Abstract
This study investigated whether a standard calorie diet that is high in glucose and deficient in dietary fibre (described as HGD [high glucose diet]) induces hepatic fat accumulation in mice. We evaluated hepatic steatosis at 7 days and 14 days after the commencement of the HGD. Hepatic triglycerides and areas of oil droplets increased in the HGD group both at day 7 and day 14, whereas weight gain, weight of epididymal fat, and plasma levels of triglycerides were unaffected by HGD consumption. A microarray analysis of the livers revealed that the expression of lipogenesis-related genes was the most affected by HGD consumption. Furthermore, HGD consumption induced the expression of hepatic proteins of fatty acid synthetase, acetyl-CoA carboxylase alpha, and stearoyl-CoA desaturase 1, which are known to be involved in the synthesis of triglyceride. These results indicate that HGD consumption causes fat accumulation in the liver, with an increase in enzymes that are involved in de novo lipogenesis without an accompanying weight or obesity phenotype. Our new findings suggest that HGD consumption could serve as a breeding ground for liver steatosis.
Keywords: High-glucose, Dietary fibre, Hepatic triglyceride, de novo lipogenesis
Highlights
-
•
A diet high in glucose and deficient in fibre (described as HGD [high glucose diet]) induces hepatic accumulation of triglyceride in a short term.
-
•
The fat accumulation in the liver does not accompanies with neither body weight gain nor increase in epididymal fat weight.
-
•
Lipogenesis-responsible enzymes including fatty acid synthase (FASN), stearoyl-CoA desaturase 1 (SCD1) and acetyl-CoA carboxylase alpha (ACCα) are suggested to be responsible factors for the fat accumulation in the liver.
1. Introduction
Obesity and diabetes are strongly associated with hepatic steatosis. Non-alcoholic fatty liver disease (NAFLD) is the form of hepatic steatosis most commonly related to obesity and diabetes, and it is also closely correlated with the increased incidence of liver fibrosis [1], hepatocellular carcinoma [2], and deaths by cardio vascular diseases (CVDs) due to atherosclerosis [3]. The increased deaths from CVDs as a consequence of NALFD is a particularly serious problem globally. Therefore, a new criterion of hepatic steatosis was proposed in 2020 to incorporate these risk factors, under the name of metabolic dysfunction associated with fatty liver disease (MAFLD) [4]. MALFD is diagnosed in patients with hepatic steatosis when they are overweight/obese and have at least two additional metabolic disorders, including hypertension, visceral fat accumulation, dyslipidaemia, and glucose intolerance [4]. The diagnostic criteria for MALFD are distinct from those for NALFD as they focus on hepatic steatosis caused due to metabolic disorders regardless of alcohol intake. In other words, liver steatosis has been recognised as a phenotype of metabolic syndrome in the liver, which can threaten human life.
A high sugar and low fibre are characteristic of modern eating habits. Akin to a high-fat diet, such a diet can potentially induce liver steatosis. An animal study has demonstrated that allowing free access to water containing high amounts of fructose or glucose while feeding them a normal diet for 10 weeks leads to liver steatosis [5]. On the other hand, dietary fibre is recognised as a dietary component that potentially suppresses hepatic steatosis. Generally, dietary fibre is defined as a polysaccharide with ten or more monomeric units that is not hydrolysed by endogenous enzymes in the human intestine [6]. Dietary fibre varies in their physicochemical properties, such as hydrophilicity, solubility, viscosity, and fermentability, which leads to various physiological effects. Since the viscous ones reduce the absorption of glucose and lipids in the intestinal tract [7,8], dietary fibre potentially contributes to the prevention of obesity, diabetes, and cardiovascular diseases (CVDs), which bear strong etiological links to metabolic disorders [9,10]. In addition, some etiological studies have shown that a lower intake of dietary fibre correlates with metabolic disorders such as obesity and diabetes [9], which are risk factors for CVDs. Given that the intake of dietary fibre is on the decline globally and is not at optimal levels (28–35 g/day for adults is recommended) [9,10], a lower intake of dietary fibre could serve as a breeding ground for liver steatosis.
In addition to these physiological effects, the intake of dietary fibre has also been shown to have protective effects on the intestines. An animal study has reported that a diet that is deficient in both insoluble and soluble fibre—achieved by replacing all carbohydrates with glucose (abbreviated as HGD [high glucose diet] hereafter)—exacerbates the colitis induced by dextran sulphate sodium (DSS) [11]. We also found that DSS-induced colitis worsened after approximately 1 week of HGD administration [33]. During this investigation, we unexpectedly found that appearance of the liver of HGD-fed mice was a little pale, which could be a sign of fat accumulation and we hypothesized that HGD could trigger fat accumulation in the liver even without an excessive caloric intake, and this change could be induced by the consumption of HGD for approximately 1 week. Therefore, in our current study, we quantified and visualized the hepatic fat accumulation mice, which were fed HGD for 7 days to examine our hypothesis. In addition, we also evaluated whether the fat accumulation increased after another 7 days of the feeding. Signs of non-hepatic metabolic disorders such as glucose, cholesterol, and free fatty acids levels in blood were also evaluated. Furthermore, we screened the molecules responsible for fat accumulation in the liver.
2. Materials and methods
2.1. Mice and diets
Five-week-old male SPF C57BL/6J mice were purchased from SLC Japan Inc. All mice were housed in the same conventional breeding room during the experiment, which included a one-week acclimation period under standard 12:12 h light-dark cycles with ad libitum access to food and water. All experiments with animals in this study were approved by the Animal Care and Use Committee of Shimane University (Approval code: IZ4-36) and complied with the ARRIVE guidelines.
The mice were randomised and housed in groups of four per cage. They were fed a normal chow diet (ND; Oriental Yeast. CO., LTD. Japan, MF) for about until they were six weeks old. Then, the mice were divided into two groups—ND group and HGD group (HGD; Research Diets, Inc. USA, cat.no. D18102202 The formula is same with previous study [[11], [33]]). Both groups were fed each diet for 7 days or 14 days. The contents of each diet are described in Table 1. The mice were sacrificed at each end of the experiment and samples were collected described as next section. The fat accumulation and lipogenesis-related gene and protein expression in the liver were compared between ND group and HGD group. In addition, changes in body weight and epididymal fat weight, blood glucose, cholesterol, non-esterified fatty acids (NEFA), aspartate aminotransferase (AST), and alanine aminotransferase (ATL) levels were also calculated to assess the signs of metabolic disorder outside the liver. Potential confounders were not controlled, but the quantity of food intake during experiment was evaluated as shown in Fig. 1. The sample size has been set to 8 akin to other similar animal experiments with consideration for the individual variations of fat accumulation in the liver. Several data were gathered from samples of less than 8 mice since it was estimated that there were no significant differences. All mice were included in the experiments since none of them displayed signs of poor health such as excess weight loss (>20 % is set to target of euthanasia as humane endpoint of this study), abnormal piloerection, diarrhoea, or blood stool after consuming the HGD. All animals were weighed every 3–4 days, and their daily food intake was determined by hand weighing the food that remained in each cage as shown in Fig. 1.
Table 1.
Composition of HGD
| Normal chow diet | HGD | |
|---|---|---|
| Fibre | 3.3 | 0 |
| Fat | 4.9 | 4 |
| Protein | 23.2 | 20 |
| Carbohydrate | 54.7 | 71 |
| Kcal/100g | 355.7 | 400 |
| Casein | Undisclosed | 20 |
| Fructose | Undisclosed | 0 |
| Dextrose | Undisclosed | 60.4 |
| Sucrose | Undisclosed | 9.6 |
| Cellurose | Undisclosed | 0 |
| Soybean Oil | Undisclosed | 2.5 |
| Lard | Undisclosed | 2 |
| Choline Bitartrate | 0.18 | 0.2 |
| Methionine | 0.44 | 0.66 |
| Cholesterol | 0.08 | 0 |
The value of each ingredient is expressed as gram which is contained 100 g of diet.
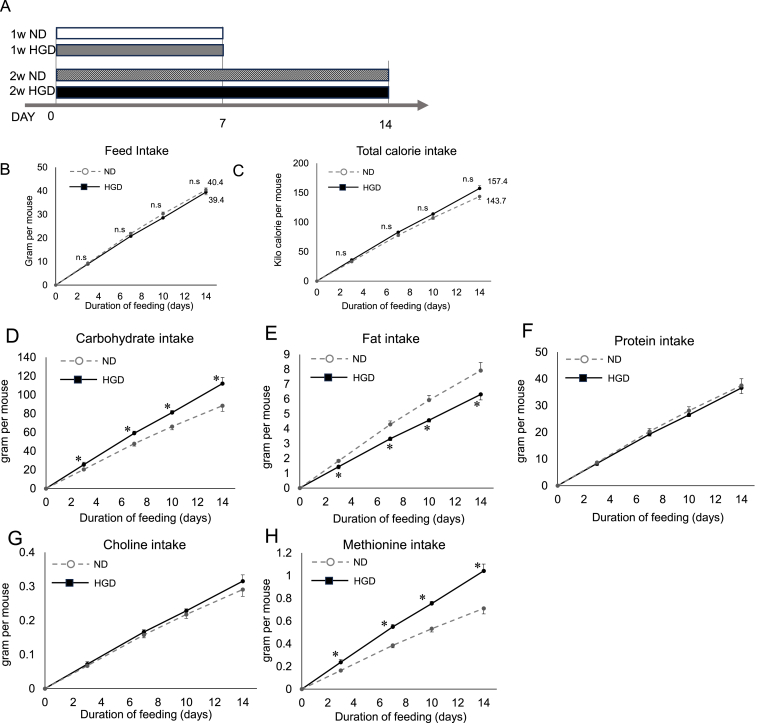
Fig. 1.
Experimental protocol and food intake quantities
(A) C57BL/6J male mice were fed each diet for 7 days or 14 days. (B) The amount of food intake (g) per mouse that was estimated by dividing the accumulated food intake per cage by the number of mice in the cage. (C) Estimated caloric intake that each mouse consumed. (D) The amounts of (D) carbohydrate intake, (E) fat intake, (F) protein intake, (G) choline intake, and (H) Methionine intake of each mouse. Each group consisted of 8 mice.
2.2. Oral glucose tolerance test (OGTT)
Oral glucose tolerance test was done according to standard procedure [12]. Glucose was dissolved in saline (10 g/mL) and equilibrated. Mice were fasted without water deprivation for 16 h. Then, blood glucose values were measured as basal values. After that, equilibrated glucose solution was administered orally to each mouse (0.1 mL/10g body weight) and the blood glucose levels were determined using a hand-held glucose monitor (FORA LAB Gluco, ForaCare Japan) at 15 min, 30min, 60min and 120 min later. Area under the curves (AUC) were calculated from the time-course graphs of blood glucose concentration.
2.3. Sample collection
At the end of the experiment, the mice were injected with a mixture of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg). They were subsequently dissected under a deep anaesthetic state. Their livers and epididymal fat tissues were collected, weighed, and immediately frozen using liquid nitrogen and stored at −80 °C until further processing and analysis. Plasma samples were obtained using centrifugation and stored at −80 °C until further processing.
2.4. Blood biochemical analyses
The blood parameters related to metabolic syndrome were measured under a non-fasting state since we were interested in the physiological state during HGD consumption. Plasma triglyceride, total cholesterol, and non-esterified fatty acids (NEFA) levels were measured using the TG E test Wako, Cholesterol E test Wako, and NEFA C test Wako (Wako, Japan, cat.no. 432–40201, cat.no. 439–17501, Code 279–75401), respectively. Plasma AST and ALT levels were measured using Transferases C-II test Wako (Fujifilm Wako Pure Chemical Corporation., Osaka, Japan, cat.no. 431–30901). Their blood glucose levels were determined using a hand-held glucose monitor (FORA LAB Gluco, ForaCare Japan), and blood was obtained from the tail vein prior to them being anesthetised. These analyses were also done on the plasma of mice, which were fasted with free access to drinking water for 16 h.
2.5. Extraction of hepatic lipids and quantification
Hepatic lipids were extracted using the Folch method [13] with minor modifications. Eighty mg of liver tissue were weighed and homogenised. Then, 1.2 mL of chloroform/methanol (vol/vol, 2:1) were added directly to the homogenised sample and vortexed vigorously for 2 min at room temperature. The mixtures were centrifuged (15,000×g for 5 min), and 1 mL of the supernatant was apportioned into a new tube. The supernatant was mixed with 200 μL of saline and centrifuged (3000 rpm for 15 min). The lower organic phase was carefully transferred into a new tube and dried at 80 °C using a block heater. The extracted fats were completely dissolved in 100 μL of 2-propanol (Wako, Japan, cat.no. 166–04836). The triglyceride, total cholesterol, and NEFA levels were determined using the kits mentioned in previous section.
2.6. Histological analyses
Ten-μm-thick cryosections were stained with Oil Red O Stain Kit (Scy Tek Laboratories, Inc., USA) and Mayer's Haematoxylin Solution (Scy Tek Laboratories, Inc., USA) [14]. The captured images were analysed using ImageJ (Fiji) [15]. The Oil Red O-stained areas were quantified in 3 microscopic fields at 20-fold magnification.
2.7. RNA extraction, quantitative RT-PCR, and microarray analyses
For the quantitative real time-PCR (RT-PCR) analysis, RNA was isolated from snap frozen liver tissue using ISOGEN II (NIPPON GENE CO., LTD, Toyama, Japan, cat.no. 311–07361). 1.5 μg of RNA was converted to cDNA using SuperScript IV VILO Master Mix with ezDNase enzyme (Invitrogen, Thermo. Fisher Scientific, Japan, cat.no. 11766050). Quantitative PCR reactions were performed with THUNDERBIRD SYBR qPCR Mix (TOYOBO, Japan, cat.no. QPS-201). The cDNA samples were amplified by Thermal Cycler Dice Real Time System TP860 (TaKaRa Bio, Japan). Then, relative mRNA expression was calculated by standard curve and normalised with the expression of β-actin as an internal control. All primers used for the quantitative RT-PCR are listed in Table 4.
Table 4.
Primer sequences for qRT-PCR amplification
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| SREBP-1c | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| FAS | CACAGTGCTCAAAGGACATGCC | CACCAGGTGTAGTGCCTTCCTC |
| SCD1 | GCAAGCTCTACACCTGCCTCTT | CGTGCCTTGTAAGTTCTGTGGC |
| ACCα | AAGGCTATGTGAAGGATG | CTGTCTGAAGAGGTTAGG |
| GPD1 | CGCATCACTGTGGTACAAGAGG | CTTGGTGTTGTCACCGAAGCCA |
| G6Pase | CCATGCAAAGGACTAGGAACAA | TACCAGGGCCGATGTCAAC |
| LPL | CTGCTGGCGTAGCAGGAAGT | CGTGGAAAGTGCCTCCATTG |
Microarray analysis of the liver mRNA was outsourced to Kamakura Techno‒Science, Inc. in Japan. They performed the analysis using the 3D-Gene® mRNA Oligo chip. We focused on the genes whose expression changed by more than twice or less than half due to HGD consumption. We conducted a Gene Ontology (GO) analysis using the DAVID cloud application (https://david.ncifcrf.gov/summary.jsp) targeting the genes that were upregulated or downregulated by HGD consumption. For the upregulated genes, a GO analysis was conducted on the genes that had absolute intensity values of 100 or higher in the HGD group. For the genes that were downregulated, a GO analysis was conducted on genes with an intensity value of 100 or higher in the ND group. The genes associated with lipogenesis were identified since they showed the highest number of altered expressions. Additionally, a heatmap was generated using Heatmapper (http://www.heatmapper.ca/) [16].
2.8. ELISA
The frozen liver tissue samples were put into a lysis buffer that contained a protease inhibitor and were homogenised with Tissue lyser II—a bead beater (QIAGEN K.K, Tokyo, Japan). This homogenisation method was the same as that followed in a previous report [17]. The homogenate was further diluted with lysis buffer. Then, the diluted suspension was applied into the wells of antibody-coated ELISA plates. We used commercially available ELISA kits and measured the expression levels of fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1), and Acetyl-CoA carboxylase α(ACCα) (FAS and SCD1: ELK6328 and ELK6972 respectively, ELK Biotechnology CO., Ltd., CO, USA.; ACCα: ab309323, Abcam plc, Cambridge, UK). The concentration of each protein was calculated after each blank value was subtracted from the 450 nm absorbance value. These protein values were normalised with total protein values, which were measured using a protein assay kit (T9300A, Takara Bio Inc., Japan).
2.9. Statistical analyses
Data are expressed as means ± standard deviation (SD). Statistical differences between the groups were determined using the Mann–Whitney U test. Data were analysed by SPSS software, version 26. A value of p < 0.05 was considered statistically significant.
3. Results
-
1.
Total amount of diet intake and estimated calories during feeding periods
The total food intake of the ND group and HGD group through the experimental period was comparable (ND vs HGD, 21.5 g vs 20.1 g per mouse at day 7; ND vs HGD, 40.4 g vs 39.4 g per mouse at day 14) (Fig. 1B). Similarly, the total caloric intake of both groups was also comparable (ND vs HGD, 77.8 ± 2.1 kcal vs 82.9 ± 1.6 kcal per mouse at day 7; 143.7 ± 4.9 kcal vs 157.4 ± 4.6 kcal at day 14 (Fig. 1C). The HGD group consumed a significantly higher amount of carbohydrates and methionine compared to the ND group through the experiments (Fig. 1D and 1H). Both groups' protein, and choline intakes were comparable (Fig. 1F and 1G). On the contrary, The HGD group's fat intake was smaller than that of the ND group (Fig. 1E).
-
2.
HGD induced fat accumulation
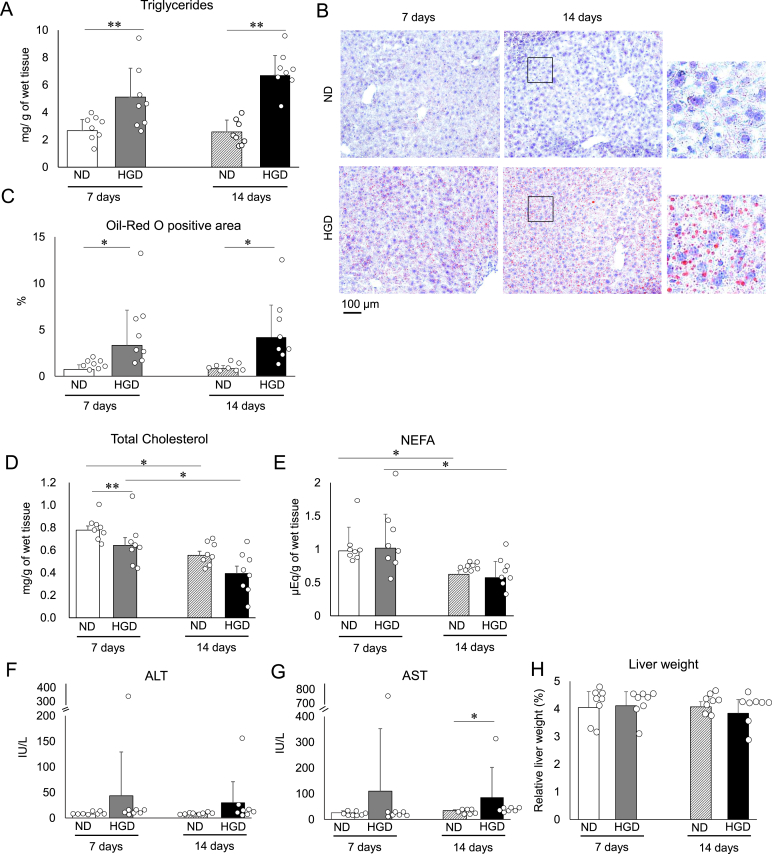
We measured the liver weights, hepatic triglyceride, total cholesterol, and NEFA (free fatty acids) levels of both groups 7 days and 14 days after the administration of their respective diets commenced. The results from day 7 showed that the HGD group's hepatic triglyceride levels were significantly higher compared to that of the ND group (Fig. 2A) whereas both group's liver weights were comparable (Fig. 2H). In addition, oil droplets were found to have accumulated in the liver histological section of the HGD group (Fig. 2B). Additionally, the oil droplets were found to have accumulated in a larger area in the HGD group in comparison to the ND group (Fig. 2C). In contrast, the total hepatic cholesterol levels of the HGD group were lower than those of the ND group (Fig. 2D). Overall, the day 14 results displayed a similar trend to the day 7 results (Fig. 2A–C and 2E) although there was an exception that both group's cholesterol levels were comparable (Fig. 2D). Focusing on the changes from day 7 to day 14, the accumulation of hepatic triglycerides did not increase for either group (Fig. 2A and 2C) as well as liver weight (Fig. 2H). However, in the case of the HGD group, the dispersion of the hepatic triglyceride values reduced, and the averages of the values increased (Fig. 2A). Additionally, the hepatic cholesterol and NEFA decreased in both groups (Fig. 2D and 2E).
Fig. 2.
The impact of HGD on liver lipid content and plasma AST and ALT levels
(A) The quantity of hepatic triglycerides. (B) Typical images of liver histological sections that were stained with Oil Red O. (C) The values of the Oil Red O-positive area in the liver histological section. Hepatic (D) total cholesterol and (E) NEFA. Plasma (F) AST and (G) ALT values. (H) Relative liver weight to body weight. Data are presented as mean ± SD. ∗p < 0.05, and ∗∗p < 0.01. Each group consisted of 8 mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We also evaluated damage to liver, which likely to be associated with liver steatosis. Plasma ALT and AST levels of both groups were compared at day 7 and day 14 (Fig. 2F and 2G). Both groups showed comparable plasma ALT and AST levels at day 7 (Fig. 2F and 2G). The day 14 results showed that both group's plasma ALT levels were comparable whereas the HGD group showed higher AST levels (Fig. 2F and 2G). In addition, at each point, there was one individual in the HGD group with high ALT and AST values (Fig. 2F and 2G). However, the degree of increase of the AST levels at day 14 was slight when statistical analysis was done after omitting the individual that displayed outstanding higher values (averages of the values; ND: 34.26 ± 3.65 vs HGD: 44.94 ± 15.41, p < 0.05). In addition, the AST and ALT values of both groups were not significantly increased or decreased from day 7 to day 14 (Fig. 2F and 2G). These results indicate that the HGD did not induce damage to the liver beyond the normal physiological state as an overall trend.
-
3.
Hepatic fat accumulation by HGD-consumption was not accompanied with the increases in body weight, peripheral fat, and blood parameters that are associated with metabolic syndrome
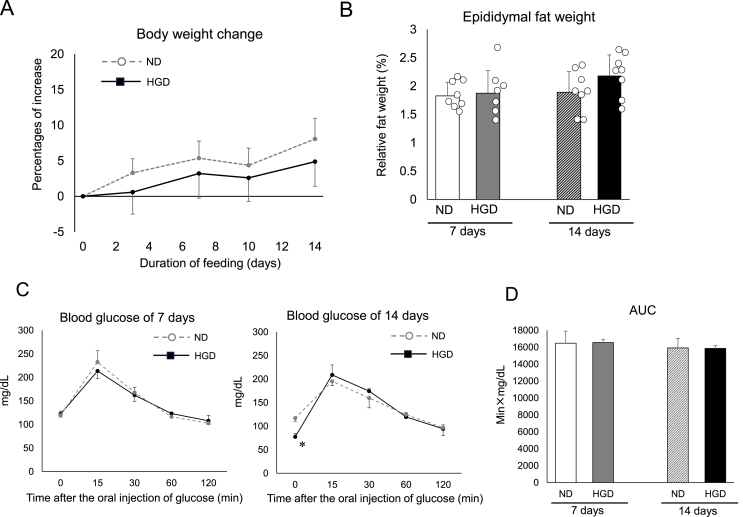
To evaluate the abnormal metabolic changes that occurred during the HGD consumption period, body weight, epididymal fat mass, blood glucose values, cholesterol values, and NEFA values were measured. The body weight changes in both groups were comparable throughout the experiment (Fig. 3A). Both groups’ epididymal fat masses were also unaffected (Fig. 3B). In essence, besides a few exceptions, the blood parameters were unaffected by HGD consumption and its duration (Table 2). As the examples of the exceptions, the blood cholesterol values of the HGD group were higher than those of ND group at day 7 (Table 2). In addition, the casual blood glucose values of the HGD group were lower than those of ND group at day 14 (Table 2). Focusing on the changes from day 7 to day 14, the casual blood glucose values of the ND group were found to have increased between day 7 and day 14. However, the change in the glucose value could be by chance since casual blood glucose levels could be wildly fluctuated. In addition, the ND group also showed lower values of blood triglycerides and blood NEFA at day 14 (Table 2).
Fig. 3.
Body weights, visceral fat mass, glucose absorption and tolerance of HGD-fed mice
(A) The body weight change from day 0 to day 14 after commencement of ND or HGD consumption. (B) Relative weight of epididymal fat to body weight. (C) Blood glucose concentration before (0 min) and during OGTT. (D) The values of AUC that were calculated from Fig. 3C. Data are presented as mean ± SD. ∗p < 0.05, and ∗∗p < 0.01. Each group consisted of 8 mice except for Fig. 3C and 3D. Each group consisted of 4 mice in the experiment of Fig. 3C and 3D.
Table 2.
Blood glucose and lipid levels after administering the HGD (non-fasted state)
| 7 days |
14 days |
|||
|---|---|---|---|---|
| ND | HGD | ND | HGD | |
| Random blood glucose (mg/dL) | 151.6 ± 20.4 | 167.9 ± 26.4 | 191.4 ± 11.5† | 160.4 ± 35.7∗ |
| Triglyceride (mg/dL) | 80.0 ± 27.7 | 61.7 ± 14.4 | 58.2 ± 5.6† | 47.3 ± 12.7 |
| Total cholesterol (mg/dL) | 74.6 ± 8.3 | 98.8 ± 31.7∗ | 81.7 ± 7.7 | 96.0 ± 28.8 |
| NEFA (mEq/L) | 0.83 ± 0.37 | 0.58 ± 0.18 | 0.54 ± 0.13† | 0.58 ± 0.14 |
Data are presented as mean ± S.D. Each group consisted of 8 mice. Comparisons between two groups, ND and HGD, ∗p < 0.05, ∗∗P < 0.01., ND 1 week and ND 2 weeks †p < 0.05.
We also examined these blood parameters after starvation as the evaluation method similar to clinical testing (Table 3). Most parameters were not affected by HGD consumption and its duration. However, the fasting blood glucose levels of the HGD group at day 14 were lower than those of ND group. These results indicate that there were no signs of metabolic syndrome, since any elevation in the values were not induced by HGD consumption.
-
4.
Absorption of glucose from intestinal tract and glucose tolerance were not affected by HGD
Table 3.
Blood glucose and lipid levels after administering the HGD (fasted state)
| 7 days |
14 days |
|||
|---|---|---|---|---|
| ND | HGD | ND | HGD | |
| Random blood glucose (mg/dL) | 119.5 ± 9.0 | 122.25 ± 6.6 | 117.25 ± 6.7 | 77.5 ± 6.8∗† |
| Triglyceride (mg/dL) | 114.7 ± 37.5 | 129.0 ± 44.0 | 84.1 ± 8.2 | 115.84 ± 34.3 |
| Total cholesterol (mg/dL) | 85.9 ± 14.3 | 114.7 ± 27.3 | 92.7 ± 4.8 | 113.2 ± 32.1 |
| NEFA (mEq/L) | 0.85 ± 0.12 | 0.86 ± 0.18 | 0.80 ± 0.06 | 1.04 ± 0.21 |
Data are presented as mean ± S.D. Each group consisted of 4 mice. Comparisons between two groups, ND and HGD, ∗p < 0.05, ∗∗P < 0.01., HGD 1 week and HGD 2 weeks †
To examine the abnormality of glucose absorption and glucose tolerance, which potentially affect hepatic fat accumulation, an OGTT test was done. The peak concentration of glucose and the AUC of both groups did not differ at day 7 (Fig. 3C and 3D). Similar results were observed at day 14 (Fig. 3C and 3D). These results indicated that HGD consumption did not provoke excess absorption of glucose and intolerance to glucose during the duration of the experiment.
-
5.
de novo lipogenesis is promoted by fibre-deficient diet, that is most important candidate of fat accumulation in liver
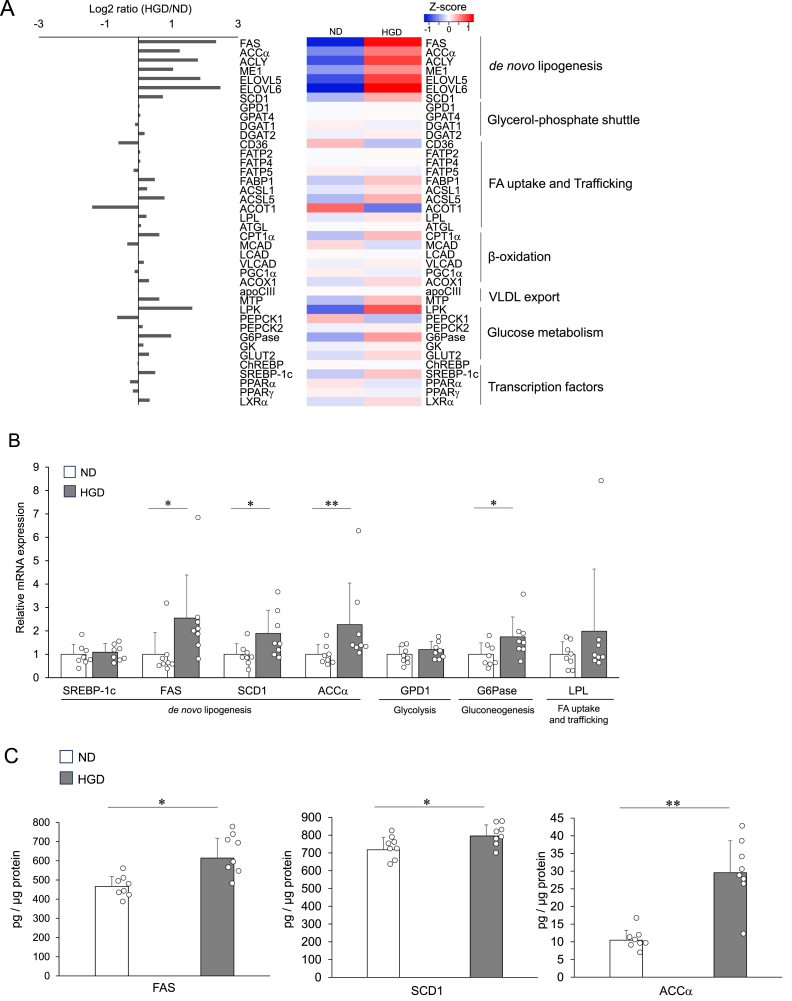
To screen the molecules which are responsible for triglyceride accumulation in the liver, a comprehensive gene profile analysis was done using a DNA microarray at day 7. Among the genes that were upregulated or downregulated by HGD consumption, the largest number of genes were related to the lipid metabolic process (Supplemental materials). Hence, we picked up the genes which are involved in lipid metabolism, as summarised in Fig. 4A. The increased genes included FAS, ACCα, ATP citrate lyase (ACLY), malic enzyme 1 (ME1), and SCD1. In contrast, the expression of carbohydrate response element binding protein (ChREBP), sterol regulatory element binding protein 1c (SREBP-1c), and other transcriptional factors were not affected by HGD consumption. In addition, genes of glucose-6-phosphatase (G6Pase) and fructose bisphosphatase 1 (FBP1), which are involved in glucogenesis, were upregulated. In other pathways, Acyl-CoA thioesterase 1 (ACOT1) and pyruvate kinase (LPK) displayed a marked change in the expression. Other genes of the fatty acid transport system, enhancement of the glycerol-phosphate shuttle, β-oxidation, and VLDL secretion were not affected.
Fig. 4.
Impact of HGD on hepatic gene and protein expression
(A) The genes upregulated or downregulated by HGD-feeding for 7 days that are related to lipid metabolic process. (B) The relative gene expression levels of the seven genes were quantified by qRT-PCR at day 7. The expression of each gene was normalised to the average expression of the endogenous reference gene β-actin. (C) Lipogenesis-related protein levels in the liver at day 7. Data are presented as mean ± SD. ∗p < 0.05, and ∗∗p < 0.01. In Fig. 4A, livers of ND group and HGD group three mice In Fig. 4B and 44C, each group consisted of 8 mice. ACCα: Acetyl-CoA carboxylase α; ACOT1: Acyl-CoA Thioesterase 1; ACOX1: acyl-CoA oxidase 1; ACSL: Long-chain acyl-CoA synthetase; ACYL: ATP citrate lyase; ApoCIII: Apolipoprotein CIII; ATGL: Adipose triglyceride lipase; ChREBP: carbohydrate response element binding protein; CPT1: carnitine palmitoyltransferase I; DGAT: Diacylglycerol O-Acyltransferase; ELOVL: elongation of very long fatty acid elongase; FABP: Fatty Acid-binding protein; FAS: fatty acid synthase; FATP: Fatty acid transport protein; FBP1: fructose bisphosphatase 1; G6Pase: glucose-6-phosphatase; GK: Glycerol kinase; GPD1: glycerol-3-phosphate dehydrogenase; GPT4: glycerol-3-phosphate acyltransferase 4; GLUT2: glucose transporter type 2; LCAD: long-chain acyl-CoA dehydrogenase; LPK: pyruvate kinase liver and red blood cell; LXR: Liver X receptor; MCAD: medium-chain acyl-CoA dehydrogenase; ME1: malic enzyme 1; MTP: microsomal triglyceride transfer protein; PEPCK: phosphoenolpyruvate carboxykinase; PGC-1α: peroxisome proliferators-activated receptor-γ co-activator-1α; PPAR: Peroxisome proliferator-activated receptor; SCD1: stearoyl-CoA desaturase 1; SREBP-1c: sterol regulatory element binding protein 1c; LPL: Lipoprotein lipase; and VLCAD: very long-chain acyl-CoA dehydrogenase. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We validated the results of the microarray by the analysis of qRT-PCR. Since FAS, SCD1, and ACCα are closely involved in the synthesis of triglycerides and increased markedly in the microarray analysis, we primarily evaluated the expression of these genes. Consistent with the results of the microarray, the HGD group displayed a higher expression of these genes in comparison to the ND group. Nevertheless, the expression of SREBP-1c, a regulator of these genes, was not affected (Fig. 4B). In addition, we also evaluated the genes that are responsible for glycolysis or glucogenesis that are along with the synthesis pathway of triglycerides. The expression of glucose-6-phosphatase (G6Pase) decreased, whereas that of Glycerol-3-phosphate dehydrogenase 1 (GPD1) was not affected by HGD consumption.
We further evaluated the protein expression of FAS, SCD1, and ACCα. As shown in Fig. 4C, HGD group showed higher expression of all these proteins than that of NG group. These results strongly suggest the acceleration of de novo lipogenesis via these molecules in the early phase of hepatic steatosis.
4. Discussion
Most rodent models of diet-induced experimental hepatic steatosis utilise diets which contain extremely high levels of fat or diets deficient in methionine and choline [[18], [19], [20]]. Basically, the HGD used in our current study is designed to be standard amounts of choline, fat and methionine although there are slight differences compared to those of standard diet. Nevertheless, as shown in Fig. 1, HGD resulted in lower intake of fat and higher methionine intake statistically. However, this nutritional intake pattern is less likely to cause hepatic steatosis, since it is rather the opposite of consuming a high-fat diet or a diet deficient in methionine and choline. Especially, methionine and choline are necessary for the synthesis of phosphatidylcholine [21,22]. Additionally, phosphatidylcholine is essential for the formation of VLDL [23], which is necessary for the excretion of triglycerides from the liver. Therefore, it is unlikely that a slightly higher intake of methionine would cause fatty liver. On the contrary, the HGD used in this study is deficient in dietary fibre while also containing a large amount of glucose. Indeed, HGD groups showed higher carbohydrate consumption compared to that of the ND group, meaning that HGD group consumed much amount of glucose. A previous animal study has shown that the administration of drinking water containing 30 % glucose with normal diet for 10 weeks induces the accumulation of triglycerides in the liver that are associated with increased FAS, SCD1, and ACCα [5]. These enzymes are directly involved in the biological pathways where glucose is catabolized and results in triglycerides synthesis [24,25]. Therefore, these enzymes were thought to be induced to catabolize overloaded glucose in the prior study. In addition, this study also shows that the AUC of glucose tolerance test is not increased or decreased at 10 weeks after the intervention [5]. This prior study and our present study have these phenotypes in common, suggesting that the accumulation of hepatic triglycerides induced by our HGD would at least partially be due to its high glucose content. Besides, the caloric intake would be higher under the experimental condition of the prior study since glucose was administered in addition to a normal diet. The results of our study are different in that the excess intake of glucose within required calories for living potentially induces hepatic fat accumulation in a relatively short time although the degree of steatosis is weak and could be reversible.
In addition, it is well-known that a traditional East Asian diet contains 70 % carbohydrates, 15 % protein, and 15 % fat per 1000 kcal, and 15 g of fibre per 1000 kcal [26]. In contrast, a western diet consists of 50 % carbohydrates, 16 % protein, and 34 % fat, and 6 g of fibre per 1000 kcal [27]. Our HGD may reflect the complete depletion of fibre from the typical East Asian dietary pattern. Therefore, such diets including ones with lower dietary fibre would affect fat deposition in the liver based on our results of the present study.
Besides, the imbalance between lipid accumulation and removal is the possible cause of triglyceride accumulation in hepatocytes [28]. Fatty acid uptake and de novo lipogenesis leads to lipid accumulation, whereas fatty acid oxidation by mitochondria and excretion as components of VLDL particles are involved in lipid removal [28]. Our microarray analysis also revealed that the effect of HGD consumption was concentrated on de novo lipogenesis pathway. Among them, the functions of upregulated genes are commonly related to the synthesis of acyl-CoA [24]. The interaction of the intermediate metabolites of glycolysis with acyl-CoA is key chemical reaction in the pathway to generate triglycerides [24,25]. Malic enzyme 1 (Me1) and ACLY are involved in the synthesis of acetyl-CoA [29], that is converted into acyl-CoA [24]. In addition, ELOVL participates in the synthesis of saturated fatty acids, which are constitutive elements of acyl-CoA [24]. These reactions and products are connected to the acyl-CoA synthesis pathway, that is mediated by FAS, SCD1 and ACCα [24,25]. Upregulation of these genes and increase in hepatic proteins of FAS, SCD1, and ACCα strongly suggest that the HGD induces triglyceride accumulation in the liver by accelerating acyl-CoA synthesis.
Our study is based on the hypothesis that HGD affects liver steatosis via any metabolic disorder. Therefore, we evaluated whether there are signs of metabolic disorder in the liver and other than liver. We found that HGD consumption did not induce typical phenotypes of metabolic syndrome, including increase in body weight, peripheral fat accumulation, increase in plasma glucose, lipids, intolerance to glucose, and the development of an abnormal intestinal ability to absorb excess glucose whereas feeding of HGD induced accumulation of hepatic triglycerides without increasing hepatic total cholesterol and NEFA. These results suggest that liver is the most affected organ and the systemic effect outside of the liver is absent or negligible as of 14 days of HGD consumption. Although the elevation of plasma total cholesterol levels was observed in HGD group at day 7 would not be recognised as a clear sign of metabolic disorder since the changes in the value were small and were only observed temporarily. Besides, there are some minor changes in lipid-related parameters in the liver and blood. Hepatic cholesterol and hepatic NEFA levels were decreased from day 7 to day 14 of the intervention in both groups. The critical reason for the decrease in these values is not clear. However, the ages of the mice used in our experiment were akin to the typical growth period used in animal feeding experiments (6 weeks old to 8 weeks old). Therefore, the drops in these values are recognised as normal physiological changes and are not specific changes induced by HGD consumption. On the other hand, fasting blood glucose levels were decreased in HGD group at day 14. The reason might be that the HGD-feeding caused a sustained high level of glucose in the intestinal lumen, which in turn might trigger a compensatory mechanism that rapidly metabolises the glucose. Although lower blood glucose levels indicate that the utilisation of glucose is under control, it is possible that the excess glucose, that was absorbed due to HGD consumption, was metabolised and subsequently accumulated in the liver as triglycerides.
Our study has a few potential limitations. We could not determine whether the effects of the fibre-deficient diet on hepatic fat deposition resulted entirely from the increased carbohydrate absorption in the small intestine. A related concern is whether colonic events are involved. The fermentation of dietary fibre may affect lipid metabolism by producing short chain fatty acids (SCFAs) that can be absorbed into portal circulation [30,31]. However, the biological effects of SCFAs remain underexplored [32]. We have found that the consumption of the HGD in our study changes microbiota and reduces the amount of SCFAs in mouse stool within a few days [33]. Therefore, we supplemented SCFAs in drinking water and examined whether SCFAs were responsible for HGD-induced hepatic fat accumulation. We found that the supplementation of SCFAs did not ameliorate the increased triglyceride values in the liver (unpublished data). We could not determine the potential relationship between colonic events and hepatic fat accumulation. Therefore, more detailed studies are needed to examine these matters.
5. Conclusion
A diet deficient in dietary fibre and high in glucose increases hepatic fat deposition in a relatively short term. This is associated with an increased expression of the molecules related to de novo lipogenesis. The present study suggests that the adoption of a low-fibre, high-glucose diet is breeding ground for fatty liver disease.
CRediT authorship contribution statement
Sonoko Karino: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Haruki Usuda: Writing – review & editing, Writing – original draft, Visualization, Supervision, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Shoma Kanda: Visualization, Supervision, Investigation, Data curation. Takayuki Okamoto: Writing – review & editing, Supervision, Formal analysis, Data curation, Conceptualization. Tomomi Niibayashi: Formal analysis, Data curation. Takahisa Yano: Writing – review & editing, Supervision, Data curation. Kohji Naora: Writing – review & editing, Supervision, Data curation. Koichiro Wada: Writing – review & editing, Writing – original draft, Supervision, Investigation, Data curation, Conceptualization. Sonoko Karino and Haruki Usuda were equally contributed to this study.
Ethical approval
All experiments with animals in this study were approved by the Animal Care and Use Committee of Shimane University (Approval code: IZ4-36).
Data availability
Summaries of microarray GO analysis are attached as supplemental material. Other data will be made available on request from the corresponding author.
Funding
This work was supported by JSPS KAKENHI Grant Number: 23K10958.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Abbreviations
- ACCa
Acetyl-CoA carboxylase alpha
- ACOT1
Acyl-CoA Thioesterase 1
- ACOX1
acyl-CoA oxidase 1
- ACSL:
Long-chain acyl-CoA synthetase
- ACYL:
ATP citrate lyase
- ALT
Alanine Aminotransferase
- ApoCIII
Apolipoprotein CIII
- AST
Aspartate aminotransferase
- ATGL:
Adipose triglyceride lipase
- ChREBP
carbohydrate response element binding protein
- CPT1
carnitine palmitoyltransferase I
- CVDs
Cardiovascular diseases
- DGAT
Diacylglycerol O-Acyltransferase
- DSS
Dextran sulphate sodium
- ELOVL:
elongation of very long fatty acid elongase
- FABP
Fatty Acid-binding protein
- FAS
fatty acid synthase
- FATP
Fatty acid transport protein
- FBP1
fructose bisphosphatase 1
- G6Pase
glucose-6-phosphatase
- GK
Glycerol kinase
- GPD1
glycerol-3-phosphate dehydrogenase
- GPT4
glycerol-3-phosphate acyltransferase 4
- GLUT2
glucose transporter type 2
- HGD
High glucose and fiber-deficient diet
- LCAD
long-chain acyl-CoA dehydrogenase
- LPK
pyruvate kinase liver and red blood cell
- LPL:
Lipoprotein lipase
- LXR
Liver X receptor
- MAFLD
Metabolic dysfunction associated with fatty liver disease
- MCAD
medium-chain acyl-CoA dehydrogenase
- ME1
malic enzyme 1
- MTP
microsomal triglyceride transfer protein
- NAFLD
Non-alcoholic fatty liver disease
- ND
Normal chow diet
- OGTT
Oral glucose tolerance test
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome proliferators-activated receptor-γ co-activator-1α
- PPAR
Peroxisome proliferator-activated receptor
- SCD1
stearoyl-CoA desaturase 1
- SREBP-1c
sterol regulatory element binding protein 1c
- VLCAD
very long-chain acyl-CoA dehydrogenase
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101848.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Seo J.A. Metabolic syndrome: a warning sign of liver fibrosis. J. Obes. Metab. Syndr. 2022;31:1–3. doi: 10.7570/jomes22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavez-Tapia N.C., Murua-Beltran Gall S., Ordonez-Vazquez A.L., Nuno-Lambarri N., Vidal-Cevallos P., Uribe M. Understanding the role of metabolic syndrome as a risk factor for hepatocellular carcinoma. J. Hepatocell. Carcinoma. 2022;9:583–593. doi: 10.2147/JHC.S283840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z., Zheng M., Lei H., Jiang Z., Chen Y., He H., Zhao G., Huang H. A clinical study of the correlation between metabolic-associated fatty liver disease and coronary plaque pattern. Sci. Rep. 2023;13:7224. doi: 10.1038/s41598-023-34462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., Kawaguchi T., Arrese M., Valenti L., Shiha G., Tiribelli C., Yki-Jarvinen H., Fan J.G., Gronbaek H., Yilmaz Y., Cortez-Pinto H., Oliveira C.P., Bedossa P., Adams L.A., Zheng M.H., Fouad Y., Chan W.K., Mendez-Sanchez N., Ahn S.H., Castera L., Bugianesi E., Ratziu V., George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N., Willoughby J., Harbison C., Fitzgerald K., Ilkayeva O., Newgard C.B., Cohen D.E., Kahn C.R. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 2017;127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commission C.A. 2021. Guidelines on Nutrition Labelling CXG 2-1985. [Google Scholar]
- 7.Giuntini E.B., Sardá F.A.H., de Menezes E.W. The effects of soluble dietary fibers on glycemic response: an overview and futures perspectives. Foods. 2022;11 doi: 10.3390/foods11233934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S.K., Rossi M., Bajka B., Whelan K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/s0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 10.Collaborators G.B.D.D. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., Young V.B., Henrissat B., Wilmes P., Stappenbeck T.S., Nunez G., Martens E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353 e1321. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Rijjal D., Wheeler M.B. A protocol for studying glucose homeostasis and islet function in mice. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Mehlem A., Hagberg C.E., Muhl L., Eriksson U., Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babicki S., Arndt D., Marcu A., Liang Y., Grant J.R., Maciejewski A., Wishart D.S. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.J., Shajib M.S., Manocha M.M., Khan W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012 doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itagaki H., Shimizu K., Morikawa S., Ogawa K., Ezaki T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int. J. Clin. Exp. Pathol. 2013;6:2683–2696. [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier M.S., Favier R., Lavoie J.M. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br. J. Nutr. 2006;95:273–281. doi: 10.1079/bjn20051635. [DOI] [PubMed] [Google Scholar]
- 20.Castaño C., Novials A., Párrizas M. Exosomes from short-term high-fat or high-sucrose fed mice induce hepatic steatosis through different pathways. Cells. 2022;12 doi: 10.3390/cells12010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstee Q.M., Day C.P. S-adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. J. Hepatol. 2012;57:1097–1109. doi: 10.1016/j.jhep.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 22.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017;1859:1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Vance D.E. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr. Opin. Lipidol. 2008;19:229–234. doi: 10.1097/MOL.0b013e3282fee935. [DOI] [PubMed] [Google Scholar]
- 24.Ishimoto K., Nakamura H., Tachibana K., Yamasaki D., Ota A., Hirano K.I., Tanaka T., Hamakubo T., Sakai J., Kodama T., Doi T. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J. Biol. Chem. 2009;284:22195–22205. doi: 10.1074/jbc.M109.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders F.W., Griffin J.L. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol. Rev. Camb. Phil. Soc. 2016;91:452–468. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Y., Wang F., Yuan J., Li D. Optimal dietary macronutrient distribution in China (ODMDC): a randomised controlled-feeding trial protocol. Asia Pac. J. Clin. Nutr. 2017;26:972–980. doi: 10.6133/apjcn.072017.06. [DOI] [PubMed] [Google Scholar]
- 27.A.R.S. United States Department of Agriculture . 2021. Usual Nutrient Intake from Food and Beverages, by Gender and Age, what We Eat in America, NHANES 2015-2018. [Google Scholar]
- 28.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrocola F., Galluzzi L., Bravo-San Pedro J.M., Madeo F., Kroemer G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metabol. 2015;21:805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Xia D., Chen J., Zhang X., Wang H., Huang L., Shen J., Wang S., Feng Y., He D., Wang J., Ye H., Zhu Y., Yang L., Wang W. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: roles of gut microbiota and short-chain fatty acid. Poultry Sci. 2022;101 doi: 10.1016/j.psj.2022.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019;20:461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de Los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S. Kanda, H. Usuda, S. Karino, T. Okamoto, T. Niibayashi, T. Yano, K. Naora, W. Koichiro, Dietary fiber deficiency accelerates colitis in mice in the short term independent of short-chain fatty acids. Gastroenterol. Insights. 2024;15:730–743. doi: 10.3390/gastroent15030052. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summaries of microarray GO analysis are attached as supplemental material. Other data will be made available on request from the corresponding author.