the highest concentration (400 ppm) of FAW third instar larvae after 72 h post-treatment.
E. globulus induced 54.51 %, 9.76 %, and 29 % of the mortality of larvae after 24, 48, and 72 h, respectively [51]. Our research results showed that E. globulus at 400 ppm concentration caused 28, 36, 48 % mortality of fall armyworm third instar larvae after 24, 48, and 72 h, respectively. Following the 24, 48, and 72-h, the LC50 values were 1011.981, 815.120, and 518.438 ppm. The findings of the study performed by Anwar et al. [52] are also consistent with the findings of our study.
The plant extracts that demonstrated the greatest efficacy against the fall armyworm were subjected to GC-MS analysis to identify specific compounds present in them, as previously described in numerous studies by Andjani et al. [53], Ahmad et al. [54], Ahmed et al. [27], Khanday & Sharma [55], and Kanmani et al. [56]. The functional groups were identified by means of FTIR spectroscopy [21]. The extraction of principal active compounds depends upon the solvent and type of plant part used. Solvents with high polarity yielded greater than those of low polarity solvents. Based on our results, ethanol yields a greater amount of plant extract, because of its high polarity. According to previous literature, ethanol produced a greater amount of extract from Melastoma malabathricum leaves as compared to other solvents [57]. Moreover, a study revealed a relatively high yield of C. sativa and C. colocynthis extract when ethanol was used as the solvent for extraction [58].
5. Conclusion
Based on results of this study, it is concluded that A. indica, C. colocynthis, E. globulus, C. sativa, N. tabacum, and P. hysterophorus had significant toxicity potential against the fall armyworm third instar larvae. GC-MS analysis demonstrated the presence of valuable bioactive compounds responsible for S. frugiperda mortality. As indicated by previous studies, an increase in the use of synthetic insecticides has led to the development of insect pest resistance and is especially harmful to the environment and beneficial organisms. Farmers should employ these kind of environmental friendly insecticides to lessen the impact of fall armyworm because these products are cheaper to use and better for the economy and the environment. These botanical extracts may be used by the farmers in both forms (crude extract or methanolic/ethanolic extracts) to minimize the damage of insect pests. However, more research is needed to purify the key molecules responsible for the mortality of fall armyworm and to determine the efficacy of these active compounds against the other insect pests.
CRediT authorship contribution statement
Usama Saleem: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Muhammad Asrar: Supervision. Farhat Jabeen: Writing – review & editing. Syed Makhdoom Hussain: Writing – review & editing, Visualization. Dilbar Hussain: Validation, Supervision, Software.
Data availability
All the relevant data will be available on a reasonable request from corresponding author.
Funding
This research did not receive any funding from public, commercial, or non-profit organizations.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to express their gratitude to the Department of Zoology at Government College University Faisalabad and Entomological Research Institute, Ayub Agricultural Research Institute Faisalabad for providing the research facilities used in this study.
References
- 1.Byerlee D. The globalization of hybrid maize, 1921–70. J. Global Hist. 2020;15(1):101–122. [Google Scholar]
- 2.Sahoo S., Adhikari S., Joshi A., Singh K.N. Use of wild progenitor teosinte in maize (Zea mays subsp. mays) improvement: present status and future prospects. Trop. Plant Biol. 2021;14(2):156–179. [Google Scholar]
- 3.Mallapur P.C., Manjunath C. Stenachroia elongella Hampson-a new threat to maize cultivation in Karnataka, India. J. Exp. Zool. 2015;18(1):327–329. [Google Scholar]
- 4.Manjunath C., Mallapur P.C., Balikai A.R. Evaluation of biopesticides/bio-control a gents against maize stem borers. Ann. Entomol. 2016;34(1/2):15–21. [Google Scholar]
- 5.Akeme N.C., Ngosong C., Sumbele A.S., Aslan A., Tening S.A., Krah Y.C., Nambangia J.O. Different controlling methods of fall armyworm (Spodoptera frugiperda) in maize farms of small-scale producers in Cameroon, in IOP Conference Series. Earth Environ. Sci. 2021;911(1) [Google Scholar]
- 6.Gamage A., Liyanapathiranage A., Manamperi A., Gunathilake C., Mani S., Merah O., Madhujith T. Applications of starch biopolymers for a sustainable modern agriculture. Sustainability. 2022;14(10):6085–6098. [Google Scholar]
- 7.Tefera T., Mugo S., Mwimali M., Anani B., Tende R., Beyene Y., Prasanna M.B. Resistance of Bt-maize (Mon810) against the stem borers Busseola fusca (Fuller) and Chilo partellus (Swinhoe) and its yield performance in Kenya. Crop Prot. 2016;89:202–208. doi: 10.1016/j.cropro.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navik O., Shylesha N.A., Patil J., Venkatesan T., Lalitha Y., Ashika R.T. Damage, distribution and natural enemies of invasive fall armyworm Spodoptera frugiperda (JE smith) under rainfed maize in Karnataka, India. Crop Prot. 2021;143 [Google Scholar]
- 9.Rahman A., Pittarate S., Perumal V., Rajula J., Thungrabeab M., Mekchay S., Krutmuang P. Larvicidal and antifeedant effects of copper nano-pesticides against Spodoptera frugiperda (JE Smith) and its immunological response. Insects. 2022;13(11):1030. doi: 10.3390/insects13111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilal A.A., Bashir L., Faheem M., Rajput A., Soomro A.J., Kunbhar S., Sahito M.G.J. First record of invasive fall armyworm [Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)] in corn fields of Sindh, Pakistan. Pakistan J. Agri. Res. 2020;33(2):247–252. [Google Scholar]
- 11.Yan X.R., Wang Z.Y., Feng S.Q., Zhao Z.H., Li Z.H. Impact of temperature change on the fall armyworm, Spodoptera frugiperda under global climate change. Insects. 2022;13(11):981. doi: 10.3390/insects13110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M.R., Gadratagi G.B., Paramesh V., Kumar P., Madivalar Y., Narayanappa N., Ullah F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy. 2022;12(9):2150. [Google Scholar]
- 13.Anilkumar G., LakshmiSoujanya P., Kumar V.D., Kumar M.V., Yathish R.K., Sekhar C.J., Jat S.H. Integrated approaches for the management of invasive fall armyworm, Spodoptera frugiperda (JE Smith) in maize. J. Plant Dis. Prot. 2024;131(3):793–803. [Google Scholar]
- 14.Paredes-Sánchez A.F., Rivera G., Bocanegra-García V., Martínez-Padrón Y.H., Berrones-Morales M., Niño-García N., Herrera-Mayorga V. Advances in control strategies against Spodoptera frugiperda. a review. Molecules. 2021;26(18):5587. doi: 10.3390/molecules26185587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mota-Sanchez D., Wise C.J. Michigan State University; 2021. The Arthropod Pesticide Resistance Database. [Google Scholar]
- 16.Ramzan M., Ilahi H., Adnan M., Ullah A., Ullah A. Observation on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on maize under laboratory conditions. Egyptian Acad. J. Biol.Sci. Entomol. 2021;14(1):99–104. [Google Scholar]
- 17.Ngegba M.P., Cui G., Khalid Z.M., Zhong G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture. 2022;12(5):600. [Google Scholar]
- 18.Perumal V., Kannan S., Pittarate S., Chinnasamy R., Krutmuang P. Essential oils from Acacia nilotica (Fabales: fabaceae) seeds: may have insecticidal effects. Heliyon. 2023;9(4) doi: 10.1016/j.heliyon.2023.e14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivekanandhan P., Alahmadi A.T., Ansari J.M., Subala P.S. Biocontrol efficacy of cajeput oil against Anopheles stephensi L. mosquito and its effect on non-target species. Front. Physiol. 2024;15 doi: 10.3389/fphys.2024.1357411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souto L.A., Sylvestre M., Tölke D.E., Tavares F.J., Barbosa-Filho M.J., Cebrián-Torrejón G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: prospects, applications and challenges. Molecules. 2021;26(16):4835. doi: 10.3390/molecules26164835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulashie K.S., Adjei F., Abraham J., Addo E. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), Case Stud. Chemical Environ. Engin. 2021;4 [Google Scholar]
- 22.David C.B., Chinedu I., Emmanuel O.O., Daniel I., Polly S.J., Sunday G.O., Ephraim H.D. Pesticide residues in vegetables: a public health concern. Int. J. Sci. Res. Inn. Stud. 2022;1(2):9–25. [Google Scholar]
- 23.Paragas S.D., Cruz K., Fiegalan R.E. Assessment of green solvents and extraction methods for biopesticide preparation from neem Azadirachta indica leaves against Oriental fruit fly Bactrocera dorsalis (Hendel) Preprints. 2018 [Google Scholar]
- 24.Singh P., Tiwari M. Review on Azadirachta indica, int. J. Pharmacogn. Life Sci. 2021;2(1):28–33. [Google Scholar]
- 25.Ali K., Sagheer M., Rashid A., Shahid M. Bioactivity of medicinal plant extracts as toxicants and enzyme inhibitors against insect pests of stored commodities. J. Crop Prot. 2021;10(1):95–109. [Google Scholar]
- 26.El-Halim A., Hend T., Ayad L.E., El-Deeb E.S. Efficiency of utilizing Citrullus colocynthis (L) essential oil alone and when it mixed with diatomaceous earth against some stored products insect pests. J. Plant Prot. Pathol. 2023;14(12):405–413. [Google Scholar]
- 27.Ahmed M., Peiwen Q., Gu Z., Liu Y., Sikandar A., Hussain D., Ji M. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.) Sci. Rep. 2020;10(1):522. doi: 10.1038/s41598-019-57092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nizamani B., Agha A.M., Ali Q., Hussain A., Manghwar H., Kamran M., Soomro M.D. Influence of indigenous plant materials on reproductive performance of Callosobruchus chinensis (L.) (Coleoptera: bruchidae) on chickpea. Int. J. Trop. Insect Sci. 2020;40:1003–1011. [Google Scholar]
- 29.Noureldeen A., Kumar U., Asad M., Darwish H., Alharthi S., Fawzy A.M., Alkashgry N. Aphicidal activity of five plant extracts applied singly or in combination with entomopathogenic bacteria, Xenorhabdus budapestensis against rose aphid, Macrosiphum rosae (Hemiptera: aphididae) J. King Saud Univ. Sci. 2022;34(8) [Google Scholar]
- 30.Pinto M., Soares C., Martins M., Sousa B., Valente I., Pereira R., Fidalgo F. Herbicidal effects and cellular targets of aqueous extracts from young Eucalyptus globulus Labill. Leaves, Plants. 2021;10(6):1159. doi: 10.3390/plants10061159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhakad K.A., Pandey V.V., Beg S., Rawat M.J., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. J. Sci. Food Agric. 2018;98(3):833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 32.Vivekanandhan P., Usha-Raja-Nanthini A., Valli G., Subramanian Shivakumar M. Comparative efficacy of Eucalyptus globulus (Labill) hydrodistilled essential oil and temephos as mosquito larvicide. Nat. Prod. Res. 2020;34(18):2626–2629. doi: 10.1080/14786419.2018.1547290. [DOI] [PubMed] [Google Scholar]
- 33.Tine S., Tine-Djebbar F., Debab A., Mesloub A., Soltani N. Insecticidal efficacy and physiological effects of Eucalyptus globulus essential oil and its constituent, 1, 8-Cineole against Tribolium confusum (Jacquelin du Val, 1868) (Coleoptera, Tenebrionidae), J. Plant Dis. Prot. 2023;130(4):769–780. [Google Scholar]
- 34.Singh D.K., Mobolade J.A., Bharali R., Sahoo D., Rajashekar Y. Main plant volatiles as stored grain pest management approach: a review. J. Agric. Food Res. 2021;4 [Google Scholar]
- 35.Mukanga M., Machuku O., Chipabika G., Matimelo M., Mumba K., Mabote D.N., D N., Lwinya K. Bio-efficacy of crude aqueous leaf extracts against the fall armyworm (Spodoptera frugiperda) and maize ear rots in Zambia. Adv. Biol. Res. 2022;3(1):38–49. [Google Scholar]
- 36.Asad M., Khan R.R., Aljuboory B.A., Rashid U.H.M., Kumar U., Haq U.I., Alharbi K. Toxic and antifeedant effects of different pesticidal plant extracts against beet. Phyton. 2023;92(4) [Google Scholar]
- 37.Sakadzo N., Makaza K., Chikata L. Biopesticidal properties of aqueous crude extracts of tobacco (Nicotiana Tabacum L.) against fall armyworm (Spodoptera frugiperda JE Smith) on maize foliage (Zea Mays L.) diets. Agric. Sci. 2020;2(1):47–55. [Google Scholar]
- 38.McPartland M.J., Sheikh Z. A review of Cannabis sativa-based insecticides, Miticides, and repellents. J. Entomol. Zool. Stud. 2018;6(6):1288–1299. [Google Scholar]
- 39.Andre M.C., Hausman F.J., Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19–34. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iseppi R., Brighenti V., Licata M., Lambertini A., Sabia C., Messi P., Benvenuti S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L.(Hemp) Molecules. 2019;24(12):2302–2312. doi: 10.3390/molecules24122302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mubeen N., Khalid A., Ullah I.M., Altaf N., Arshad M., Amin L., Sadaf A. Effect of Metarhizium anisopliae on the nutritional physiology of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), Egypt. J. Biol. Pest Control. 2022;32(1):73–85. [Google Scholar]
- 42.S.W. Abbott, A method of computing the effectiveness of an insecticide, J. Econ. Entomol. 18(2) 265-267.
- 43.Idrees A., Afzal A., Chohan A.T., Hayat S., Qadir A.Z., Gaafar Z.R.A., Li J. Laboratory evaluation of selected botanicals and insecticides against invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) J. King Saud Univ. Sci. 2023;35(7) [Google Scholar]
- 44.Harte J.S., Bray P.D., Nash-Woolley V., Stevenson C.P., Fernández-Grandon M.G. Antagonistic and additive effect when combining biopesticides against the fall armyworm, Spodoptera frugiperda. Sci. Rep. 2024;14(1):6029. doi: 10.1038/s41598-024-56599-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altaf N., Arshad M., Majeed Z.M., Ullah I.M., Latif H., Zeeshan M., Afzal M. Comparative effectiveness of chlorantraniliprole and neem leaf extract against fall armyworm, Spodoptera frugiperda (JE smith) (Lepidoptera: Noctuidae) Sarhad J. Agric. 2022;38(3):833–840. [Google Scholar]
- 46.Hassam A.U., Gulzar A., Rasool B., Zafar S., Younis T., Shakeel M., Hafeez M. Efficacy of Citrullus colocynthis seed extract on Earias vittella, Fabricius, (Lepidoptera: Noctuidae): environment sustainable approach. Brazilian J. Biol. 2022;84 doi: 10.1590/1519-6984.254479. [DOI] [PubMed] [Google Scholar]
- 47.Kardinan A., Maris P. Effect of botanical insecticides against fall armyworm Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) IOP Conference Series: Earth Environ. Sci. 2021;653(1) [Google Scholar]
- 48.Yogesh W., Deepali G., Sachin P. Plant weeds Parthenium hysterophorus and Ageratina adenophora for fall armyworm control. Int. J. Life Sci. 2013;1(1):71–75. [Google Scholar]
- 49.Arey C.N. Doctoral Dissertation, Louisiana State University and Agricultural & Mechanical College) 2022. Identifying the components of a hemp (Cannabis sativa L.) (rosales: cannabaceae) integrated pest management program in Louisiana. [Google Scholar]
- 50.Prvulovic D., Gvozdenac S., Latković D., Peić Tukuljac M., Sikora V., Kiprovski B., Ovuka J. Phytotoxic and insecticidal activity of industrial hemp (Cannabis sativa L.) Extracts against Plodia interpunctella Hübner—a potential sunflower grain protectant. Agronomy. 2023;13(10):2456. [Google Scholar]
- 51.Tenazoa N.M.P., Khan A., Aslam S., Ahmed I., Zaryab S., Ullah S., Begum S., Salman M., Ahmad B., Ashfaque M., Khan I., Qazi I., Muhammad M. The life cycle of fall Armyworm (FAW), Spodoptera frugiperda and toxicity of botanicals against 2nd instar larvae on maize. Biosci. Res. 2022;12(9):1223–1228. [Google Scholar]
- 52.Anwar F., Khan J.H., Tiwana U.A., Kashif M., Khan M.S., Ali M., Jabeen N., Tenazoa N.M.P., Jamil M. Biology and management of early instar larvae of fall armyworm, Spodoptera frugiperda (Lepidopter: Noctuidae) with selected plant extracts. Biosci. Res. 2022;12(9):968–973. [Google Scholar]
- 53.Andjani N.H., Sentosa Y., Yati K., Jufri M., Fauzantoro A., Gozan M. Determination of LC50 value of Nicotiana tabacum L. extract against Gryllus bimaculatus imago and Galleria mellonella larvae. AIP Conf. Proc. 2019;293(1) [Google Scholar]
- 54.Ahmad S., Zafar R., Khan H.I., Javaid A., Intisar A. Assessment of toxicity of Parthenium hysterophorus L. extract against larvae of Trogoderma granarium. Plant Prot. 2022;6(3):239–245. [Google Scholar]
- 55.Khanday S., Sharma D.G. GC-MS analysis and antifeedant activity of Azadirachta indica-leaf extract. Stechnolock Plant Biol. Res. 2021;1:1–15. [Google Scholar]
- 56.Kanmani S., Kumar L., Raveen R., Tennyson S., Arivoli S., Jayakumar M. Toxicity of tobacco Nicotiana tabacum Linnaeus (Solanaceae) leaf extracts to the rice weevil Sitophilus oryzae Linnaeus 1763 (Coleoptera: Curculionidae) The J. Basic Appl. Zool. 2021;82:1–12. [Google Scholar]
- 57.Awang A.M., Aziz R., Sarmidi R.M., Abdullah L., Yong K.P., Musa F.N. Comparison of different solvents on the extraction of Melastoma malabathricum leaves using soxhlet extraction method, Der Pharm. Lett. 2017;8(4):153–157. [Google Scholar]
- 58.Ahmed M., Ji M., Qin P., Gu Z., Liu Y., Sikandar A., Javeed A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019;17(3) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data will be available on a reasonable request from corresponding author.