Graphical abstract
Keywords: Cervical cancer, Neoadjuvant chemotherapy, Pregnancy, Individualized therapy
Highlights
-
•
There has not yet been established standardized regimen for neoadjuvant chemotherapy treatment of cervical cancer during pregnancy
-
•
Individual chemotherapeutic agents have known potential adverse effect to the fetus and the toxicity is often dose dependent
-
•
Individualized treatment approaches with sequential administration can reduce the risk for the fetus while ensuring optimal anticancer treatment for the pregnant patient
Abstract
The incidence of cancer during pregnancy is steadily rising because of the postponement of plans for childbearing. One of the most common cancers diagnosed during pregnancy is cervical cancer. Diagnosis of most cases usually occurs in the early stages, but there are still cases of tumors staged IB2 and higher. In these cases, the treatment strategy entails administration of neoadjuvant chemotherapy. However, a universally recognized standardized regimen for neoadjuvant chemotherapy treatment of cervical cancer during pregnancy has yet to be established. The chemotherapy agents used during treatment are known for their fetal adverse effects. The aim of the therapy is to attain full-term pregnancy while minimizing fetal toxicity and decreasing tumor size. In this case report, we present a first-time sequential chemotherapy administration to minimize the cumulative toxicity of individual regimens and demonstrate the benefits for the patient and fetus.
1. Introduction
The estimated incidence of cancer during pregnancy is approximately 17–25 cases per 100,000 pregnancies. The occurrence of cancer during pregnancy has increased in recent years, mainly attributed to the trend of postponing plans for childbirth (Amant et al., 2019 Oct 1, Eibye et al., 2013 Sep). The literature reports the crude incidence of cervical cancer as 27.2 cases per 100,000 pregnancies. Among individuals aged 25 to 29 years, every fifth instance of cervical cancer is linked to pregnancy (Eibye et al., 2013 Sep, de Haan et al., 2018 Mar). Most cervical cancers are identified in the initial stages; however, regrettably, there are instances during pregnancy where clinically evident cases with tumors larger than 2 cm (stage IB2 and above) are observed. The management of newly diagnosed cervical cancer depends on the extent of the disease, gestational age, and the cooperation of a fully informed patient. If the patient does not wish to continue with the pregnancy, in the most European countries including Czechia, it is possible to terminate the pregnancy till 24 weeks of gestational age. Surgical procedures carry an elevated risk of pregnancy loss and severe prematurity. Observational procedures leading up to acceptable term delivery are associated with the risk of disease progression. Consequently, physicians frequently opt for neoadjuvant chemotherapy as a treatment strategy to achieve term pregnancy while simultaneously reducing tumor size (Amant et al., 2019 Oct 1, Cintra et al., 2023). In the context of neoadjuvant treatment for cervical cancer in pregnant patients, there is a broad range of therapeutic agents, and a universally accepted standardized regimen has yet to be established (Amant et al., 2019 Oct 1, Babkova et al., 2024 Apr). Various treatment options for neoadjuvant therapy during pregnancy have been described in the literature. Bernardini et al., for instance, described a platinum-based neoadjuvant chemotherapy treatment for cervical cancer during pregnancy. This treatment involved three protocols: cisplatin only, cisplatin/paclitaxel, and carboplatin/paclitaxel (Bernardini et al., 2022 Aug 14). Two other studies elucidated the use of neoadjuvant therapy involving a combination of cisplatin and ifosfamide (Babkova et al., 2024 Apr, Halaska et al., 2014 Mar). In their study, Huang et al. detailed the use of platinum-based treatment protocols, namely cisplatin and paclitaxel, for the management of cervical cancer in pregnant patients (Huang et al., 2021 Aug 13). Adverse effects are a well-known characteristic of individual chemotherapeutic agents. The most severe fetal complication associated with the use of platinum agents, particularly cisplatin, is ototoxicity, which is dose dependent (Vandenbroucke et al., 2020 Oct, Clemens E, van den Heuvel-Eibrink MM, Mulder RL, Kremer LCM, Hudson MM, Skinner R, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol [Internet]., 2019). Ifosfamide is known for its dose-dependent hematotoxicity and the potential to induce fetal anemia during cancer treatment (Babkova et al., 2024 Apr). In contrast, paclitaxel is considered a less toxic agent, and its limited transplacental transfer mitigates the adverse effects of the anticancer treatment. Its tendency to cause peripheral neurotoxicity is well documented, and this effect is also dose-dependent (Van Calsteren, 2010).
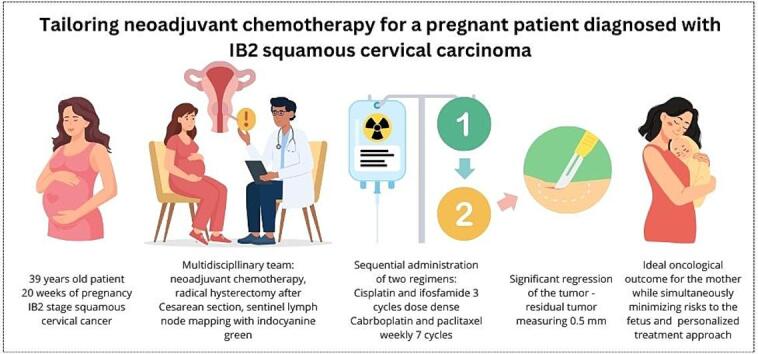
We present a squamous cell cervical FIGO stage IB2 in pregnant patient based on these discoveries. During treatment, we tailored the neoadjuvant therapy to avoid the potential fetal adverse effects of the chemotherapeutic agents and ensure the pregnant patient's optimal anticancer treatment. This patient was treated sequentially with two therapeutic regimens: three cycles of cisplatin with ifosfamide, followed by seven cycles of weekly carboplatin with paclitaxel.
2. Case
2.1. Case history
A 39-year-old patient was referred to our center for consultation. The patient underwent a biopsy of the cervix at a local clinic, which revealed squamous cell carcinoma grade 3. During that period, the patient was in her second pregnancy at 20 weeks gestation and experienced vaginal bleeding. The patient had a history of cervical dysplasia and was treated with conization (LLETZ – large loop excision of transformation zone) in 2017 at a different center. Otherwise, her medical history was unremarkable: she had one unassisted childbirth in 2020 and received regular examinations by a certified gynecologist with no abnormal findings including normal Pap smears.
2.2. Physical examination results
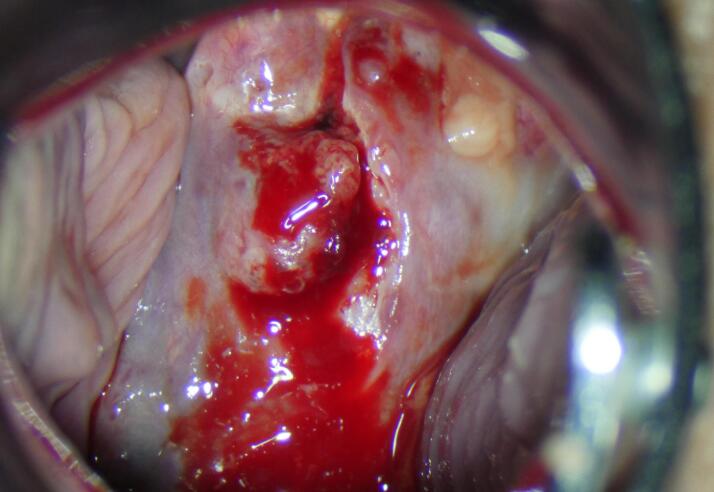
The colposcopy examination revealed a cervical tumor measuring approximately 20x30 mm (Fig. 1). The palpation examination indicated tumor-free parametria and an enlarged uterus consistent with a pregnancy of 20 weeks.
Fig. 1.
Colposcopy at the time of the diagnosis (Archive of University Hospital Kralovske Vinohrady).
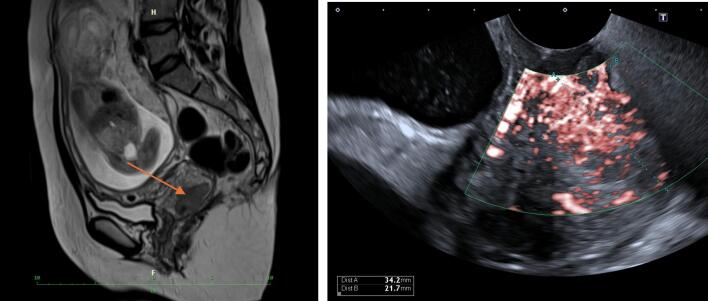
MRI and expert onco-gynecological and obstetrical ultrasound examinations were performed. The ultrasound examination detected a hypoechogenic tumor measuring 34x20x29 mm in the right region of the cervix. At the number 10–12, the tumor almost reached the pericervical fascia. Cervical length measurement was 40 mm long. No suspicious lymph nodes were visible. The MRI showed a well-circumscribed tumor measuring 34x18x18 mm, free parametria and no suspicious lymph nodes (Fig. 2). Serum SCC was 2.2 ug/l. A complete blood count before treatment showed mild pregnancy-associated anemia with a hemoglobin level of 116 g/l. The fetus was eutrophic along with euhydramnion, and the EFW (estimated fetal weight) corresponded to 20 weeks of pregnancy. The placenta was located on the posterior uterine wall. Screening of the anatomy anomalies was without any pathology. The patient had a strong pregnancy wish.
Fig. 2.
MRI and US at the time of the diagnosis (Archive of University Hospital Kralovske Vinohrady).
After discussing with the pregnant patient and her partner, a treatment plan involving neoadjuvant chemotherapy, radical hysterectomy after Cesarean section, and sentinel lymph node mapping with indocyanine green was proposed following the guidelines (Amant et al., 2019 Oct 1). According to the patient‘s wish bilateral salpingo-oophorectomy was planned.
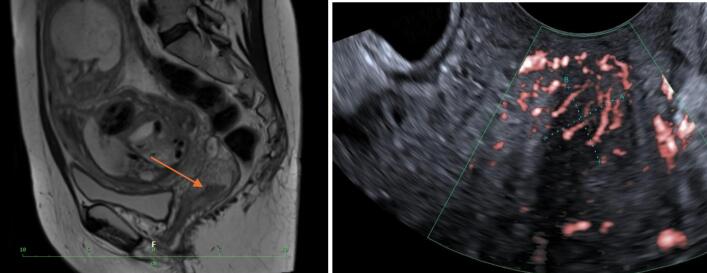
Based on the published studies from our center, we proposed an individualized treatment regimen that involves the sequential administration of two regimens to minimize toxicity. The proposed strategy involved the administration of three cycles of cisplatin and ifosfamide at two-week intervals, using the standard dosing of cisplatin (70 mg/m2) and ifosfamide (1500 mg/m2). This regimen would be followed by seven cycles of weekly carboplatin 2AUC and paclitaxel at 50 mg/m2. The first cycle of chemotherapy was administered at a gestational age of 21 weeks 2 days at the end of March 2024, with a dosing of 120 mg of cisplatin and 3 g of ifosfamide. Fetal middle cerebri artery peak systolic velocity was monitored right before the chemotherapy administration and on the 10th day after treatment according to our recommendation (Babkova et al., 2024 Apr). There were no signs of fetal anemia throughout the three administered cycles. After the completion of the third cycle of chemotherapy, a second MRI and expert onco-gynecological ultrasound were performed. The MRI and ultrasound examinations revealed a reduction in tumor size by more than 50 %, measuring 17x9x19 mm in diameter (Fig. 3).
Fig. 3.
MRI and US after three cycles of chemotherapy (Archive of University Hospital Kralovske Vinohrady).
After three cycles of cisplatin with ifosfamide, the treatment protocol was modified to include paclitaxel and carboplatin. The weekly dosage consisted of 86 mg of paclitaxel and 265 mg of carboplatin. Peak systolic velocity of fetal arteria cerebri media was measured immediately before the administration of chemotherapy. There were no signs of fetal anemia within all seven administered cycles. After the last cycle, another MRI and expert ultrasound round were repeated. Both methods exhibited consensus and no discernible tumor persistence (Supplementary Fig. S1). Expert colposcopy showed regression and was consistent with microcarcinoma (Supplementary Fig. S2).
The final cycle was administered in June 2024, concluding the process. The patient was 34 weeks and 4 days pregnant. A cesarean section was conducted at a gestational age of 37 weeks and 2 days, immediately followed by a radical abdominal hysterectomy. The indocyanine green was administered after delivery of the fetus and placenta and complete suture of the uterotomy. The hysterectomy involved removing C1 in the left paracervix and C2 in the right paracervix, along with bilateral salpingo-oophorectomy. Sentinel lymph node mapping was also performed using indocyanine green (Supplementary Fig. S3). Systemic pelvic lymphadenectomy was also performed. The surgical procedure was completed without any complications, and blood loss was within the range of 1000 ml (800 ml from the cesarean section and 200 ml from the radical hysterectomy). There were no significant findings in the postoperative assessment. The newborn had physiological postpartum adaptation, achieving Apgar scores of 9, 10, and 10 with a pH of 7.3. The infant's birthweight measured 2390 g, indicating the 9th percentile. The hemoglobin level of the newborn was 185 g/l (i.e., within the normal range). The patient was breastfeeding.
The histopathological examination conducted after the surgery revealed considerable regression, indicating the presence of a residual tumor measuring 0.5 mm in depth, located 1.5 mm beneath the surface epithelium, and exhibiting lymphovascular space invasion. Two sentinel lymph nodes were identified on both sides, and the left sentinel showed ITCs (isolated tumor cells); the other seven lymph nodes were negative. Upon deliberation with the patient, the multidisciplinary panel concluded that supplementary treatment is warranted. The patient has been scheduled for external radiotherapy and routine post-treatment surveillance. The infant will also receive follow-up care as recommended by the INCIP guidelines for children exposed to prenatal chemotherapy (Amant et al., 2019 Oct 1).
3. Discussion
The establishment of standardized therapeutic guidelines for the treatment of cervical cancer during pregnancy is still pending, resulting in using multiple therapeutic regimens (Amant et al., 2019 Oct 1, Babkova et al., 2024 Apr, Bernardini et al., 2022 Aug 14, Huang et al., 2021 Aug 13). At our onco-gynecological center, the treatment regimen of cisplatin with ifosfamide is routinely used for managing cervical cancer in pregnant patients. A recently published study detailed the association between this regime and a higher risk of developing fetal anemia, causing the necessity of chemotherapy postponing. Severe fetal anemia can lead to hydrops fetalis and, ultimately, fetal demise (Babkova et al., 2024 Apr, Barr JJ, Mari G. Cerebral Blood Flow Velocity Waveforms and Fetal Anemia. In: Maulik D, Lees CC, editors. Doppler Ultrasound in Obstetrics and Gynecology [Internet]. Cham: Springer International Publishing;, 2023). The presence of fetal anemia could potentially be associated with the administration of ifosfamide, a drug recognized for its notable myelotoxicity (Babkova et al., 2024 Apr, Gangireddy and Ifosfamide, 2023). The administration of cisplatin is linked to the risk of platinum-induced hearing loss in the fetus as the most severe side effect. The risk of ototoxicity increases with a higher total cumulative dose of cisplatin (Clemens E, van den Heuvel-Eibrink MM, Mulder RL, Kremer LCM, Hudson MM, Skinner R, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol [Internet]., 2019, Geijteman et al., 2014). In vitro studies have shown a relatively low transplacental transfer of cisplatin, a phenomenon that escalates with the progression of gestational age (Al-Saleh et al., 2008 Oct). Taking this information into account, the decision was made to implement three cycles of cisplatin combined with ifosfamide during the initial phase of treatment. Subsequently, a weekly regimen of paclitaxel with a carboplatin therapeutic approach was adopted to mitigate these negative effects and extend the duration of therapy for achieving a full-term pregnancy (Amant et al., 2019 Oct 1). Paclitaxel is a chemotherapeutic agent with minimal side effects and a low fetal plasma concentration (Van Calsteren, 2010). Carboplatin is known for its risk of fetal hematotoxicity, but has a lower risk of platinum-induced hearing loss (Clemens et al., 2019). Considering the scheduled timeframe of chemotherapy, specifically from the 21st week to the 35th week of pregnancy, we chose sequential administration to reduce the cumulative toxicity associated with individual regimens. To date, there is no evidence in the existing literature of the application of this combination and sequential regimen. Regarding our patient, we illustrate the benefits for both the patient and the fetus.
4. Conclusion
There is no universally accepted standardized regimen for cervical cancer treatment during pregnancy, and there are numerous potential chemotherapy agents for neoadjuvant chemotherapy during pregnancy. The primary goal is to achieve a satisfactory oncological outcome for the mother while simultaneously minimizing risks to the fetus, reducing treatment-related side effects, and, as in our specific case, prolonging the treatment duration to achieve term pregnancy. We propose a personalized treatment approach that sequentially administers two neoadjuvant chemotherapy regimens to attain the most favorable result.
Patient consent, ethics statement
The protocol received approval from the Institutional Review Board of Charles University in Prague and the University Hospital Kralovske Vinohrady (EK 259/08 on March 12, 2008, and EK VP/05/0/2019 on February 6, 2019). Before enrolling in the study, all participating patients provided informed consent by affixing their signatures to the requisite documentation. As part of their informed consent, all study participants were provided with information about the course of the study and the anonymous processing of results.
Funding and support
This study was supported by the Cooperation program, Maternal and Childhood Care 207035, 3rd Faculty of Medicine, Charles University.
CRediT authorship contribution statement
Anna Babkova: Writing – original draft, Project administration, Methodology. Helena Robova: Formal analysis. Hana Malikova: Visualization. Jana Drozenova: Formal analysis. Tomas Pichlik: Investigation. Michael J. Halaska: Supervision. Lukas Rob: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2024.101532.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al-Saleh E., Al-Harmi J., Nandakumaran M., Al-Shammari M. Transport kinetics of cisplatin in the perfused human placental lobule in vitro. J Matern Fetal Neonatal Med. 2008 Oct;21(10):726–731. doi: 10.1080/14767050802276542. [DOI] [PubMed] [Google Scholar]
- Amant F., Berveiller P., Boere I.A., Cardonick E., Fruscio R., Fumagalli M., et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol. 2019 Oct 1;30(10):1601–1612. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- Babkova A., Rob L., Kubecova M., Hruda M., Halaska M.J. Middle cerebral artery peak systolic velocity monitoring of fetal anemia during chemotherapy in pregnancy. Acta Obstet Gynecol Scand. 2024 Apr;103(4):660–668. doi: 10.1111/aogs.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JJ, Mari G. Cerebral Blood Flow Velocity Waveforms and Fetal Anemia. In: Maulik D, Lees CC, editors. Doppler Ultrasound in Obstetrics and Gynecology [Internet]. Cham: Springer International Publishing; 2023 [cited 2024 Jan 7]. p. 297–311. Available from: https://doi.org/10.1007/978-3-031-06189-9_18.
- Bernardini F., Ferrandina G., Ricci C., Fagotti A., Fanfani F., Cavaliere A.F., et al. Neoadjuvant Chemotherapy in Pregnant Patients with Cervical Cancer: A Monocentric Retrospective Study. Curr Oncol. 2022 Aug 14;29(8):5702–5714. doi: 10.3390/curroncol29080450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra GF, Derchain SFM, Bicalho DS, Filho AL da S, Primo WQSP. Cervical cancer in pregnancy. Rev Bras Ginecol Obstet [Internet]. 2023 Jun 20 [cited 2024 Aug 19];45(5):293–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10281765/. [DOI] [PMC free article] [PubMed]
- Clemens E, van den Heuvel-Eibrink MM, Mulder RL, Kremer LCM, Hudson MM, Skinner R, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol [Internet]. 2019 Jan [cited 2023 Dec 26];20(1):e29–41. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7549756/. [DOI] [PMC free article] [PubMed]
- de Haan J., Verheecke M., Van Calsteren K., Van Calster B., Shmakov R.G., Mhallem Gziri M., et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018 Mar;19(3):337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- Eibye S., Kjær S.K., Mellemkjær L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol. 2013 Sep;122(3):608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- Gangireddy M, Nookala V. Ifosfamide. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Dec 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK542169/.
- Geijteman ECT, Wensveen CWM, Duvekot JJ, van Zuylen L. A child with severe hearing loss associated with maternal cisplatin treatment during pregnancy. Obstet Gynecol. 2014 Aug;124(2 Pt 2 Suppl 1):454–6. [DOI] [PubMed]
- Halaska M.J., Komar M., Vlk R., Tomek V., Skultety J., Robova H., et al. A pilot study on peak systolic velocity monitoring of fetal anemia after administration of chemotherapy during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2014 Mar;174:76–79. doi: 10.1016/j.ejogrb.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Huang H., Quan Y., Qi X., Liu P. Neoadjuvant chemotherapy with paclitaxel plus cisplatin before radical surgery for locally advanced cervical cancer during pregnancy: A case series and literature review. Medicine (baltimore). 2021 Aug 13;100(32):e26845. doi: 10.1097/MD.0000000000026845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Calsteren K. Chemotherapy during pregnancy: pharmacokinetics and impact on foetal neurological development. Facts Views vis Obgyn. 2010;2(4):278–286. [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke T., Verheecke M., van Gerwen M., Van Calsteren K., Halaska M.J., Fumagalli M., et al. Child development at 6 years after maternal cancer diagnosis and treatment during pregnancy. Eur J Cancer. 2020 Oct;138:57–67. doi: 10.1016/j.ejca.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.