Abstract
An ideal biomaterial should create a customized tissue-specific microenvironment that can facilitate and guide the tissue repair process. Due to its good biocompatibility and similar biochemical properties to native tissues, decellularized extracellular matrix (dECM) generally yields enhanced regenerative outcomes, with improved morphological and functional recovery. By utilizing various decellularization techniques and post-processing protocols, dECM can be flexibly prepared in different states from various sources, with specifically customized physicochemical properties for different tissues. To initiate a well-orchestrated tissue-regenerative response, dECM exerts multiple effects at the wound site by activating various overlapping signaling pathways to promote cell adhesion, proliferation, and differentiation, as well as suppressing inflammation via modulation of various immune cells, including macrophages, T cells, and mastocytes. Functional tissue repair is likely the main aim when employing the optimized dECM biomaterials. Here, we review the current applications of different kinds of dECMs in an attempt to improve the efficiency of tissue regeneration, highlighting key considerations on developing dECM for specific tissue engineering applications.
Keywords: Decellularized extracellular matrix biomaterials, Physicochemical properties, Underlying mechanisms on dECM-mediated regeneration process, Tissue engineering
Graphical abstract

1. Introduction
Over the past few decades, researchers have been dedicated to delineating the processes and mechanisms involved in tissue repair. It is well-documented that each tissue type can generate a tissue-specific microenvironment for its resident cell lineages, which supports their growth, differentiation and homeostasis; as well as provides complex structural and biochemical cues essential for their functioning during the regeneration process [1]. Based on the above paradigm, surgical treatment and autologous tissue transplantation have long been the preferred treatment regime for reconstructing defect areas. Despite the evident advantages of using autologous tissue, there are still several obstacles restricting its general applications: e.g., donor site morbidity, insufficient quantity of tissue for grafting and possible unsatisfactory aesthetic outcomes [2,3]. Due to their tunable mechanical properties and capacity to provide structural support, artificial synthetic polymers have often been considered to be an alternative grafting material, especially for large-scale and load-bearing tissue regeneration. However, the lack of bioactivity and biodegradability increased the risks of failure after implantation. As the key role of extracellular matrix (ECM) is well recognized during the tissue regeneration process, decellularized extracellular matrix (dECM)-based graft biomaterials have been developed through various decellularization techniques, which can compensate for the deficiencies of bioinert synthetic polymers [4].
By utilizing a series of physical, chemical and enzyme-mediated bioprocessing techniques, the immunogenic cellular component within the tissue is completely removed, while the bulk of tissue-specific bioactive molecules and collagen network can be retained within the dECM [5]. Biomaterials inspired by dECM are characterized by the recapitulation of a conducive microenvironment similar to native target tissues. These can provide biochemical and structural support to specialized cell types and promote basic cellular processes including cell proliferation, migration, and differentiation. Due to the aforementioned advantages, dECM-based biomaterials have been widely applied in almost all types of tissue damage, indicating much promise for tissue regeneration. However, the content and quantity of active ingredients preserved in dECM vary according to different sources. Recent studies have focused on the in-depth profiling of dECM proteome, so that subtle differences can be identified within the dECM proteomic atlas, facilitating the design of optimized dECM biomaterials for specific tissue regeneration applications [6]. There is also a dire need to develop dECM that can flexibly adapt to the shape and pathological conditions of individual defects and wound sites. Hence, it was initially intended for dECM to serve as physical scaffolds for promoting cell adhesion and ingrowth, while micronized dECM powders, together with reconstituted dECM within injectable or bio-printable hydrogels were later developed to fill irregular defects [[7], [8], [9]]. The combination with bioinert polymers is also a good idea to improve the structural stability and mechanical properties of dECM. These diverse forms significantly expanded the clinical applications of dECM-based biomaterials.
In this review, we focused on integrating current knowledge from the scientific literature to further our understanding of dECM-based biomaterials for specific tissue regeneration applications. More specifically, we discussed the preparation and properties of dECM derived from different sources and their fabrication in various forms. Furthermore, the underlying mechanisms by which dECMs modulate cellular behaviors associated with tissue regeneration were systematically analyzed from three aspects: inflammatory response, cell adhesion and proliferation, as well as cell differentiation. Finally, we critically examined representative applications of dECM according to different sources and forms in both soft and hard tissue regeneration. This review thus aims to deepen understanding of dECM-based biomaterials, guiding researchers to select appropriate sources and application forms of dECM for specific tissue regeneration needs, to maximize tissue regeneration efficacy.
2. Source-dependent preparation and biological properties of dECMs
The commonly used dECM for tissue regeneration can be broadly classified into two types: cell-derived and tissue-derived. The majority of cell-derived dECM (CDM) are produced by culturing lab-grown cells in vitro, while tissue-derived dECM (TDM) are generated from homologous or heterologous animal models. Decellularization is carried out by the combined use of various chemical, physical and biological methods [10]. Additionally, it must be noted that relatively short and gentle protocols and reagents are adequate for producing CDM with simple composition, as compared to TDM which requires complex processing techniques. The dECM derived from cells and tissues vary in quantity and characteristics so that sources of decellularized materials from different materials can be selected depending on specific experimental designs and aims [11]. TDM has the advantage of preserving the physical characteristics of the native tissue, such as structural and mechanical properties. Given that the end products often need to be tuned extensively for the intended purpose, CDM with high tunability and consistency should be considered [12]. In any case, the sources selected and properties required would depend on the final target. CDM and TDM are two common starting materials, both of which have various advantages and shortcomings that have to be taken into consideration in light of the specific aims and context (Fig. 1).
Fig. 1.
Advantages and shortcomings of dECM derived from cells, hard and soft tissues.
2.1. Cell-derived dECM
During the culture process, cells can secrete ECM proteins to remodel the surrounding environment, making it more physiologically conducive for cell proliferation and differentiation as soon as they adhere to the surface. Upon decellularization, these functional ECM proteins are reconstituted as CDM. CDM derived from different cell types can have a similar chemical composition but exhibit differences in protein concentration and species attributed to individual cell lineage function. Stem cells facilitate tissue renewal as well as regeneration by renewing themselves through division [13]. External changes in the niche can induce active responses of stem cells, tailoring the ECM output to meet regenerative demands. Therefore, stem cells are regarded as one of the most valuable cell types to be used for CDM production and this cell-free approach is considered as a safer and less problematic alternative compared to direct transplantation [14,15].
The biological composition and function of dECM derived from stem cells might vary with different stem cell types. To investigate this issue, a previous study compared the chondrogenic potential of dECM extracted from synovium-derived stem cells, adipose-derived stem cells and urine-derived stem cells. The results showed that infrapatellar fat pad-derived stem cells (IPFSC) achieved the best chondrogenesis outcome when grown on the dECM scaffold derived from urine-derived stem cells. The advantageous presence of basement membrane proteins rather than fibrillar matrix components might be a possible reason why dECM derived from urine-derived stem cells better facilitated mesenchymal-epithelial transition, leading to enhanced chondrogenesis and improved regeneration [16]. Another study also noted that adult progenitor cells isolated from bone marrow (hBMSCs), human adipose (hADSCs) and articular cartilage (hCDPCs) exhibited individual ECM protein profiles and displayed different chondrogenic efficiency. dECM scaffold derived from hADSCs generated a fibrillar-like ECM pattern (high FN & COL1) and resulted in the quick formation of fibrous fillings, while dECMs derived from hBMSCs and hCDPCs provided a chondrogenic-inductive environment enriched with aggrecan and COL3, leading to better cartilage-like tissue regeneration [17]. As seen from these findings, carefully selected cell sources to produce dECM may pave the way for better tissue regeneration outcomes.
2.2. Tissue-derived dECM
In contrast to CDM, TDM refers to biomaterials derived from homologous or heterologous tissues or organs. It has been reported that TDM can transmit pathogens and initiate inflammatory or anti-host immune responses when applied in experimental animal models and clinical trials [18]. To ensure its biocompatibility and safety, a longer and more rigorous protocol should be considered during the decellularization process [19]. Tissue sections or pieces are a conventional starting point to be decellularized. The bigger the tissue pieces, the longer time it takes for chemical and enzymatic reactions to achieve complete decellularization. Cellular/lipid content and density should also be taken into account when selecting appropriate types of decellularization agents [20,21]. For the simple tissues, such as small intestines and gallbladders, they can be processed by a chemical bath, while solid organs (e.g.: skin, nerves and cardiac valves) often require a combination of chemical bath and mechanical treatment [22]. When preparing the dECM derived from hard tissue such as bone, much effort should be made on optimizing decellularizing solution to penetrate the tissue [21]. It is recommended to adopt freeze–thaw cycles at the beginning of the decellularization to enhance the penetration efficiency of chemical agents as well as reduce disruptions in the tissue ultrastructure [23]. Besides that, demineralization and lipid removal are also put on account for the bone tissue. Similar to the adipose tissue, there is a high fat content of the bone which may slow down the decellularization because of its poor wettability. Thus, lipid removal should be carried out prior to the cell removal step by using non-polar solvents such as acetone and chloroform. Although the decellularization step is relatively intricate and complex for producing TDM, biomaterials made from TDM are regarded as reservoirs of tissue-specific bioactive molecules and complex vascular networks, which could be advantageous for complicated and large-scale tissue regeneration [24,25]. Multiple ECM-cell interactions can be modulated by subtle microenvironmental cues provided by the interior architecture retained in TDM (e.g.: morphology of pores & alignment of collagen fibers) [26]. Upon loading certain initiating materials or specific cells, the resulting refunctionalized TDM biomaterial is suitable for generating tissue-engineered grafts with enhanced repair capacity. Currently, several refunctionalized TDMs have been successfully translated into clinical applications according to the Food and Drug Administration of the United States, making them a promising biomaterial for tissue and organ regeneration [[27], [28], [29]].
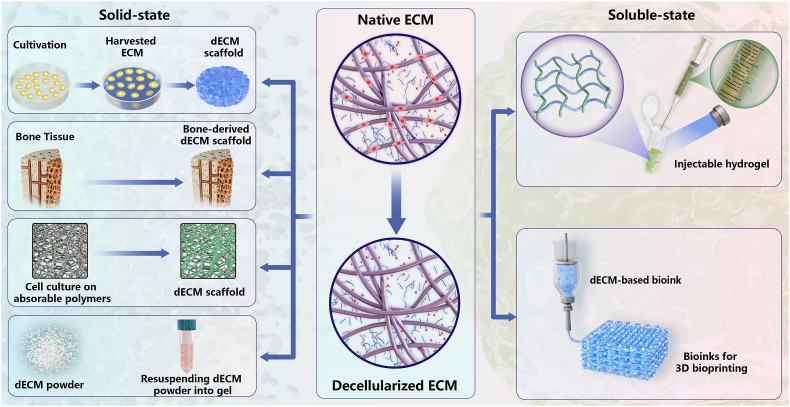
3. The flexibility of dECMs in various states and their physicochemical properties
Generally, dECM is prepared according to two main strategies: whole organ/tissue decellularization and loose tissue decellularization [10]. The three-dimensional structure retained in the former is both an advantage and also somewhat of a limitation due to conformational adaptation and potential host response [30]. Therefore, this chapter will mainly focus on loose tissue decellularization and discuss in detail its physicochemical properties in both solid-state and soluble-state. Solid dECM biomaterials can be ground into microparticles or directly applied as a scaffold without further disrupting the structure, while soluble dECM with ideal viscosity is favorable for forming injectable hydrogels or self-assembly bioinks. When compared with soluble dECM, solid dECM has the advantage of accessibility and ease of handling. The mechanical strength and ECM architecture inside solid dECM ensure its physical integrity and allow for re-cellularization. On the other hand, soluble dECM can be used to encapsulate and culture cells directly by providing a 3D microenvironment similar to that in vivo, making it easily adaptable to defect areas with different shapes. When preparing for the soluble dECM type, an additional step of solubilization is required to dissolve dECM into a liquid form. It is crucial to adjust the physiological pH and temperature during this process. Depending on the different physical and biochemical properties required, the desired application of dECM varies with the type forms (Fig. 2).
Fig. 2.
Two common dECM-based biomaterial types: solid-state and soluble-state. (ECM, extracellular matrix; dECM, decellularized extracellular matrix; 3D, three-dimensional).
3.1. Solid-state dECM
After cell removal using physical, chemical and enzymatic methods, the decellularized matrix is treated by snap freezing and lyophilization to prepare a dECM scaffold, which can be subsequently ground into a powder by milling. Variations in different stages of the fabrication process might impact the biological component, mechanical strength and microstructure of the final products (Fig. 3) [31]. For example, the physical and biological integrity may be disturbed during the milling. However, dECM powder shows its superiority as the optimized “tissue paper” when introduced to a solvent mixture and being converted into a kind of injectable gel or bioink [32].
Fig. 3.
The notable physicochemical properties of four solid-state dECMs. (ECM, extracellular matrix; dECM, decellularized extracellular matrix).
3.1.1. dECM scaffold
There are three strategies for producing a dECM scaffold. As compared to the other two strategies, cell-derived dECM scaffolds are looser and have better plasticity, as discussed above [33]. Owing to the loss of mechanical strength, they are more suitably used to enhance the regeneration of tissues that are not exposed to mechanical stress, such as articular cartilage and ligament. Sometimes, in order to obtain adequate amount of dECM within a limited timeframe, extrinsic factors are employed to increase the production of cartilage-like ECM, such as growth factors, dexamethasone, interleukin-8 (IL-8), mechanical stimuli and hypoxia culture condition [[34], [35], [36], [37], [38], [39]]. As for anterior cruciate ligament reconstruction, stem cell sheet-derived dECM scaffold was found to considerably enhance osteogenic induction and angiogenic capacity via macrophage M2 polarization and MMP/TIMP expression [40].
On the other hand, tissue-derived dECM scaffolds are preferred for providing biomechanical support for load-bearing tissue regeneration such as bone and fibrocartilage. With the help of preexisting Haversian canal structures, a dECM scaffold produced from the entire cortical bone can be designed to accelerate angiogenesis during the regeneration of large bone defects. As expected with dECM scaffolds, vessel ingrowth was observed along the Haversian canals, while newly formed bone-like tissue spread from the inner side to the outer part. The vascularized decellularized cortical bone scaffold proved to be a valuable bone substitute for overcoming the size limitations of decellularized allogeneic bone chips [41]. Inspired by interleaving book shape, native fibrocartilage tissue was sectioned into a book shape before decellularization. Then, adipose-derived stromal cell sheets were sandwiched by the book-shaped fibrocartilage dECM scaffold, which might be favorable for loading transplanted cells, promoting cell infiltration and ECM deposition. The interleaving book-shaped design displayed satisfactory performances in cell attachment, proliferation and chondrogenic inducibility in vitro, and these findings were replicated in vivo with abundant ECM deposition and good interface integration [42].
Another processing strategy has been developed to further enhance the mechanical properties and enable control over the physical properties of dECM scaffolds. It is helpful to improve the regeneration efficacy of tissues associated with extracellular stiffness. In this case, cells were first distributed onto a scaffold template that is mainly composed of absorbable polymers and allowed to deposit ECM. Upon decellularization, cell-derived dECM functioned as a bioactive surface coating on the 3D scaffold. The characterization of ECMs obtained from different seeded cell types on the hybrid scaffolds displayed overlapping but distinct compositions. As one study indicated, polycarbonate fiber mats coated by dECM from chondrocytes could promote the chondrogenic differentiation of human mesenchymal stem cells, while osteogenic differentiation was activated in the fiber mats coated by dECM from co-culture of osteoblasts and chondrocytes [43]. Polycaprolactone could also be incorporated as fibrous scaffolds through electrospinning. A fibrocartilage-mimetic scaffold was constructed with a combination of polycaprolactone, albumin and chondrocyte-derived dECM. The polymeric template structure provided mechanical strength and stiffness, while dECM and albumin were found to increase osteoblast proliferation, differentiation and mineral deposition [44].
3.1.2. dECM microparticles
Although dECM scaffolds could build a 3D platform offering biomechanical signals, tunable dECM powders provided versatility in a way that could either be applied as fillers or converted into gel or ink. When resuspending these powders into synthetic or natural gels, powdered suspensions might also better maintain ECM biophysical properties than soluble dECM that is completely dissolved as a solution [45]. However, special attention should be given to the size and concentration of the powder. As compared to micro-dECM powders ranging between 100 and 1000 μm, ultrafine dECM powders (ranging between 1 and 50 μm) enabled relatively faster biodegradability and displayed a reasonable residence time of 7 days on the ocular surface after subconjunctival injection. Despite in vivo results demonstrating an equally promising regeneration efficiency of two powder sizes during corneal wound healing, the smaller size was potentially more desirable for uniform size distribution and immune recognition for drug delivery [46]. In another study that used decellularized bone matrix particles (DCBs) with a size of 40 μm, a 3D-printed hybrid scaffold was fabricated for bone tissue engineering. Polycaprolactone provided mechanical support, while DCBs were selected due to their osteo-inductivity and osteo-conductivity. According to the result of Alizarin Red Staining, the intensity of the red stain increased as the concentration of DCBs was increased, whereas the print quality dramatically dropped as the concentration increased to 85 %. Hence, the balance between printability and bioactivity should also be considered [47].
3.2. Soluble-state dECM
It is difficult for solid scaffolds to recapitulate natural ECM-cell interactions within native ECM, which is in a fluid state. To overcome this deficiency, dECM powder was further solubilized by enzymatic digestion or more recently by ultrasonic cavitation to form a soluble-state dECM. The ideal solution might allow cells to proliferate and spread in a fluid environment similar to that of the original tissue [48]. This notable property makes soluble-state dECM a promising candidate for cell transplantation including MSCs and hepatocyte [49,50]. Co-transplantation of cells with dECM hydrogel not only improved the survival and retention rate of engrafted cells, but also displayed therapeutic effects [51]. When compared with dECM powder resuspended in gels, solubilized dECM with high concentration still maintained stable viscosity, making it easy to spray. Soluble dECM is also versatile from the processing perspective. Injectable and 3D printable dECM hydrogel can readily be implanted within irregularly-shaped defects in a minimally invasive manner (Fig. 4). Several factors for meeting application requirements are thus highlighted as follows.
Fig. 4.
The notable physicochemical properties of two soluble-state dECMs. (dECM, decellularized extracellular matrix).
3.2.1. Injectable dECM hydrogel
Further solubilization could result in the complete disruption of the inner structure and mechanical properties of dECM. The self-assembly of collagen might only provide physical crosslinking with weak mechanical strength and rapid degradation. Hence, dECM hydrogels are often functionalized with additional chemical and biological crosslinking. For example, dECM hydrogel derived from bone was linked with methacrylate groups to form a photo-cross-linkable hydrogel, which elastic modulus was increased with an increase in light exposure time. When crosslinked for 15 s, the elastic modulus of this methacrylated bone-derived dECM hydrogel started at 0.9 kPa and increased gradually to 1.3 kPa and 1.5 kPa at 30 s and 45 s, respectively. The rheological assessment evaluated by Real-time in situ photorheometry revealed a lower gradual slope in methacrylated dECM hydrogel as compared to commercial GelMA. It was demonstrated that methacrylated dECM hydrogel was inherently a soft material and suitable for tissue regeneration when lower stiffness was needed [52]. By optimizing the methacrylate concentration and photo-initiator concentration, the mechanical properties of methacrylated dECM could also be tailored [53]. However, the potential toxicity of chemical cross-linking agents might accumulate with increasing concentration and impair the biocompatibility of dECM-based biomaterials. Since dECM is enriched with collagen, genipin, extracted from a traditional Chinese medicine - Eucommia ulmoides, can biologically crosslink collagen-based dECM hydrogels by binding with the free amine groups of lysine or hydroxylysine [54]. Due to its low cytotoxicity and mechanical-enhancing properties, genipin has attracted attention in bioengineering research. Genipin cross-linked dECM hydrogels display different elastic modulus and resistance according to enzymatic hydrolysis, positively associated with genipin concentration. One nucleus pulposus-derived dECM hydrogel crosslinked by genipin with a concentration range of 0.02%–0.04 % demonstrated improved stability and mechanical properties, which was comparable to human nucleus pulposus (about 11 kPa). Scanning electron microscopy further confirmed the decreased mesh area with increasing genipin concentration [55]. Nevertheless, crosslinked dECM hydrogels are still too soft and brittle to be applied as tissue adhesives. For this reason, genipin-terminated 4 arm-poly (ethylene glycol) (GeniPEG) was developed as a novel genipin-based gelator, by which the resulting hydrogel could be crosslinked within a few seconds in response to pH changes. When compared to free genipin crosslinking, dECM-GeniPEG hydrogel exhibited greater tissue adhesive strength while still retaining satisfactory biocompatibility and biodegradability. It has promising applications as an organ-specific tissue adhesive for preventing post-operative complications [56].
3.2.2. Bioprintable dECM hydrogels
When compared to purely injectable dECM hydrogels, spatially-stable hydrogels could be bioprinted through 3D bioprinting technology and allow for more precise control over the deposited dECM biomaterials and cell positioning [57]. By embedding cells within the hydrogels, the resulting dECM-based bioinks should not only be cytocompatibility but also processable for manufacturing cell-laden 3D constructs. During this process, the biochemical and viscoelastic properties of dECM bioinks are given special attention. In this section, we will mainly introduce one of the most commonly utilized 3D bioprinting methods for dECM-based tissue regeneration. Extrusion-based 3D bioprinting enables specific bioinks with shear thinning properties to be stacked layer by layer at a relatively slow printing speed (700 mm/s−1∼10 μm/s−1) to ensure good vertical structure fidelity [58]. Notably, the viscosity of desirable bioinks should decrease under shear strains so that the extruded shear force acting on cells would be decreased and possible clogging could be prevented, thereby leading to a high cell viability after bioprinting. However, the very low viscosity of dECM-derived bioinks generally resulted in weak moldability. Incorporation with other shear-thinning biomaterials has been proposed to address this issue. In one study, to be extruded through the needle with an inner diameter of 260 μm, 10 % (w/v) GelMA mixed with 5 mg/mL dECM derived from dental follicle tissue was prepared as the final concentration to achieve suitable shear thinning properties, degradation and shape fidelity [59]. Additionally, a double network crosslinked-bioink was developed using a mixed solution of methacrylated hyaluronic acid (MeHA) and dECM produced form porcine hyaline cartilages, which could be crosslinked under visible light and 37 °C, respectively. It was confirmed that the addition of 10 mg/mL dECM improved the mechanical property and cell viability [60]. The above studies demonstrate the feasibility and importance of the combination of dECM solution with relatively high-viscosity biomaterials. The resulting bioprintable dECM-derived bioinks yielded high efficiency of cell delivery and improved regenerative potential.
4. The sequence of events and underlying molecular mechanisms associated with the dECM-mediated regeneration process
Previous studies have indicated single or multiple ECM molecules as key factors within the tissue microenvironment that provides biochemical and physical cues regulating the differentiation process, lineage fate and other cell-tissue interactions. Indeed, ECM is composed of a diverse array of matrix proteins including the core matrisome (glycoproteins, collagen and proteoglycans) and matrisome-associated proteins (regulators, secreted factors and ECM-affiliated proteins). Assembled ECM can trigger and orchestrate multiple intracellular signaling cascades, in a way that would stimulate corresponding cellular responses and regulate cellular behavior. Comprehensive investigations on what cells would do on dECM will shed light on developing decellularized materials that match the defect microenvironment. In this section, we examine the salient role of dECM biomaterials in regulating complex cellular behaviors and phenotypes implicated in the inflammatory response, cell adhesion, proliferation and differentiation (Fig. 5). Prior to the explanation of specific mechanisms, general regulation mechanisms of cell functions by dECM should also be noted. It has been well reviewed that standard ECM receptors such as integrins and DDR tyrosine kinase receptors are signal transduction receptors in close contact with cell adhesion and migration [61]. For example, DDR1-SHP2-STATs signaling has been discovered to mediate collagen-induced cell migration and epithelial-branching morphogenesis [62,63]. ECM-integrin also transduce signals into cells to regulate cell adhesion and proliferation by binding with laminins, GFOGER-like sequences in collagen and the tripeptide motif Arg-Gly-Asp in ECM proteins [64]. Besides that, ECM proteins can bind soluble growth factors and regulate their activation and presentation to cells, thereby multivalent growth factor signals such as Wnt-GSK3 signaling are integrated to indirectly mediate cell activities [65]. As mentioned, VEGF binds to fibronectin III domains in promoting cell proliferation [66], while collagen II, the major collagen of cartilage, binds TGF-β1 and BMP-2 to enhance chondrogenesis. Cells perceive their extracellular microenvironment not only through biochemical cues but also through some physical and mechanical signals. YAP/TAZ are identified as sensors of mechanical cues instructed by ECM stiffness and cell geometry to induce the differentiation of mesenchymal stem cells and survival of endothelial cells [67]. Another important mechanosensitive cation channels such as Piezo1 can sense the blood vessels stiffness, and convert it into chemical signals [68]. Additionally, Piezo1 and Ca2+ influx have been reported to sense stiff matrix and enhance inflammatory activation in macrophages by mediating F-actin formation [69].
Fig. 5.
Illustration of dECM to promote pro-regeneration immune response, cell adhesion, proliferation and differentiation via triggering various biochemical signals. (MMPs, metalloproteinases).
4.1. Regulatory effects of dECM on immune-related cells that suppress inflammation
Upon implantation, immune responses regulated by a variety of immune cells profoundly impact the outcome of dECM regeneration, contributing to either inflammatory or regenerative tissue remodeling events. The cellular behaviors of macrophages, T cells and mast cells are the most widely investigated targets. Among these, macrophages have received considerable attention in dECM-based regeneration events, as these cells are implicated in the entire immune system with context-dependent phenotypes due to their specific functional plasticity [70]. Indeed, the alteration of macrophage phenotype, also named polarization, plays a crucial role in tissue remodeling at different stages. Generally, early macrophages accumulating at the inflammation site might be polarized towards an M1 phenotype to phagocytose antigens, degrade the dECM and activate tissue-resident fibroblasts. Pro-regenerative M2 macrophages are involved in the recruitment of stem cells and angiogenesis by secreting anti-inflammatory cytokines. Therefore, the majority of present dECM-based studies aim to achieve favorable regulation of the immune system by manipulating the transition from the M1 to the M2 phenotype. It has been widely reported that dECM-based biomaterials could be effective in increasing M2 macrophage polarization while decreasing the ratio of M1/M2 during the early stage of injury, which was partially responsible for improved neuronal outgrowth, chondrogenic differentiation and osteoinductivity [[71], [72], [73]]. By optimizing the freeze-thaw cycles, the micromechanical properties of dECM could be enhanced, which has been found to positively promote M2 macrophage polarization via the mechanotransduction pathways [74]. Nevertheless, M1 macrophage polarization is still essential for initiating vascularization through pro-inflammatory signaling as well as degrading grafts by matrix metalloproteinases (MMPs). Premature M1 to M2 phenotype transition did not always lead to better tissue regeneration [75]. Hence, exploring underlying mechanisms by which dECM can dynamically modulate this balance will be the focus of future investigations.
When it comes to T cells, dECM has been reported to activate the proliferative activity of sensitized T-cells and participate in the transition to the Th2 phenotype, which is mainly associated with the pro-regenerative process [76]. Furthermore, different dECM protein ultrastructure and degrees of cross-linking had effects on regulating T-helper cells in the immune response [77]. As for effects on mast cells, few studies have been done owing to the lack of representative in vivo conditions. A previous study employed dECM hydrogel derived from porcine dermis as a 3D platform to study mast cell biology. As compared to collagen type I, human mast cells displayed upregulated metabolic activity, cell viability, and expression of IgE receptors (associated with the maturation of mast cells) upon the culture of dECM hydrogel [78]. In some cases, fibroblasts have also been implicated in the regulation of immune responses by interacting with dECM. When dECM obtained from WI-38 human lung fibroblasts was incorporated within the collagen-based bioactive scaffolds, the mRNA expression levels of TIMP2 and proinflammatory MMPs in dermal fibroblasts were downregulated, while the expression level of MMP3, known to be a remodeling MMP, was increased [79].
4.2. dECM enhancement of cell attachment and proliferation
Providing an inductive environment for cell adhesion and proliferation is the most essential prerequisite for biomaterials. Owing to the various signaling pathways involved, this section only focuses on the three most studied cell types: cells implicated in nerve regeneration, stem cells and highly differentiated cardiomyocytes. It has been reported that dECM derived from hBMSCs might facilitate initial cell adhesion and guidance through activation of the P13K-Akt signaling pathway during nerve regeneration. Pathway enrichment analysis also revealed several upregulated hub genes that play key roles in the regulation of axonal guidance and cell attachment, such as gastrulation brain homeobox 2 (Gbx2), Tenascin R (Tnr) and ATPase Na+/K+ transporting subunit alpha 3 (Atp1a3) [80]. As for stem cells, there exists a stable balance between the quiescent and proliferative state in physiologic conditions. Once triggered by the inflammation-induced oxidative stress, premature senescence will be induced in stem cells, thereby compromising their regenerative potential. A previous study utilized the protective effects of dECM to resist the cellular senescence caused by H2O2. The findings revealed that collagen type I, rather than fibronectin in the dECM, partially led to the mitigation of premature senescence via the SIRT1-dependent signaling pathway. A superior osteogenic differentiation potential was also identified even with a senescent stem cell phenotype [81]. As for cardiac tissue regeneration, lack of self-repair capacity within the aging myocardium has long been an intractable challenge. Interestingly, cross-transplantation of fetal heart-derived dECM could otherwise drive cardiomyocytes toward a proliferative phenotype [82]. To explore the underlying mechanism, dECM hydrogel derived from fetal and adult porcine hearts were injected respectively into low-regenerative capacity hearts with myocardial infarction. It was found that fetal dECM could stimulate cell cycle activity in cardiomyocytes by increasing nuclear YAP localization [83]. In an in vivo myocardial infarct model, another possible association with decreased expression of CLCA2, a member in the family of calcium-dependent chloride channel regulators, was further discovered upon injecting dECM derived from fetal porcine hearts [84]. By investigating the anti-fibrotic effects of fetal dECM, they also clarified the function of CAPG, a cytoskeletal-related signaling protein, which might mediate decreased fibroblast activation resulting from the fetal dECM treatment [85].
4.3. dECM enhances cell differentiation
Several studies have evaluated the effects of dECM on stem cell differentiation and lineage fate while attempting to clarify the underlying mechanisms. Some well-known signaling pathways have been suggested to play a role in dECM-induced stem-cell differentiation towards chondrogenesis, such as the glycogen synthase kinase-3 beta (GSK3β), Wnt and MAPK signaling pathways [86]. Other intracellular signaling pathways, including the pERK/ERK and pFAK/FAK signaling axes, pYAP/YAP and beta-catenin signaling pathways, were involved in the tri-lineage differentiation (adipogenic, osteogenic, and chondrogenic) when using dECM derived from immortalized adipose-derived mesenchymal stem cells [87]. To further explore the exact dECM component that potentially induces stem cell differentiation, one study compared the osteogenic differentiation ability of two dECMs composed of different COL4A2 contents. Both in vitro and in vivo findings showed greater proliferation and osteogenic differentiation of periodontal ligament stem cells (PDLSCs) in dECM enriched with COL4A2, whereas decreasing expression of COL4A2 in dECM by siRNA contributed to the opposite results. At the end of this study, COL4A2 was identified as a key factor in influencing PDLSCs differentiation towards osteogenic lineage via the negative regulation of the canonical Wnt/β-catenin pathway [88]. However, the matrix microenvironment could sometimes influence but could not solely determine the tissue-specific stem cell differentiation preference. For example, human adipose-derived MSCs (ADSCs) and synovium-derived MSCs (SDSCs) were cultured separately on the same dECM scaffold but responded in opposite ways with respect to chondrogenic and osteogenic differentiation, which may be attributed to tissue specificity. ADSCs originate from adipose tissues, while SDSCs are adult stem cells pre-disposed to chondrogenesis. Even after culturing on a modified dECM that significantly improved the chondrogenic potential of SDSCs, there was no significant enhancement of chondrogenic differentiation using ADSCs. Besides that, the dynamic fluctuation of TWIST1 expression was explored in SDSCs during their expansion and differentiation phase, providing hints for further investigations of the underlying mechanisms [89].
In summary, previous studies focused on characterizing the effects of dECM on cellular behaviors have been reviewed from the aforementioned three aspects. Despite such efforts, the multitude of signaling pathways implicated in dECM-based regeneration have not been rigorously validated and widely recognized. Despite that, understanding the feedback mechanisms on the inflammatory response, cell attachment, proliferation and differentiation, can be useful for selecting a customized dECM for specific tissue engineering applications. Since dECM is not a single molecule but a mixture of protein compositions, subsequent experiments should be carried out using various approaches to comprehensively elucidate the complex network of molecular interactions between its various components and cellular activities.
5. Application of dECMs in tissue regeneration
CDM and TDM can be molded and shaped into various forms as mentioned above. The following sub-sections focus on recent applications of CDM and TDM in tissue regeneration with both solid states (e.g., scaffold and microparticles) and soluble states (e.g., hydrogel and 3D bioprinting).
5.1. CDM for cartilage, skeletal muscle and bone tissue regeneration
Mesenchymal stem cells (MSCs) appear to be the preferred cell source for producing CDM. To date, there are increasing publications on MSC preconditioning and combination with different polymeric materials for the synthesis of a tailor-made ECM. The differentiation-inducing properties of dECM-based biomaterials have been widely studied. For example, a human mesenchymal cell line was lentivirally transduced to constitutively express BMP-2 and then employed to produce the customized CDM scaffold enriched with BMP-2. Based on their results, the resulting construct could enhance chondrogenesis, forming a morphogen-enriched ECM to activate chondrogenic regenerative processes (Fig. 6A). Its osteoinductive property was also confirmed using rat mandibular defects in vivo, which was better than synthetic delivery of BMP-2 or even live tissue grafts (Fig. 6B–C) [90]. In another study, CDM was acquired from adipose-derived mesenchymal stem cells and a porous nanocomposite was engineered by combining with Se@SiO2. A continuous and gradual release of selenium effectively diminished ROS production and boosted mitochondrial function. Benefiting from the concurrent application of SeNPs and dECM-coating, myogenic differentiation was increased during skeletal muscle tissue engineering [91]. Similar dECM-based composites can also be fabricated by adding bioactive glass and poly(lactide-co-glycolide). Enhanced cell metabolic activity and osteogenic differentiation were achieved as compared to the CDM alone (Fig. 6D–F) [92]. The weight of evidence from these reports concludes that CDM derived from stem cells can be conveniently modified or fabricated as a versatile biomaterial depending on different regenerative purposes. Further studies still need to be carried out in an attempt to refine the selection of cell sources and manufacturing processes for achieving specific therapeutic effects.
Fig. 6.
Application of cell-derived dECM in tissue engineering. (A) hTERT-immortalized human mesenchymal line with constitutive expression of human BMP-2 transgene (MB cells) was dynamically seeded, differentiated to the chondrogenic lineage and subsequently induced to apoptosis and lyophilized to produce dECM. (B) After 6-week implantation into the rat mandibular defect, much more new bone formation in both the inner and outer regions could be observed with the above dECM. (C) Quantitative assessment of osteointegration and new bone volume formation. Reproduced with permission [90]. Copyright 2021, Wiley. (D) Bioactive glass–Poly(lactide-co-glycolide) (BG-PLG) composite scaffolds were coated with mesenchymal stem cells (MSC)-secreted extracellular matrix (ECM). (E) ECM coating enhanced MSC metabolic activity and (F) increased cell-secreted osteocalcin. Reproduced with permission [92]. Copyright 2016, ACS.
5.2. TDM for soft and hard tissue regeneration
5.2.1. dECMs derived from cardiac tissue, skin and skeletal muscle
Limited self-regenerative capacity represents a formidable challenge during cardiac tissue repair. By contrast, specific dECM biomaterials derived from cardiac tissues possess pro-regenerative properties that can direct the differentiation of various stem cells into cardiomyocytes or stimulate cardiomyocyte cell cycle activity. Differentiation towards the adipogenic, endothelial and chondrogenic lineages was also reported using decellularized cardiac tissue. Besides that, the preservation of vasculature trees within native heart tissues provides the dECM with a tremendous potential in angiogenesis, making it favorable for fabricating vascularized constructs in cardiovascular tissue regeneration [93,94]. A myocardial dECM hydrogel laden with adipose-derived stem cells was developed to improve regenerative capacity after myocardial infarction. Stem cells loaded into 2.0 % dECM hydrogel not only exhibited an even distribution with high viability but also displayed enhanced angiogenic potential, suggesting promising regenerative capabilities. The myocardial infarction rat model further demonstrated the synergistic effects of this cell-laden dECM hydrogel in reducing fibrosis and infarct size, achieving enhanced recovery of cardiac function [95]. However, lacking structural and mechanical similarity to the native tissue limited the application of dECM hydrogel. To overcome the above problem, soluble dECM is reconstituted as a bioactive bioink that can deposit cells layer-by-layer to reproduce the intricate structure of the tissue through 3D bioprinting. One dECM-based bioink laden with primary cardiomyocytes was sequentially printed using an extrusion-based 3D bioprinter (Fig. 7A). The quantitative evaluation revealed enhanced cardiomyocyte differentiation within the bioprinted construct (Fig. 7B) [96]. 3D bioprinting makes it possible to customize dECM-based constructs with specific shapes and locations. Compared to soluble dECM, dECM microparticles not only retained similar enhancing effects on cardiomyocyte activity, vessel density and cardiac function, but a slower degradation rate also made it suitable to be employed as macromolecular loading [97]. Sometimes, the shortcomings of dECM itself can also be improved when it is used in combination with other biomaterials and therefore, is better suited for extended applications. For example, it was reported that a biohybrid scaffold could be fabricated by integrating dECM-derived from pericardium with poly(propylene fumarate) (PPF) and translated as a small-diameter vascular graft. Due to the additional introduction of PPF, this dECM-based scaffold showed a reduced degradation and swelling rate, which was comparable to that of tissue regeneration, indicating a stable platform for re-endothelialization and tissue growth [98].
Fig. 7.
Tissue-derived dECM for cardiac and skeletal muscle tissue engineering. (A) Primary cardiomyocytes isolated from neonatal rats were encapsulated in bioinks composed of hdECM. The bioinks were sequentially printed using an extrusion-based 3D bioprinter and cultured either statically or dynamically. Enhanced maturation of cardiomyocytes in hdECM was observed. (B) Enhanced maturation of cardiomyocytes in dECM when cultured dynamically. Reproduced with permission [96]. Copyright 2019, Elsevier. (C) Schematic illustration of 3D skeletal muscle construct generated using a combination of direct cellular reprogramming, decellularization of muscle tissue, and fibril alignment technique. After 4-week implantation into a volumetric muscle loss injury mouse mode, (D) HE, Masson's trichrome staining and (E) quantitative measurement suggested that this aligned muscle constructs significantly reduced fibrosis while promoting de novo muscle regeneration in damaged muscle tissue. Reproduced with permission [106]. Copyright 2021, Wiley.
Different from cardiac tissues, skin tissues have relatively high self-healing capacity under normal conditions without external stimuli. However, healing on their own becomes less effective in the case of extensive burns or diabetic wounds caused by chronic diseases. Although several skin substitutes have successfully been translated into clinical applications, these are more likely to concentrate on the standard of care [99]. Therefore, dECMs derived from skin (sdECMs) have been fabricated in various forms, such as an injectable hydrogel or a scaffold, for advanced applications in wound healing. It is generally accepted that the addition of growth factors into biomaterials can be a realistic strategy to improve and expedite diabetic wound healing. For example, additional incorporation with sacchachitin nanofibers (SCNFs) and platelet-rich plasma (PRP) showed better wound healing efficiency in diabetic rats as compared to dECM alone. SCNFs added into the hydrogel formulation exerted a chemotactic effect on inflammatory cells, while the inclusion of PRP, containing copious amounts of growth factors, further accelerated diabetic wound healing by facilitating angiogenesis and epithelialization [100]. Another antimicrobial study loaded usnic acid into the sdECM, which demonstrated an enhanced anti-biofilm property when treating infected wounds [101]. Additionally, 3D bioprinting technology is helpful for constructing multi-layered biomimetic structures to enhance skin regeneration. In one study, acellular dermal matrix (ADM) was used as a bioink to produce a full-thickness skin construct where three types of cells (human epidermal cells, fibroblasts and umbilical vein vascular endothelial cells) were, respectively loaded into 20 % GelMA, 1.5 % ADM and 10 % GelMA to simulate the stratified structure of the natural skin. This artificial 3D construct was shown to maintain cell viability and support epidermis reconstruction by in vitro testing. When implanted in vivo, the scaffold not only stimulated dermal ECM secretion but also promoted angiogenesis, accelerating re-epithelization and therefore enhancing wound healing quality [102]. Utilizing a similar strategy, gingival fibroblasts were encapsulated within ADM to form a promising graft substitute for keratinized gingiva augmentation and clinical periodontal tissue regeneration [103]. In summary, the aforementioned studies highlight sdECM as an ideal biomaterial for providing structural support and a conducive microenvironment enriched with growth factors, which supports the repopulation of a variety of cell types.
Similar to cardiac muscle, anisotropic skeletal muscle is mainly made up of highly oriented and densely-packed myofibers, which structure and arrangement may guide myogenic cell differentiation and influence contractile muscle function. After decellularization, the naturally aligned pattern of ECM is primarily preserved in dECM derived from skeletal muscle (mdECM), which has a significant impact on guiding myogenic cell differentiation. C2C12 myoblast cells encapsulated in mdECM scaffolds displayed increased cell proliferation and myogenic differentiation in vitro. After being implanted into rabbit tibialis anterior muscle defects, such mdECM-based structure achieved a greater number of newly-formed myofibers when compared with the collagen group after 1 and 2 months [104]. Primary structural alignment inside the mdECM can be further optimized by topographical contribution using electrospinning. A topographically aligned micro-/nanostructure can be fabricated with a high degree of control over alignment/anisotropy and diameter. Appropriate degrees of cross-linking and fiber alignment were confirmed to increase cell adhesion, early growth and myofiber formation [105]. The elastomeric chip was noted as another method for creating anisotropically-aligned mdECM nanofibrils, which showed high therapeutic efficacy on both volumetric muscle loss and muscular dystrophy (Fig. 7C). In vivo implantation of this aligned cell-laden construct resulted in promoted myofiber formation, restored dystrophin expression and de novo formation of muscle tissues (Fig. 7D–E) [106]. Together, these studies underscore the significant impacts of mdECM alignment on cell behavior and function during skeletal muscle tissue regeneration.
5.2.2. dECM derived from bone and elasmoid scales
Demineralized bone matrix (DBM) has long been utilized as a bone graft substitute to overcome the limited availability of autografts as well as to avoid potential disease transmission and immunogenic responses associated with allografts. Nevertheless, three commercially available DBM materials have been reported to induce stronger inflammatory responses than synthetic hydroxyapatite compounds within a murine air pouch biocompatibility model [107]. It remained unclear whether DBMs themselves or the carrier provoked the inflammatory reactions. For this reason, an additional decellularization step was employed on DBMs. As compared to DBM, the proliferation of mouse primary calvarial cells was enhanced when cultured with dECM derived from bovine bone (bdECM). However, trypsin treatment during decellularization reduced the mechanical properties of bdECM, of which the storage modulus (∼150 Pa at 6 mg/mL) was significantly lower than that of bone (8–11 GPa) [108]. Adequate strength and proper architecture are key factors to be considered especially in force-bearing tissue regeneration. Hence, bdECM was milled into a uniform micron-sized powder and its addition into the hydrogel can elevate elastic modulus to about 1000 Pa, which enabled BMSCs differentiation towards the chondrogenic lineage, followed by endochondral ossification over time (Fig. 8A–C) [109]. Besides that, bdECM was able to be modified by methacryloyl (MA-bdECM). Upon the addition of 0.5 % MA-bdECM into alginate, a chemically and physically dual-crosslinkable bioink was developed to fabricate a cell-laden mesh structure, of which storage modulus reached about 3000 Pa. The improved mechanical properties were responsible for its good performance in enhancing osteogenic differentiation [110]. To further design a biomimetic scaffold, researchers used raw materials (PCL/PLGA/HA = 1:1:4) coated with bdECM to produce a bone tissue-engineering zone that exhibited a Young's modulus close to 2.5 GPa to support osteogenic differentiation [111]. Considering the similar biological components between bone and dentin, bdECM was also utilized as a biochemical cue to simulate dental‐specific microenvironments for dental tissue engineering. Human dental pulp stem cells embedded in the bdECM-based construct maintained their viability, growth and were induced to osteo/odontogenic differentiation [112]. Another interesting application of bdECM was seen in periodontal ligament (PDL) reconstruction. Mouse mandible bone containing periodontal ligament was decellularized to serve as a tooth-supporting tissue. In vivo study using the subrenal capsule in rats demonstrated its potential in reconstructing PDL tissue by controlling host cell migration [113].
Fig. 8.
Tissue-derived dECM for bone tissue engineering. (A) Schematic illustration of the bilayered construct design (upper: cartilage layer; bottom: bone layer), and the pathway of chondrogenesis and endochondral ossification. (B) Representative histological images of H&E, Safranin-O/fast green, Masson, type Ⅱ, I collagen and OCN staining of the osteochondral defect area at 12 weeks after surgery. IF: interface; NA: native area; RC: repaired cartilage; RB: repaired bone. (C) Expression of cartilage-related genes and bone-related genes of the regenerated osteochondral tissue at 12 weeks post-surgery. Reproduced with permission [109]. Copyright 2022, Elsevier. (D) Decellularized fish scale and chitosan were combined to produce a highly porous bio-composite scaffold with enhanced osteogenic activity. (E) SEM images of the biomineralized matrix on the scaffolds. Reproduced with permission [114]. Copyright 2019, Elsevier.
Elasmoid scales are part of the dermal skeleton that covers the body of teleost fish. The highly ordered collagen arrangement and similar biochemical compositions to bone tissue make it possible to use elasmoid scales in bone tissue regeneration. The addition of dECM derived from fish scale (fdECM) improved the mechanical properties and osteoinductivity of chitosan-based scaffolds (Fig. 8D–E) [114]. Nevertheless, more substantial research studies and further efforts are required before such methods become more widely used and accepted in orthopedic therapy.
6. Conclusion and outlook
Owing to their good biocompatibility and low immunogenicity, dECM-based biomaterials continue to be the most promising substrata in the field of regenerative medicine. With further more intensive research, there will hopefully be greater clarity on the physiobiological properties of dECM biomaterials with respect to their sources and application forms. Some key cellular processes were also found to be positively regulated during dECM-based tissue engineering that can enhance tissue healing. Based on the aforementioned developments, this review thus sheds much light on the key considerations in designing customized dECM tailored for specific tissue engineering applications.
The sources of dECM should be selected as required. For example, dECM can be produced from matrix secreted by cells for better tunability and consistency, or from tissues for complex biochemical and mechanical cues. Although dECM derived from cells is mainly deposited by stem cells, stem cells from different sources differ from each other, which might dictate their differentiation bias when applied to regulate cell fate. Further studies should be carried out to explore subtle differences in their proteomic profiles. As for dECM derived from tissues, researchers should pay much attention to tissue sources and complete decellularization to reduce possible disease transmission risks and anti-host immune responses. There may be a kind of dECM suitable for a certain type of tissue regeneration. For example, the acellular dermal matrix can be used for either the wound healing or the periodontal tissue regeneration. Nevertheless, tissue-specific dECM displays its prominent superiority especially for the tissue regeneration with specific composition and function. It has been recently reported that uterus-derived decellularized ECM demonstrated a significantly improved endometrial regenerative effect as compared to using the non-reproductive organ derived dECMs (kidney- and stomach-dECM) [115]. To date, minimum requirements have been proposed to control the remaining nuclear material, dsDNA content and DNA fragment size for achieving adequate decellularization. However, these would not be suitable for all tissues and some important cellular components, such as the levels of residual mitochondria, MHC-1 proteins, phospholipids, etc., also need to be taken into account. It is recommended that standardized quality control of dECM according to the ECM source should be established to reduce the risk of any immune response and disease transmission after implantation.
Currently, there is no consensus on which application form of dECM is better. Depending on the physical and biochemical properties required, different dECM forms are developed. The prevailing view is that dECM transferred into particles has good portability, while dECM hydrogel stands out for its injectability and minimally invasive delivery mode. Thanks to the development of tissue engineering, the strategy of combining 3D bioprinting with dECM has significantly enhanced the physicochemical properties of dECM-based biomaterials, showing great potential in tissue regeneration. Other surface modification techniques can also be combined with dECM to provide biological cues. Hence, integrating various fabrication technologies would confer versatility to application forms of dECM-based composite biomaterials, thus expanding their range of clinical applications (Table 1).
Table 1.
The brief summary of dECM properties and their target application.
| origin | Application form | fabrication process | THE target Application |
|---|---|---|---|
| Cell-derived | Cell-derived dECM scaffold | Relatively short and gentle protocols and reagents, such as:
|
Tissues that are not exposed to mechanical stress |
| Hybrid dECM scaffold (Cell-derived dECM functions as a bioactive surface coating on the scaffold template) |
|
||
| Tissue-derived | Tissue-derived dECM scaffold | The longer and more rigorous protocol, such as:
|
|
| dECM microparticle | Additional milling followed by snap freezing and lyophilization |
|
|
| Injectable dECM hydrogel | Further solubilization by enzymatic digestion, such as hydrochloric acid-pepsin digestion, or more recently by ultrasonic cavitation |
|
|
| Bioprintable dECM hydrogel | Further solubilization by enzymatic digestion. The viscoelastic properties are given special attention for its printability |
|
Although the effects of dECM on cellular behavior and functions have been extensively investigated, a deeper understanding of the underlying mechanisms has not yet been achieved. For example, functional dECM biomaterials are known to be involved in inhibiting inflammation via the modulation of mast cells, macrophages and T cells. Some transitional signaling pathways are implicated in dECM-induced cell adhesion, proliferation and differentiation, including ERK phosphorylation and pYAP/YAP signaling. Nevertheless, identifying the exact dECM component that promotes tissue regeneration and its underlying cellular and molecular mechanisms is still challenging but meaningful for further advancing the field of dECM-based regenerative medicine. In conclusion, selecting an appropriate dECM in a context-dependent manner and elucidating the detailed mechanisms of dECM-cell interaction can facilitate optimizing the regenerative outcome.
CRediT authorship contribution statement
Shihan Zhang: Writing – review & editing, Writing – original draft, Conceptualization. Yaru Guo: Writing – review & editing, Conceptualization. Yixuan Lu: Resources, Investigation. Fangyong Liu: Writing – review & editing. Boon Chin Heng: Visualization, Supervision. Xuliang Deng: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
We have nothing to declare.
Acknowledgements
Shihan Zhang and Yaru Guo contributed equally to this work. This work was supported by the National Natural Science Foundation of China (81991505, 82201123) and China Postdoctoral Science Foundation (2021M700279 and 2023T160029).
Biographies

Shihan Zhang received the degree of Master of Stomatological Medicine at Peking University, Beijing, China, in 2021. She is currently a Ph.D. candidate in prosthodontics at Peking University School and Hospital of Stomatology, China. Her research interests are focused on stem cells and bone tissue engineering.

Yaru Guo is an endodontics specialist and clinical consultant at the Department of Geriatric Dentistry of Peking University School and Hospital of Stomatology. She was conferred the degree of Ph.D. from Peking University. Her research interests are focused on oral biomaterials and regenerative medicine.

Xuliang Deng is a Professor of Prothodontics, and also a researcher of dental materials and tissue engineering at the Peking University School and Hospital of Stomatology. Since 2014, he has served as the Associate Dean of Research at the Peking University School and Hospital of Stomatology. At present, he is the chair of the “NMPAKey Laboratory for Dental Materials”, the deputy director of the “National Engineering Laboratory for Digital and Material Technology of Stomatology”, and the deputy director of the “Beijing Laboratory of Biomedical Materials”.
Data availability
Data will be made available on request.
References
- 1.Cramer M.C., Badylak S.F. Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann. Biomed. Eng. 2020;48(7):2132–2153. doi: 10.1007/s10439-019-02408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin T.J., Cheung W.S., Zavras A.I., Damoulis P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006;77(12):2070–2079. doi: 10.1902/jop.2006.050296. [DOI] [PubMed] [Google Scholar]
- 3.Bertl K., Melchard M., Pandis N., Müller-Kern M., Stavropoulos A. Soft tissue substitutes in non-root coverage procedures: a systematic review and meta-analysis. Clin Oral Investig. 2017;21(2):505–518. doi: 10.1007/s00784-016-2044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown M., Li J., Moraes C., Tabrizian M., Li-Jessen N.Y.K. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121786. [DOI] [PubMed] [Google Scholar]
- 5.Golebiowska A.A., Intravaia J.T., Sathe V.M., Kumbar S.G., Nukavarapu S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: advances, challenges and clinical prospects. Bioact. Mater. 2023;32:98–123. doi: 10.1016/j.bioactmat.2023.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asthana A., Tamburrini R., Chaimov D., Gazia C., Walker S.J., Van Dyke M., Tomei A., Lablanche S., Robertson J., Opara E.C., Soker S., Orlando G. Comprehensive characterization of the human pancreatic proteome for bioengineering applications. Biomaterials. 2021;270 doi: 10.1016/j.biomaterials.2020.120613. [DOI] [PubMed] [Google Scholar]
- 7.Yu C., Kornmuller A., Brown C., Hoare T., Flynn L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials. 2017;120:66–80. doi: 10.1016/j.biomaterials.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland A.J., Converse G.L., Hopkins R.A., Detamore M.S. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv Healthc Mater. 2015;4(1):29–39. doi: 10.1002/adhm.201400165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masaeli E., Karamali F., Loghmani S., Eslaminejad M.B., Nasr-Esfahani M.H. Bio-engineered electrospun nanofibrous membranes using cartilage extracellular matrix particles. J. Mater. Chem. B. 2017;5(4):765–776. doi: 10.1039/c6tb02015a. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury D., Yee M., Sheng Z.L.J., Amirul A., Naing M.W. Decellularization systems and devices: state-of-the-art. Acta Biomater. 2020;115:51–59. doi: 10.1016/j.actbio.2020.07.060. [DOI] [PubMed] [Google Scholar]
- 11.Chan W.W., Yu F., Le Q.B., Chen S., Yee M., Choudhury D. Towards Biomanufacturing of cell-derived matrices. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick L.E., McDevitt T.C. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater. Sci. 2014;3:12–24. doi: 10.1039/C4BM00246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205) doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 14.Madrigal M., Rao K.S., Riordan N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014;12 doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular Vesicles: toward cell-free therapeutic applications. Mol. Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei M., Pei Y.A., Zhou S., Mikaeiliagah E., Erickson C., Giertych B., Akhter H., Wang L., Stewart A., Parenti J., Wang B., Wen S., Sim S., Quenneville E., Hansen K.C., Frisch S., Hu G. Matrix from urine stem cells boosts tissue-specific stem cell mediated functional cartilage reconstruction. Bioact. Mater. 2022;23:353–367. doi: 10.1016/j.bioactmat.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y., Tuan R.S. Bioactivity of human adult stem cells and functional relevance of stem cell-derived extracellular matrix in chondrogenesis. Stem Cell Res. Ther. 2023;14(1) doi: 10.1186/s13287-023-03392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skóra J., Pupka A., Dorobisz A., Barć P., Korta K., Dawiskiba T. Evaluation of the humoral and cellular immune responses after implantation of a PTFE vascular prosthesis. Postepy Hig. Med. Dosw. 2012;66:469–474. doi: 10.5604/17322693.1002205. [DOI] [PubMed] [Google Scholar]
- 19.Mendibil U., Ruiz-Hernandez R., Retegi-Carrion S., Garcia-Urquia N., Olalde-Graells B., Abarrategi A. Tissue-specific decellularization methods: Rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabirian F., Mozafari M. Decellularized ECM-derived bioinks: prospects for the future. Methods. 2020;171:108–118. doi: 10.1016/j.ymeth.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McInnes A.D., Moser M.A.J., Chen X. Preparation and Use of decellularized extracellular matrix for tissue engineering. J. Funct. Biomater. 2022;13(4) doi: 10.3390/jfb13040240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert T.W. Strategies for tissue and organ decellularization. J. Cell. Biochem. 2012;113(7):2217–2222. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 24.Rana D., Zreiqat H., Benkirane-Jessel N., Ramakrishna S., Ramalingam M. J. Tissue Eng. Regen. Med. 2017;11:942–965. doi: 10.1002/term.2061. [DOI] [PubMed] [Google Scholar]
- 25.Ullah I., Busch J.F., Rabien A., Ergün B., Stamm C., Knosalla C., Hippenstiel S., Reinke P., Kurtz A. Adult tissue extracellular matrix determines tissue Specification of human iPSC-derived Embryonic stage Mesodermal Precursor cells. Adv. Sci. 2020;7(5) doi: 10.1002/advs.201901198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safari F., Fani N., Eglin D., Alini M., Stoddart M.J., Baghaban Eslaminejad M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. 2019;107(8):1793–1802. doi: 10.1002/jbm.a.36698. [DOI] [PubMed] [Google Scholar]
- 27.Beres A., Christison-Lagay E.R., Romao R.L., Langer J.C. Evaluation of Surgisis for patch repair of abdominal wall defects in children. J. Pediatr. Surg. 2012;47:917–919. doi: 10.1016/j.jpedsurg.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 28.Cosentino M., Kanashiro A., Vives A., Sanchez J., Peraza M.F., Moreno D., Perona J., De Marco V., Ruiz-Castañe E., Sarquella J. Surgical treatment of Peyronie's disease with small intestinal submucosa graft patch. Int. J. Impot. Res. 2016;28:106–109. doi: 10.1038/ijir.2016.10. [DOI] [PubMed] [Google Scholar]
- 29.Darrien J.H., Kasem H. Successful closure of gastrocutaneous fistulas using the Surgisis(®) anal fistula plug. Ann. R. Coll. Surg. Engl. 2014;96:271–274. doi: 10.1308/003588414X13814021677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar L., Altamimi A., García Sánchez M., Tamburrinia R., Asthana A., Gazia C., Orlando G. Utility of extracellular matrix powders in tissue engineering. Organogenesis. 2018;14(4):172–186. doi: 10.1080/15476278.2018.1503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reing J.E., Brown B.N., Daly K.A., Freund J.M., Gilbert T.W., Hsiong S.X., Huber A., Kullas K.E., Tottey S., Wolf M.T., Badylak S.F. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31(33):8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakus A.E., Laronda M.M., Rashedi A.S., Robinson C.M., Lee C., Jordan S.W., Orwig K.E., Woodruff T.K., Shah R.N. "Tissue papers" from organ-specific decellularized extracellular matrices. Adv. Funct. Mater. 2017;27(3) doi: 10.1002/adfm.201700992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H., Hoshiba T., Kawazoe N., Koda I., Song M., Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32(36):9658–9666. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 34.Tang C., Jin C., Li X., Li J., Du X., Yan C., Lu S., Wei B., Xu Y., Wang L. Evaluation of an autologous bone mesenchymal stem cell-derived extracellular matrix scaffold in a rabbit and Minipig model of cartilage repair. Med Sci Monit. 2019;25:7342–7350. doi: 10.12659/MSM.916481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augustyniak E., Trzeciak T., Richter M., Kaczmarczyk J., Suchorska W. The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int. Orthop. 2015;39(5):995–1003. doi: 10.1007/s00264-014-2619-0. [DOI] [PubMed] [Google Scholar]
- 36.Sekiya I., Koopman P., Tsuji K., Mertin S., Harley V., Yamada Y., Shinomiya K., Nifuji A., Noda M. Dexamethasone enhances SOX9 expression in chondrocytes. J. Endocrinol. 2001;169(3):573–579. doi: 10.1677/joe.0.1690573. [DOI] [PubMed] [Google Scholar]
- 37.Park M.S., Kim Y.H., Jung Y., Kim S.H., Park J.C., Yoon D.S., Kim S.H., Lee J.W. In situ recruitment of human bone marrow-derived mesenchymal stem cells using Chemokines for articular cartilage regeneration. Cell Transplant. 2015;24(6):1067–1083. doi: 10.3727/096368914X681018. [DOI] [PubMed] [Google Scholar]
- 38.Leipzig N.D., Shoichet M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y., Malladi P., Chiou M., Bekerman E., Giaccia A.J., Longaker M.T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13(12):2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 40.Yao S., Liang Z., Lee Y.W., Yung P.S.H., Lui P.P.Y. Bioactive decellularized Tendon-derived stem cell sheet for promoting graft healing after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2023;51(1):66–80. doi: 10.1177/03635465221135770. [DOI] [PubMed] [Google Scholar]
- 41.Kim C.W., Ha H.J., Yang J.Y., Hwang E. New bone formation in the whole decellularized cortical bone scaffold using the model of Revitalizing a Haversian system. J. Craniofac. Surg. 2022;33(3):962–968. doi: 10.1097/SCS.0000000000008072. [DOI] [PubMed] [Google Scholar]
- 42.Chen C., Liu F., Tang Y., Qu J., Cao Y., Zheng C., Chen Y., Li M., Zhao C., Sun L., Hu J., Lu H. Book-shaped acellular fibrocartilage scaffold with cell-loading capability and chondrogenic inducibility for tissue-engineered fibrocartilage and bone-Tendon healing. ACS Appl. Mater. Interfaces. 2019;11(3):2891–2907. doi: 10.1021/acsami.8b20563. [DOI] [PubMed] [Google Scholar]
- 43.Mao Y., Hoffman T., Wu A., Goyal R., Kohn J. Cell type-specific extracellular matrix guided the differentiation of human mesenchymal stem cells in 3D polymeric scaffolds. J. Mater. Sci. Mater. Med. 2017;28(7) doi: 10.1007/s10856-017-5912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Junka R., Zhou X., Wang W., Yu X. Albumin-coated polycaprolactone (PCL)-Decellularized extracellular matrix (dECM) scaffold for bone regeneration. ACS Appl. Bio Mater. 2022;5(12):5634–5644. doi: 10.1021/acsabm.2c00686. [DOI] [PubMed] [Google Scholar]
- 45.Spang M.T., Christman K.L. Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater. 2018;68:1–14. doi: 10.1016/j.actbio.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Chung L., Hooks J., Maestas D.R., Jr., Lebid A., Andorko J.I., Huleihel L., Chin A.F., Wolf M., Remlinger N.T., Stepp M.A., Housseau F., Elisseeff J.H. Type 2 immunity induced by bladder extracellular matrix enhances corneal wound healing. Sci. Adv. 2021;7(16) doi: 10.1126/sciadv.abe2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung B.P., Naved B.A., Nyberg E.L., Dias M., Holmes C.A., Elisseeff J.H., Dorafshar A.H., Grayson W.L. Three-dimensional printing of bone extracellular matrix for Craniofacial regeneration. ACS Biomater. Sci. Eng. 2016;2(10):1806–1816. doi: 10.1021/acsbiomaterials.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kort-Mascort J., Flores-Torres S., Peza-Chavez O., Jang J.H., Pardo L.A., Tran S.D., Kinsella J. Decellularized ECM hydrogels: prior use considerations, applications, and opportunities in tissue engineering and biofabrication. Biomater. Sci. 2023;11(2):400–431. doi: 10.1039/d2bm01273a. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X., Li Q., Guo Z., Li Z. Constructing a cell microenvironment with biomaterial scaffolds for stem cell therapy. Stem Cell Res. Ther. 2021;12(1) doi: 10.1186/s13287-021-02650-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Udagawa D., Nagata S., Yagi H., Nishi K., Morisaku T., Adachi S., Nakano Y., Tanaka M., Hori S., Hasegawa Y., Abe Y., Kitago M., Kitagawa Y. A novel approach to Orthotopic hepatocyte transplantation engineered with liver hydrogel for fibrotic Livers, enhancing cell-cell interaction and angiogenesis. Cell Transplant. 2024;33 doi: 10.1177/09636897241253700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kojima H., Kushige H., Yagi H., Nishijima T., Moritoki N., Nagoshi N., Nakano Y., Tanaka M., Hori S., Hasegawa Y., Abe Y., Kitago M., Nakamura M., Kitagawa Y. Combinational treatment involving decellularized extracellular matrix hydrogels with mesenchymal stem cells increased the efficacy of cell therapy in Pancreatitis. Cell Transplant. 2023;32 doi: 10.1177/09636897231170437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parthiban S.P., Athirasala A., Tahayeri A., Abdelmoniem R., George A., Bertassoni L.E. BoneMA-synthesis and characterization of a methacrylated bone-derived hydrogel for bioprinting ofin-vitrovascularized tissue constructs. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/abb11f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monteiro N., He W., Franca C.M., Athirasala A., Bertassoni L.E. Engineering Microvascular networks in LED light-Cured cell-laden hydrogels. ACS Biomater. Sci. Eng. 2018;4(7):2563–2570. doi: 10.1021/acsbiomaterials.8b00502. [DOI] [PubMed] [Google Scholar]
- 54.Zhu S.Q., Zheng L.Y., Pan A.P., Yu A.Y., Wang Q.M., Xue A.Q. The efficacy and safety of Posterior Scleral Reinforcement using genipin cross-linked Sclera for Macular Detachment and Retinoschisis in highly Myopic Eyes. Br. J. Ophthalmol. 2016;100(11):1470–1475. doi: 10.1136/bjophthalmol-2015-308087. [DOI] [PubMed] [Google Scholar]
- 55.Yu L., Liu Y., Wu J., Wang S., Yu J., Wang W., Ye X. Genipin cross-linked decellularized nucleus pulposus hydrogel-like cell delivery system induces differentiation of ADSCs and Retards Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.807883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishiguchi A., Taguchi T. A pH-driven genipin gelator to engineer decellularized extracellular matrix-based tissue adhesives. Acta Biomater. 2021;131:211–221. doi: 10.1016/j.actbio.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 57.Roy A., Saxena V., Pandey L.M. 3D printing for cardiovascular tissue engineering: a review. Mater. Technol. 2018;33:433–442. [Google Scholar]
- 58.Cui X., Li J., Hartanto Y., Durham M., Tang J., Zhang H., Hooper G., Lim K., Woodfield T. Advances in extrusion 3D bioprinting: a focus on Multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9(15) doi: 10.1002/adhm.201901648. [DOI] [PubMed] [Google Scholar]
- 59.Yang X., Ma Y., Wang X., Yuan S., Huo F., Yi G., Zhang J., Yang B., Tian W. A 3D-bioprinted functional Module based on decellularized extracellular matrix bioink for periodontal regeneration. Adv. Sci. 2023;10(5) doi: 10.1002/advs.202205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C., Zheng Z., Jia J., Zhang W., Qin L., Zhang W., Lai Y. Preparation and characterization of photocurable composite extracellular matrix-methacrylated hyaluronic acid bioink. J. Mater. Chem. B. 2022;10(22):4242–4253. doi: 10.1039/d2tb00548d. [DOI] [PubMed] [Google Scholar]
- 61.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;27(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu K.K., Trcka D., Bendeck M.P. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc. Pathol. 2011;20(2):71–76. doi: 10.1016/j.carpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Wang C.Z., Su H.W., Hsu Y.C., Shen M.R., Tang M.J. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol. Biol. Cell. 2006;17(6):2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang X., He X., Qiu Z., Zhang H., Xie R., Liu Z., Gu Y., Zhao N., Xiang Q., Cui Y. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8(1) doi: 10.1038/s41392-022-01259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albrecht L.V., Tejeda-Muñoz N., De Robertis E.M. Cell Biology of canonical Wnt signaling. Annu. Rev. Cell Dev. Biol. 2021;37:369–389. doi: 10.1146/annurev-cellbio-120319-023657. [DOI] [PubMed] [Google Scholar]
- 66.Wijelath E.S., Rahman S., Namekata M., Murray J., Nishimura T., Mostafavi-Pour Z., Patel Y., Suda Y., Humphries M.J., Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 2006;99(8):853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 68.Beech D.J., Kalli A.C. Force sensing by Piezo channels in cardiovascular Health and disease. Arterioscler. Thromb. Vasc. Biol. 2019;39(11):2228–2239. doi: 10.1161/ATVBAHA.119.313348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atcha H., Jairaman A., Holt J.R., Meli V.S., Nagalla R.R., Veerasubramanian P.K., Brumm K.T., Lim H.E., Othy S., Cahalan M.D., Pathak M.M., Liu W.F. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-23482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Hong J.Y., Seo Y., Davaa G., Kim H.W., Kim S.H., Hyun J.K. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020;101:357–371. doi: 10.1016/j.actbio.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Zeng J., Huang L., Xiong H., Li Q., Wu C., Huang Y., Xie H., Shen B. Injectable decellularized cartilage matrix hydrogel encapsulating urine-derived stem cells for immunomodulatory and cartilage defect regeneration. NPJ Regen Med. 2022;7(1):75. doi: 10.1038/s41536-022-00269-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.García-García A., Pigeot S., Martin I. Engineering of immunoinstructive extracellular matrices for enhanced osteoinductivity. Bioact. Mater. 2022;24:174–184. doi: 10.1016/j.bioactmat.2022.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo P., Huang R., Wu Y., Liu X., Shan Z., Gong L., Deng S., Liu H., Fang J., Wu S., Wu X., Liu Q., Chen Z., Yeung K.W.K., Qiao W., Chen S., Chen Z. Tailoring the multiscale mechanics of tunable decellularized extracellular matrix (dECM) for wound healing through immunomodulation. Bioact. Mater. 2023;28:95–111. doi: 10.1016/j.bioactmat.2023.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long J., Qin Z., Chen G., Song B., Zhang Z. Decellularized extracellular matrix (d-ECM): the key role of the inflammatory process in pre-regeneration after implantation. Biomater. Sci. 2023;11(4):1215–1235. doi: 10.1039/d2bm01204a. [DOI] [PubMed] [Google Scholar]
- 76.Ozpinar E.W., Frey A.L., Arthur G.K., Mora-Navarro C., Biehl A., Snider D.B., Cruse G., Freytes D.O. Dermal extracellular matrix-derived hydrogels as an in vitro substrate to study mast cell maturation. Tissue Eng Part A. 2021;27(15–16):1008–1022. doi: 10.1089/ten.tea.2020.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakraborty J., Roy S., Ghosh S. Regulation of decellularized matrix mediated immune response. Biomater. Sci. 2020;8(5):1194–1215. doi: 10.1039/c9bm01780a. [DOI] [PubMed] [Google Scholar]
- Ozpinar E.W., Frey A.L., Arthur G.K., Mora-Navarro C., Biehl A., Snider D.B., Cruse G., Freytes D.O. Dermal extracellular matrix-derived hydrogels as an in vitro substrate to study mast cell maturation. Tissue Eng Part A. 2021;27(15–16):1008–1022. doi: 10.1089/ten.tea.2020.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H.S., Hwang H.J., Kim H.J., Choi Y., Lee D., Jung H.H., Do S.H. Effect of decellularized extracellular matrix Bioscaffolds derived from fibroblasts on skin wound healing and remodeling. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.865545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S., Wang H., Lu P., Gong L., Gu X., Li M. Mechanisms underlying the cell-matrixed nerve grafts repairing peripheral nerve defects. Bioact. Mater. 2023;31 doi: 10.1016/j.bioactmat.2023.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou L., Chen X., Liu T., Zhu C., Si M., Jargstorf J., Li M., Pan G., Gong Y., Luo Z.P., Yang H., Pei M., He F. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12(2):1008–1021. doi: 10.1002/term.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bejleri D., Davis M.E. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater. 2019;8(5) doi: 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Senapati S., Akinbote A., Gnanasambandam B., Park P.S., Senyo S.E. Microenvironment stiffness requires decellularized cardiac extracellular matrix to promote heart regeneration in the neonatal mouse heart. Acta Biomater. 2020;113:380–392. doi: 10.1016/j.actbio.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X., Pierre V., Senapati S., Park P.S., Senyo S.E. Microenvironment stiffness Amplifies post-ischemia heart regeneration in response to Exogenous extracellular matrix proteins in neonatal Mice. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.773978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Pierre V., Liu C., Senapati S., Park P.S., Senyo S.E. Exogenous extracellular matrix proteins decrease cardiac fibroblast activation in stiffening microenvironment through CAPG. J. Mol. Cell. Cardiol. 2021;159:105–119. doi: 10.1016/j.yjmcc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi S.H., Lee K., Han H., Mo H., Jung H., Ryu Y., Nam Y., Rim Y.A., Ju J.H. Prochondrogenic effect of decellularized extracellular matrix secreted from human induced pluripotent stem cell-derived chondrocytes. Acta Biomater. 2023;167:234–248. doi: 10.1016/j.actbio.2023.05.052. [DOI] [PubMed] [Google Scholar]
- 87.Novoseletskaya E., Grigorieva O., Nimiritsky P., Basalova N., Eremichev R., Milovskaya I., Kulebyakin K., Kulebyakina M., Rodionov S., Omelyanenko N., Efimenko A. Mesenchymal stromal cell-produced components of extracellular matrix potentiate Multipotent stem cell response to differentiation stimuli. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen Y., Yang H., Wu J., Wang A., Chen X., Hu S., Zhang Y., Bai D., Jin Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics. 2019;9(15):4265–4286. doi: 10.7150/thno.35914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pei Y.A., Mikaeiliagah E., Wang B., Zhang X., Pei M. The matrix microenvironment influences but does not dominate tissue-specific stem cell lineage differentiation. Mater Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pigeot S., Klein T., Gullotta F., Dupard S.J., Garcia Garcia A., García-García A., Prithiviraj S., Lorenzo P., Filippi M., Jaquiery C., Kouba L., Asnaghi M.A., Raina D.B., Dasen B., Isaksson H., Önnerfjord P., Tägil M., Bondanza A., Martin I., Bourgine P.E. Manufacturing of human tissues as off-the-Shelf grafts Programmed to induce regeneration. Adv Mater. 2021;33(43) doi: 10.1002/adma.202103737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y.C., Yang Y.X., Liu Y., Liu X.J., Dai J.H., Gao R.S., Hu Y.Y., Fei W.Y. Combining porous Se@SiO2 nanocomposites and dECM enhances the myogenic differentiation of adipose-derived stem cells. Int J Nanomedicine. 2023;18:7661–7676. doi: 10.2147/IJN.S436081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harvestine J.N., Vollmer N.L., Ho S.S., Zikry C.A., Lee M.A., Leach J.K. Extracellular matrix-coated composite scaffolds promote mesenchymal stem cell Persistence and Osteogenesis. Biomacromolecules. 2016;17(11):3524–3531. doi: 10.1021/acs.biomac.6b01005. [DOI] [PubMed] [Google Scholar]
- 93.Wang B., Williams L.N., de Jongh Curry A.L., Liao J. Preparation of acellular myocardial scaffolds with well-preserved cardiomyocyte lacunae, and method for applying mechanical and electrical simulation to tissue construct. Methods Mol. Biol. 2014;1181:189–202. doi: 10.1007/978-1-4939-1047-2_17. [DOI] [PubMed] [Google Scholar]
- 94.Taylor D.A., Sampaio L.C., Gobin A. Building new hearts: a review of trends in cardiac tissue engineering. Am. J. Transplant. 2014;14:2448–2459. doi: 10.1111/ajt.12939. [DOI] [PubMed] [Google Scholar]
- 95.Qiao L., Kong Y., Shi Y., Sun A., Ji R., Huang C., Li Y., Yang X. Synergistic effects of adipose-derived stem cells combined with decellularized myocardial matrix on the treatment of myocardial infarction in rats. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.116891. [DOI] [PubMed] [Google Scholar]
- 96.Das S., Kim S.W., Choi Y.J., Lee S., Lee S.H., Kong J.S., Park H.J., Cho D.W., Jang J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 97.Wang X., Ansari A., Pierre V., Young K., Kothapalli C.R., von Recum H.A., Senyo S.E. Injectable extracellular matrix microparticles promote heart regeneration in Mice with post-ischemic heart injury. Adv Healthc Mater. 2022;11(8) doi: 10.1002/adhm.202102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kimicata M., Allbritton-King J.D., Navarro J., Santoro M., Inoue T., Hibino N., Fisher J.P. Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater. 2020;110:68–81. doi: 10.1016/j.actbio.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parmaksiz M., Dogan A., Odabas S., Elçin A.E., Elçin Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016;11 doi: 10.1088/1748-6041/11/2/022003. [DOI] [PubMed] [Google Scholar]
- 100.Lin C.J., Lin H.L., You W.C., Ho H.O., Sheu M.T., Chen L.C., Cheng W.J. Composite hydrogels of Ultrasound-Assisted-Digested formic acid-decellularized extracellular matrix and sacchachitin nanofibers incorporated with platelet-rich plasma for diabetic wound treatment. J. Funct. Biomater. 2023;14(8) doi: 10.3390/jfb14080423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandika P., Khan F., Heo S.Y., Kim Y.M., Yi M., Jung W.K. Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly(ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold. Biomater. Adv. 2022;140 doi: 10.1016/j.bioadv.2022.213046. [DOI] [PubMed] [Google Scholar]
- 102.Jin R., Cui Y., Chen H., Zhang Z., Weng T., Xia S., Yu M., Zhang W., Shao J., Yang M., Han C., Wang X. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 2021;131:248–261. doi: 10.1016/j.actbio.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 103.Liu P., Li Q., Yang Q., Zhang S., Yi K., Zhang G., Tang Z. Evaluation of the effect of 3D-bioprinted gingival fibroblast-encapsulated ADM scaffolds on keratinized gingival augmentation. J. Periodontal. Res. 2023;58(3):564–574. doi: 10.1111/jre.13126. [DOI] [PubMed] [Google Scholar]
- 104.Lee H., Ju Y.M., Kim I., Elsangeedy E., Lee J.H., Yoo J.J., Atala A., Lee S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods. 2020;171:77–85. doi: 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 105.Smoak M.M., Hogan K.J., Grande-Allen K.J., Mikos A.G. Bioinspired electrospun dECM scaffolds guide cell growth and control the formation of myotubes. Sci. Adv. 2021;7(20) doi: 10.1126/sciadv.abg4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin Y., Jeon E.J., Jeong S., Min S., Choi Y.S., Kim S.H., Lee J.S., Shin J., Yu J.H., Ahn D.H., Kim Y.G., Yang H.S., Kang T.J., Cho S.R., Choi N., Cho S.W. Reconstruction of muscle Fascicle-like tissues by anisotropic 3D patterning. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 107.Markel D.C., Guthrie S.T., Wu B., Song Z., Wooley P.H. Characterization of the inflammatory response to four commercial bone graft substitutes using a murine biocompatibility model. J. Inflamm. Res. 2012;5:13–18. doi: 10.2147/JIR.S21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sawkins M.J., Bowen W., Dhadda P., Markides H., Sidney L.E., Taylor A.J., Rose F.R., Badylak S.F., Shakesheff K.M., White L.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9(8):7865–7873. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hua Y., Huo Y., Bai B., Hao J., Hu G., Ci Z., Wu X., Yu M., Wang X., Chen H., Ren W., Zhang Y., Wang X., Zhou G. Fabrication of biphasic cartilage-bone integrated scaffolds based on tissue-specific photo-crosslinkable acellular matrix hydrogels. Mater Today Bio. 2022;17 doi: 10.1016/j.mtbio.2022.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee J., Hong J., Kim W., Kim G.H. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr. Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116914. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X., Song W., Han K., Fang Z., Cho E., Huangfu X., He Y., Zhao J. Three-dimensional bioprinting of a structure-, composition-, and mechanics-Graded biomimetic scaffold coated with specific decellularized extracellular matrix to improve the Tendon-to-bone healing. ACS Appl. Mater. Interfaces. 2023;15(24):28964–28980. doi: 10.1021/acsami.3c03793. [DOI] [PubMed] [Google Scholar]
- 112.Kim D., Lee H., Lee G.H., Hoang T.H., Kim H.R., Kim G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng Transl Med. 2022;7(3) doi: 10.1002/btm2.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakamura N., Ito A., Kimura T., Kishida A. Extracellular matrix induces periodontal ligament reconstruction in vivo. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kara A., Tamburaci S., Tihminlioglu F., Havitcioglu H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019;130:266–279. doi: 10.1016/j.ijbiomac.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 115.Ahn J., Sen T., Lee D., Kim H., Lee J.Y., Koo H.S., Kim J.Y., Kim J., Jang J., Kan Y.-J., Cho D.W. Uterus-derived decellularized extracellular matrix-mediated endometrial regeneration and Fertility enhancement. Adv. Funct. Mater. 2023;33 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.