Abstract
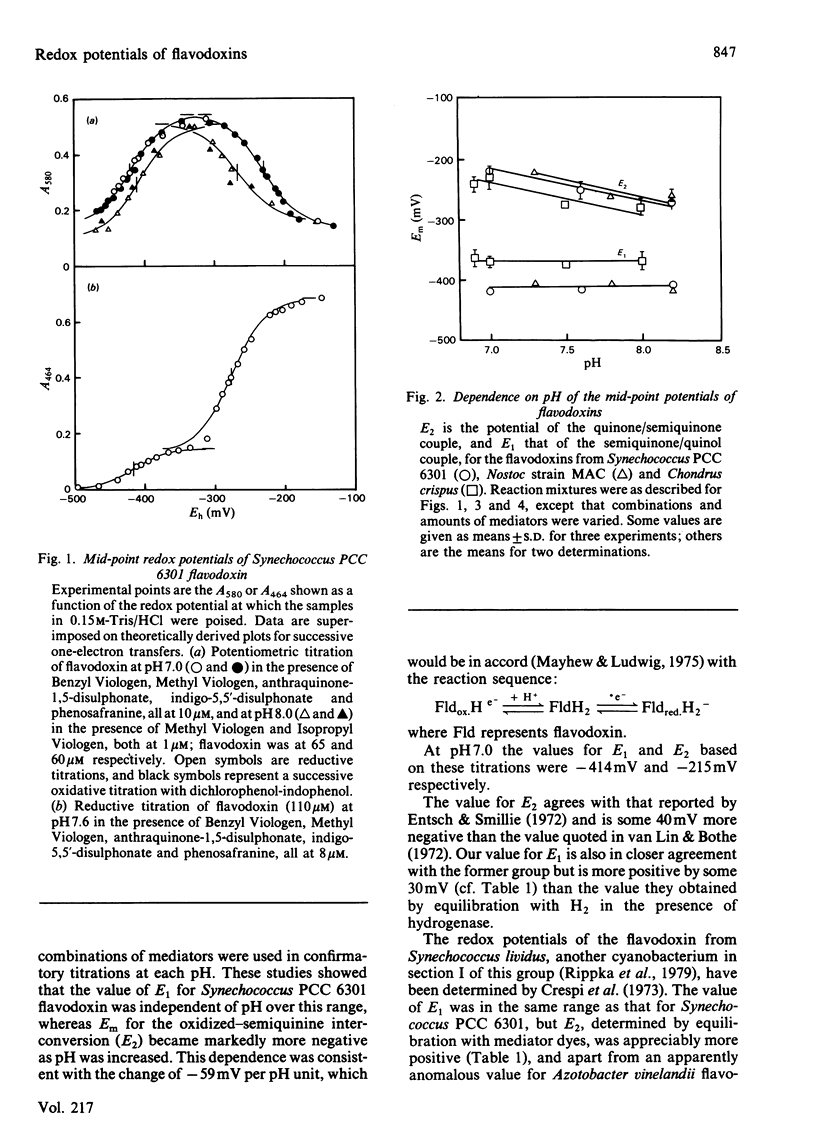
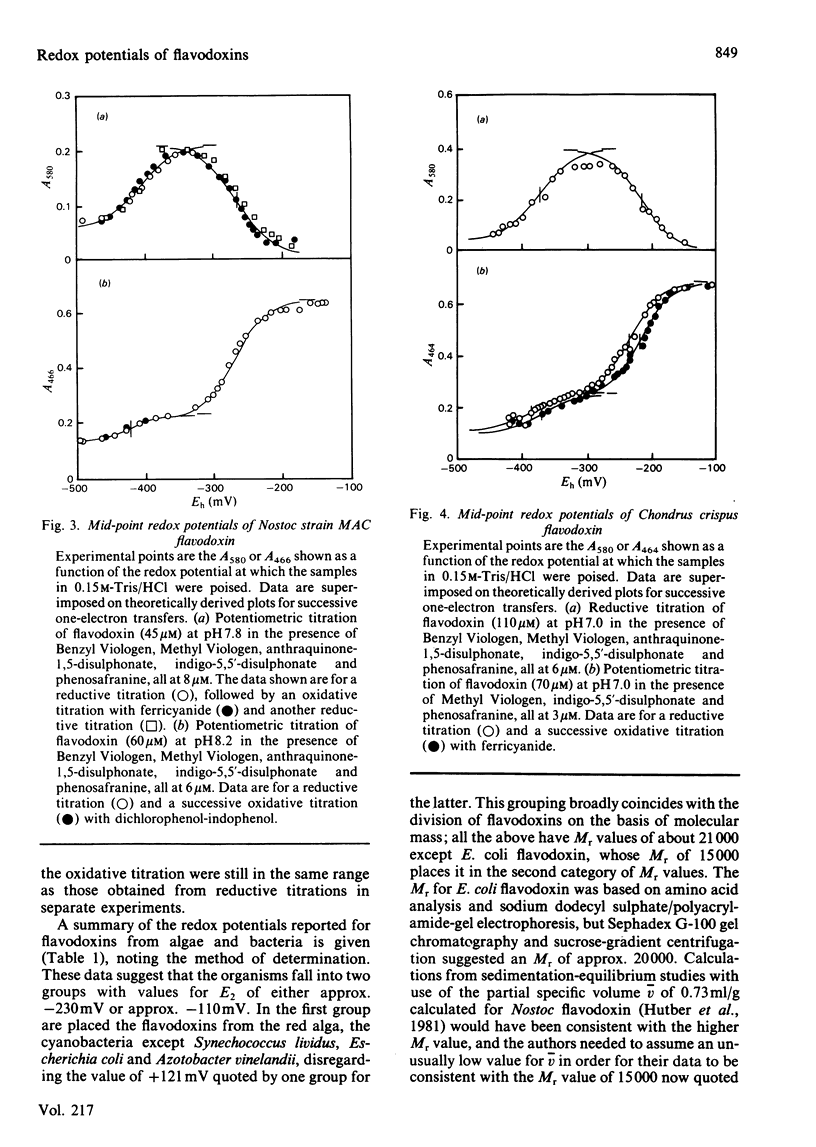
The redox potentials of flavodoxins from the cyanobacteria Synechococcus PCC 6301 (formerly Anacystis nidulans) and Nostoc strain MAC, and from the red alga Chondrus crispus, were determined by potentiometric titration. For the oxidized-semiquinone interconversion the potentials at pH 7.0 of the three flavodoxins were between -210 and -235 mV, and these were pH-dependent over the range pH 6.9-8.2. For the semiquinone-reduced interconversion the potentials of the cyanobacterial flavodoxins were close to -414 mV, and that for the algal flavodoxin, -370 mV, is the highest reported in this group of flavoproteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barman B. G., Tollin G. Flavine-protein interactions in flavoenzymes. Thermodynamics and kinetics of reduction of Azotobacter flavodoxin. Biochemistry. 1972 Dec 5;11(25):4755–4759. doi: 10.1021/bi00775a019. [DOI] [PubMed] [Google Scholar]
- Bothe H., Falkenberg B. The reduction of flavodoxin from Azotobacter vinelandii by pyruvate. Z Naturforsch B. 1972 Sep;27(9):1090–1094. doi: 10.1515/znb-1972-0930. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Norris J. R., Bays J. P., Katz J. J. ESR and NMR studies with deuterated flavodoxin. Ann N Y Acad Sci. 1973 Dec 31;222:800–815. doi: 10.1111/j.1749-6632.1973.tb15306.x. [DOI] [PubMed] [Google Scholar]
- Crespi H. L., Smith U., Gajda L., Tisue T., Ammeraal R. M. Extraction and purification of 1 H, 2 H, and isotope hybrid algal cytochrome, ferredoxin and flavoprotein. Biochim Biophys Acta. 1972 Mar 16;256(3):611–618. doi: 10.1016/0005-2728(72)90196-x. [DOI] [PubMed] [Google Scholar]
- Dubourdieu M., le Gall J., Favaudon V. Physicochemical properties of flavodoxin from Desulfovibrio vulgaris. Biochim Biophys Acta. 1975 Mar 20;376(3):519–532. doi: 10.1016/0005-2728(75)90172-3. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Entsch B., Smillie R. M. Oxidation--reduction properties of phytoflavin, a flavoprotein from blue-green algae. Arch Biochem Biophys. 1972 Aug;151(2):378–386. doi: 10.1016/0003-9861(72)90512-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. P., Husain A., Rogers L. J. A constitutive flavodoxin from a eukaryotic alga. Biochem Biophys Res Commun. 1978 Mar 30;81(2):630–635. doi: 10.1016/0006-291x(78)91582-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. P., Rogers L. J., Rao K. K., Hall D. O. Efficiency of ferredoxins and flavodoxins as mediators in systems for hydrogen evolution. Biochem J. 1980 Nov 15;192(2):665–672. doi: 10.1042/bj1920665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. P., Sykes G. A., Rogers L. J. Apoflavodoxin aggregation following dissociation of flavin. Biochim Biophys Acta. 1980 Sep 23;625(1):127–132. doi: 10.1016/0005-2795(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Irie K., Kobayashi K., Kobayashi M., Ishimoto M. Biochemical studies on sulfate-reducing bacteria. XII. Some properties of flavodoxin from Desulfovibrio vulgaris. J Biochem. 1973 Feb;73(2):353–366. [PubMed] [Google Scholar]
- Mayhew S. G., Foust G. P., Massey V. Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):803–810. [PubMed] [Google Scholar]
- Mayhew S. G. Properties of two clostridial flavodoxins. Biochim Biophys Acta. 1971 May 12;235(2):276–288. doi: 10.1016/0005-2744(71)90206-3. [DOI] [PubMed] [Google Scholar]
- Moura I., Moura J. J., Bruschi M., Le Gall J. Flavodoxin and rubredoxin from Desulphovibrio salexigens. Biochim Biophys Acta. 1980 Jun 10;591(1):1–8. doi: 10.1016/0005-2728(80)90215-7. [DOI] [PubMed] [Google Scholar]
- Stankovich M. T. An anaerobic spectroelectrochemical cell for studying the spectral and redox properties of flavoproteins. Anal Biochem. 1980 Dec;109(2):295–308. doi: 10.1016/0003-2697(80)90652-1. [DOI] [PubMed] [Google Scholar]
- Van Lin B., Bothe H. Flavodoxin from Azotobacter vinelandii. Arch Mikrobiol. 1972;82(2):155–172. doi: 10.1007/BF01890407. [DOI] [PubMed] [Google Scholar]
- Vetter H., Jr, Knappe J. Flavodoxin and ferredoxin of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):433–446. doi: 10.1515/bchm2.1971.352.1.433. [DOI] [PubMed] [Google Scholar]
- Watt G. D. An electrochemical method for measuring redox potentials of low potential proteins by microcoulometry at controlled potentials. Anal Biochem. 1979 Nov 1;99(2):399–407. doi: 10.1016/s0003-2697(79)80024-x. [DOI] [PubMed] [Google Scholar]
- Yoch D. C. The electron transport system in nitrogen fixation by azotobacter. IV. Some oxidation-reduction properties of azotoflavin. Biochem Biophys Res Commun. 1972 Oct 17;49(2):335–342. doi: 10.1016/0006-291x(72)90415-9. [DOI] [PubMed] [Google Scholar]


