Abstract
Aim
Central liver resections are considered to be high‐risk procedures due to postoperative biliary complications. However, anatomical aspect‐related causes are underreported. Focusing upon right anterior sectionectomy (H58) and central bisectionectomy (H458), we assessed risk factors for postoperative biliary complications.
Methods
We retrospectively reviewed patients who underwent H58 or H458 in our hospital between April 2008 and June 2023 (n = 58). We conducted univariate and multivariate analysis of risk factors of postoperative biliary complications among perioperative factors and anatomical factors including the branching type of the right posterior hepatic duct (RPHD) and the length of the right hepatic duct (RHD).
Results
Twenty‐six patients (44.8%) had postoperative biliary complications. Potent risk factors in univariate analysis were the tumor proximity to the right anterior Glissonean branch and longer RHD (both P < 0.01). In multivariate analysis, longer RHD was the only independent risk factor and its hazard (95% confidence interval [CI] was 1.19 (1.05–1.35). Receiver operating characteristics curve (ROC) analysis and the area under the ROC showed that 10 mm was the optimal cutoff value with high discriminatory power (0.72). Considering intraoperative procedures of the right anterior segment Glissonean branch dissection, mass ligation at the second‐order branch had marginal risk, especially in patients with RHD >10 mm; its hazard (95% CI) was 5.83 (0.95–35.7).
Conclusion
Anatomical factors of RPHD and RHD influenced postoperative biliary complications in this cohort. The supraportal with RHD type was most common anatomy but considered to be hazardous if the RHD was >10 mm.
Keywords: biliary complications, biliary anatomy, liver resection
Biliary anatomical factors were shown in this study to influence biliary complications after central liver resection. Importantly, the supraportal with right hepatic duct type is the most common anatomy and can be hazardous if it is >10 mm in length.
1. INTRODUCTION
In liver surgery, postoperative biliary complications often prolong postoperative hospital stays and require additional interventions for management. Severe situations can become fatal. 1 , 2 , 3
Patient‐based risk factors for postoperative biliary complications include old age, lower body mass index (BMI), Child–Pugh B liver functional status, and history of preoperative chemotherapy. Also, certain types of major liver resection have been reported as high‐risk procedures for postoperative biliary complications; for example, central bisectionectomy or right anterior sectionectomy. 2 , 4 , 5 According to a nationwide survey based in Japan, compared with 7.2% in the overall cohort, there were higher incidences of postoperative biliary complications in these two procedures: 20% for central bisectionectomy and 11.3%, for right anterior sectionectomy. 5 These procedures will be summarized as procedures requiring broad exposure of the hilar Glissonean sheath, and this might be a cause of postoperative biliary complications. 6 However, there is a lack of studies of these high‐risk hepatectomy procedures with proper consideration of the biliary anatomical aspects in relation to postoperative biliary complications. 7 This study therefore aimed to investigate the risk factors of postoperative biliary complications with respect to anatomical aspects, tumor situation, and surgical procedures among high‐risk hepatectomy procedures for postoperative biliary complications.
2. METHODS
2.1. Study design
The protocol for this retrospective observational study was approved by the Wakayama Medical University Research Ethics Committee and it conforms to the provisions of the Declaration of Helsinki. The institutional approval number was #3612. The Wakayama Medical University Research Ethics Committee waived the requirement for informed patient consent. Instead, an opt‐out approach was used to obtain consent to participation. We reviewed the clinical records of patients who underwent central bisectionectomy or right anterior sectionectomy without biliary reconstruction in our hospital between April 2008 and June 2023.
2.2. Data collection and definition
We recorded the following preoperative parameters: age, ASA status, BMI, albumin (g/dL), prothrombin time (%), total bilirubin (mg/dL), tumor diagnosis (hepatocellular carcinoma/metastatic tumor/intrahepatic, cholangiocarcinoma/gallbladder carcinoma), maximum tumor size (cm), tumor proximity to the right anterior Glissonean pedicle (yes/no), anatomical variation of the right hepatic bile duct, 8 length of the right main hepatic duct (RHD, mm), liver resection types (right anterior sectionectomy; H58, central bisectionectomy; H458), 9 the type of procedures when cutting the right anterior Glissonean branch (mass ligation at second‐order branch/individual ligation at third‐order branches), use of a stapler device when cutting the right anterior Glissonean branch (yes/no), performance of bile leak test (yes/no), intraoperative bile duct suture repair (yes/no), operating time (min), blood loss (mL), postoperative biliary complications (yes/no), the Clavien–Dindo classification grade, 10 and the length of postoperative hospital stay (days). We defined bile leakage, bile duct stricture, and formation of biloma as postoperative biliary complications. Bile leakage was confirmed according to the International Study Group of Liver Surgery guidelines. 11 Postoperative bile duct stricture and biloma formation were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) 7 d postoperatively and again at around 6 mo postoperatively. Their severity was evaluated by Clavien–Dindo classifications. 10 In this study, all grades of severity were recorded as outcome events.
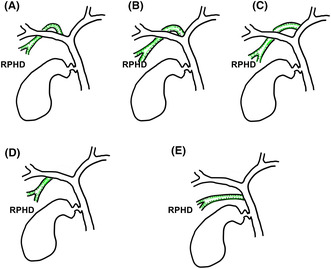
To assess anatomical variation of the right hepatic duct branches, our hospital routinely performed a preoperative MRI study, so we used hepatocyte images of gadoxetate disodium (Primovist, Bayer Yakuhin, Osaka, Japan) enhanced MRI or magnetic resonance cholangiopancreatography images. Patients who did not undergo MRI were therefore excluded from our analysis. To classify the RPHD variation, the criteria reported by Ohkubo et al were used in this study as follows: supraportal type with RHD (type A), supraportal type without RHD (type B; trifurcation type, type C; RPHD jointing left hepatic duct type), infraportal type with RHD (type D), infraportal type without RHD (type E) (Figure 1 ). 8 The RHD length was measured between bifurcation of the common hepatic duct and the right posterior hepatic duct branch (RPHD). 7 In case of RHD absent anatomy type (types B, C, and E), the length was defined as 0 mm.
FIGURE 1.
Variation of right posterior hepatic duct anatomy. (A) Supraportal type with right hepatic duct; type A. (B) Supraportal type without right hepatic duct (trifurcation type); type B. (C) Supraportal type without right hepatic duct (RPHD jointing left hepatic duct type); type C. (D) Infraportal pattern with RHD; type D. (E) Infraportal type without RHD; type E. Other combined types or rare branching types were excluded from this cohort.
Regarding tumor proximity to the right anterior Glissonean branch, we defined it as nearly in contact with the root of the right anterior Glissonean branch (<3 mm). Values of albumin, bilirubin, and prothrombin time were binarized based on cutoff values of 3.5 g/dL, 2.0 mg/dL, and 80%, respectively.
2.3. Statistics
Continuous values were expressed as median (25th and 75th percentiles). Statistical significance was determined by a two‐sided Mann–Whitney U test for continuous variables. Categorical variables were evaluated by a two‐sided chi‐squared test or Fisher's exact probability test, as appropriate.
In evaluating risk factors for postoperative biliary complications, we performed univariate and multivariate logistic regression analysis. Variables with P < 0.1 by univariate analysis were considered potent risk factors and were entered into multivariate analysis. Moreover, receiver operating characteristic (ROC) curve analysis was added for significant continuous risk variables. The area under the ROC curves (AUROC) were calculated and the optimal cutoff value was determined according to the Youden index. Selected risk variables were scored and a Cochran–Armitage test was performed to assess for the presence of trend association between risk scores and incidence of biliary complications. Regarding prognostic analysis, disease‐free survival was evaluated in patients with and without biliary complications by the Kaplan–Meier method and the difference was estimated by a log‐rank test. All P values were two‐tailed and P < 0.05 was considered statistically significant. All analyses were performed using JMP Pro 16 (SAS Institute, Cary, NC, USA).
3. RESULTS
During the surveyed period, 69 patients underwent H58 or H458. Of them, 11 patients were excluded from analysis due to biliary tumor thrombus requiring biliary duct repair or reconstruction (n = 3), lack of MRI study (n = 5), or due to rare biliary duct anomaly, such as a right‐sided round ligament (n = 3). As a result, 58 patients who underwent H58 (n = 33) and H458 (n = 25) were enrolled in analysis. Among these patients, there were 15 bile leakages, 12 postoperative RPHD strictures, and we observed 15 biloma formations. Twenty‐four patients (41.4%) had these biliary complications. As for severity, Grade 3 or more biliary complications were observed in 14 patients (24.1%) (Table 1).
TABLE 1.
Anatomical patterns of right posterior hepatic duct branching and biliary complications.
| Anatomy of RPHD | Supraportal type | Infraportal type | |||
|---|---|---|---|---|---|
| RHD+ | RHD− | RHD+ | RHD− | ||
| Type A | Type B | Type C | Type D | Type E | |
| Number of patients | 39 | 9 | 5 | 1 | 4 |
| RHD length, mm | 12.0 (7.0, 15.0) | 0 | 0 | 12.0 | 0 |
| Patients with biliary complications | 19 | 4 | 0 | 1 | 0 |
| RPHD stricture | 9 | 2 | 0 | 1 | 0 |
| Bile leakage | 12 | 2 | 0 | 1 | 0 |
| Biloma formation | 10 | 4 | 0 | 1 | 0 |
Abbreviations: RHD, right hepatic duct; RPHD, right posterior hepatic duct.
3.1. Distribution of anatomical patterns of right posterior hepatic duct branching
The relationships between the RPHD anatomy and incidence of biliary complications are shown in Table 1. The most common anatomy was type A (n = 39, 67.2%) followed by type B (n = 9, 15.5%), type C (n = 5, 8.6%), type E (n = 4, 6.9%), and then type D (n = 1, 1.7%). Regarding the RHD length, the median value was 12.0 mm among those with type A anatomy. Among the 24 patients who had postoperative biliary complications, 19 had type A anatomy (79.2% of those with complications).
3.2. Baseline characteristics of patients with and without postoperative biliary complications
Baseline characteristics of this cohort are shown in Table 2. The most common disease in this cohort was hepatocellular carcinoma (n = 37), followed by colorectal liver metastasis (n = 13), intrahepatic cholangiocarcinoma (n = 6), and gall bladder cancer (n = 2). As for tumor location, 25 patients (43.1%) had a tumor proximal to the right anterior Glissonean branch. Liver function was well preserved and all patients had Child–Pugh grade A liver functional status. There were significant differences between patients with and without postoperative biliary complications in tumor proximity, RPHD branching type, presence of RHD, RHD length, and postoperative hospital stays.
TABLE 2.
Baseline characteristics of patients in our cohort with and without postoperative biliary complications.
| With postoperative biliary complications (n = 24) | Without postoperative biliary complications (n = 34) | P Value | |
|---|---|---|---|
| Age, years old | 74 (67, 80) | 70 (66, 76) | 0.27 |
| Gender, male/female | 16/8 | 26/8 | 0.41 |
| ASA physical status, 3/2 and 1 | 7/17 | 5/29 | 0.18 |
| BMI, kg/m2 | 22.7 (19.5, 24.1) | 22.3 (20.6, 24.7) | 0.97 |
| Albumin, g/dL >/≤3.5 g/dL | 21/3 | 32/2 | 0.64 |
| Total bilirubin, mg/dL </≥2 mg/dL | 24/0 | 34/0 | |
| Prothrombin time, >/≤80% | 19/5 | 33/1 | 0.72 |
| Child–Pugh grade, A/B | 24/0 | 34/0 | NE |
| Diagnosis, HCC/Met/ICC/GBCa | 17/4/3/0 | 20/9/3/2 | 0.47 |
| Tumor size, cm | 6.1 (3.0, 10.0) | 5.0 (4.0, 6.6) | 0.27 |
| Tumor proximity, yes/no | 15/9 | 10/24 | 0.01 |
| RPHD branching type a , type A/B/C/D/E | 19/4/0/1/0 | 20/5/5/0/4 | 0.02 |
| Supraportal type, yes/no | 23/1 | 30/4 | 0.39 |
| RHD, +/− | 20/4 | 20/14 | 0.08 |
| RHD length, mm | 12 (7, 17) | 5 (0, 11.6) | <0.01 |
| Surgical procedures, H458 /H58 b | 11/13 | 14/20 | 0.72 |
| Abdominal approach, laparoscopic/open | 1/23 | 3/31 | 0.34 |
| Dissecting procedures of the right anterior Glissonean branch, mass ligation at second‐order branch/ individual ligation at third‐order branches | 21/3 | 23/11 | 0.12 |
| Use of a stapler device during cutting the right anterior Glissonean branch, yes/no | 10/12 | 14/22 | 0.62 |
| Bile leakage test, yes/no | 18/6 | 23/11 | 0.54 |
| Intraoperative bile duct repair, yes/no | 14/10 | 12/22 | 0.08 |
| Operating time, min | 388 (325, 539) | 399 (343, 454) | 0.75 |
| Blood loss, mL | 518 (309, 867) | 405 (165, 1119) | 0.36 |
| Postoperative hospital stays, days | 22 (13, 42) | 13 (11, 16) | <0.01 |
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; Met, metastatic liver tumor; ICC, intrahepatic cholangiocarcinoma; GBCa, gall bladder carcinoma; RPHD, right posterior hepatic duct; RHD, right hepatic duct; NE, not estimated.
Defined by Ohkubo et al. 8
Defined by Nagino et al. 9
3.3. Risk factor analysis of postoperative biliary complications
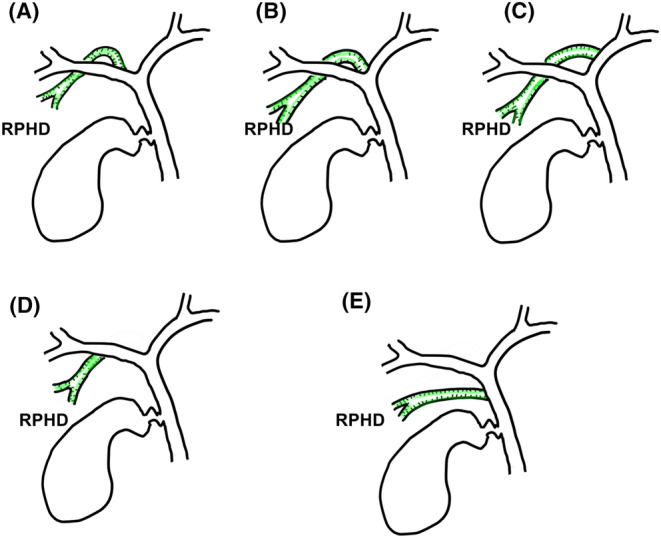
Univariate and multivariate risk factor analysis of postoperative biliary complications are shown in Table 3. In univariate analysis, longer RHD length (P < 0.01), and tumor proximity to the right anterior Glissonean pedicle (P = 0.01) were selected as significant risk factors. Multivariate analysis revealed that longer RHD length was the only independent risk factor, its hazard (95% confidence interval, CI) was 1.19 (1.05–1.35). Next to it, although without significance, mass ligation at the second‐order branch had a marginal risk and its hazard (95% CI) was 6.29 (0.84–46.9). To optimize the hazardous RHD length for biliary complications, we performed ROC curve analysis (Figure 2 ). The AUROC was 0.78, and the optimal cutoff value was determined to be 10 mm.
TABLE 3.
Risk factor analysis of postoperative biliary complications.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazards (95% CI) | P Value | Hazards (95% CI) | P Value |
| Age, per 1 year | 1.02 (0.97–1.07) | 0.41 | ||
| Gender, male/female | 0.62 (0.19–1.97) | 0.41 | ||
| ASA, 3/2, 1 | 2.39 (0.65–8.71) | 0.18 | ||
| BMI, per 1 kg/m2 | 1.04 (0.92–1.19) | 0.49 | ||
| Albumin, ≤/>3.5 g/dL | 2.29 (0.07–2.84) | 0.38 | ||
| Prothrombin time, ≤/>80% | 8.68 (0.94–79.9) | 0.06 | 8.91 (0.71–111.9) | 0.09 |
| Tumor size, per 1 cm | 1.15 (0.99–1.33) | 0.06 | 1.06 (0.85–1.32) | 0.59 |
| Tumor proximity, yes/no | 4.00 (1.32–12.1) | 0.01 | 2.08 (0.48–8.95) | 0.33 |
| Supraportal type, yes/no | 3.07 (0.32–29.3) | 0.33 | ||
| RHD length, per 1 mm | 1.15 (1.05–1.27) | <0.01 | 1.19 (1.05–1.35) | <0.01 |
| Surgical procedures, H458/H58 a | 1.21 (0.42–3.47) | 0.72 | ||
| Abdominal approach, laparoscopic/open | 0.45 (0.04–4.60) | 0.50 | ||
| Dissecting procedures of the right anterior Glissonean branch, mass ligation at second‐order branch/individual ligation at third‐order branches | 3.34 (0.82–13.7) | 0.09 | 6.29 (0.84–46.9) | 0.07 |
| Intraoperative bile leakage test, yes/no | 1.43 (0.45–4.62) | 0.55 | ||
| Bile duct repair suture, yes/no | 2.56 (0.88–7.51) | 0.09 | 0.89 (0.20–4.03) | 0.89 |
| Operating time, per 1 h | 1.12 (0.86–1.45) | 0.40 | ||
| Blood loss, per 100 mL | 1.67 (0.10–27.9) | 0.72 | ||
Abbreviations: BMI, body mass index; CI, confidence interval; RHD, right hepatic duct.
Defined by Nagino et al. 9
FIGURE 2.
Receiver operating characteristics curve between right hepatic duct length and postoperative biliary complications.
3.4. Relationship between the type of procedures in cutting the right anterior Glissonean branch and postoperative biliary complications in high‐risk situations
Dissection procedures of the right anterior Glissonean branch could be a possible contributing factor that can be intervenable during surgery. Mass ligation at the second‐order branch had marginal risk for biliary complications, so we compared the incidence of biliary complications between these two procedures under the high‐risk situation (RHD > 10 mm).
Among patients with RHD > 10 mm (n = 26), postoperative biliary complications were observed in 14 of the patients (77.8%) who underwent mass ligation at the second‐order branch. Three patients (37.5%) underwent individual ligation at third‐order branches (P = 0.08 by Fisher's exact test). In that situation, the risk ratio (95% CI) of mass ligation at the second‐order branch on postoperative biliary complications was 5.83 (0.95–35.7).
Additionally, we assessed the influence of the use of a stapling device on biliary complications when cutting the right anterior Glissoenan pedicle. There were 22 patients with a stapler device used for cutting, while other patients underwent ligation and cut (n = 33) or cut and hand‐sewn suture (n = 3). In most of the patients in whom a stapler device was used, there was mass ligation at the second‐order branch (n = 21). Among the patients with mass ligation (n = 44), the incidence of biliary complications with a stapler device was 47.6% (10/21) and 47.8% (11/23) without the use of a stapler device (P = 0.99). The use of a stapler device did not therefore influence biliary complications during mass ligation of the right anterior Glissonean pedicle.
However, focusing on the biliary stricture in patients with RHD > 10 mm (n = 26), its incidence with or without a stapler device was 61.5% (8/13) or 7.7% (1/13), respectively (P < 0.01). In patients with RHD ≤ 10 mm, it was 0% with a stapler device (0/9) and 13% without a stapler device (3/23) (P = 0.54).
3.5. Prognostic outcomes in patients with and patients without biliary complications
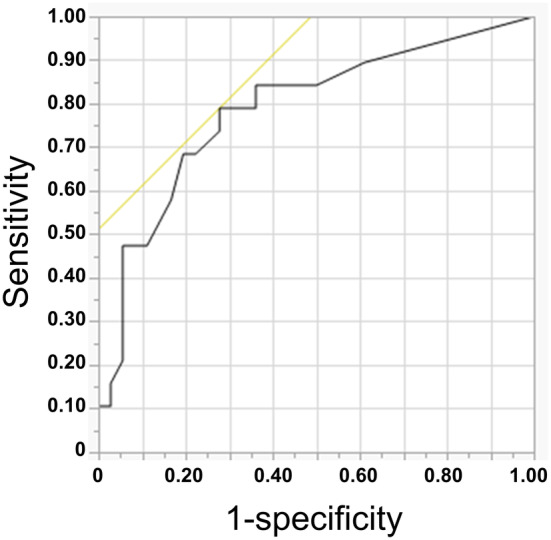
Disease‐free interval was estimated in patients with and without biliary complications (Figure 3A). There were no significant differences between the two groups. The median disease‐free survival periods (95% CI) were 10.8 (4.7–34.1) and 10.8 (5.4–29.8) mo in patients with and without biliary complications, respectively (P = 0.99). Regarding overall survival (Figure 3B), median survival periods (95% CI) were 42.4 (17.7–88.0) and 44.2 (26.5–not reached) mo, respectively (P = 0.60).
FIGURE 3.
Disease‐free survival curves (A) and overall survival curves (B) of patients with (dotted line) and without (solid line) postoperative biliary complications.
4. DISCUSSION
Among patients who underwent anterior sectionectomy or central bi sectionectomy, an independent risk factor for postoperative biliary complications was a long RHD. Moreover, considering a hazardous RHD length, 10 mm was calculated as the optimal cutoff value. The RHD length depends on the anatomy of RPHD branching type, so anatomical factors were thought to influence postoperative biliary complications in our cohort.
In general, right anterior sectionectomy or central bisectionectomy are considered to be difficult liver surgery procedures due to complicated resection lines and the need for exposure of the hilar plate, resulting in a high incidence of postoperative biliary complications. 5 , 12 Our subjective cohort was therefore originally considered to be at high risk for postoperative biliary complications. However, the reason for postoperative biliary complications in relation to biliary anatomy has not been widely indicated. The current study revealed that longer RHD length, an anatomical factor, was an influential risk factor.
As all‐severity of Clavien–Dindo classifications was included in outcome measurements, the incidence of postoperative biliary complications was as high as 44.8% in this cohort. However, when limited to severity of grade 3 or more, the incidence in 14 patients (24.1%) was similar to that of previous reports. 5
Biliary stricture will be the most troublesome to manage of the biliary complications because it is sometimes combined with refractory bile leak or cholangitis. Twelve patients in this cohort had RPHD stricture. Among them, four patients were asymptomatic and were simply observed without any interventions. Among the eight symptomatic patients, six underwent endoscopic retrograde cholangiography (ERC). Just two of these patients could achieve selective drainage tube insertion to the RPHD and could be treated by successful drainage management, while it failed in four patients. These four patients and the remaining two patients who did not receive ERC were treated by long‐lasting percutaneous drainage management until bile leakage was stopped or reduced. Assessment of the risk factors and performance of preventative measures against postoperative biliary complications are therefore required.
Patient factors including sarcopenia, lower BMI, and Child–Pugh class B cirrhosis or surgical factors including repeat liver resection and prolonged surgery have been reported as risk factors for postoperative biliary complications. 2 , 13 , 14 , 15 However, our cohort was limited to patients who undergone complicated anatomical liver resection; all patients had well‐preserved liver function and moderate BMI and also originally prolonged surgical procedures, and there were no cases of repeated liver resection. Reported patient and surgical factors might not therefore have been identified as risks in this cohort.
As for postoperative right posterior hepatic duct stricture, 12 of our patients (20.7%) had this complication and it was more frequent in the group with >10 mm length of RHD (P = 0.02 by Fisher's exact test). Concerning this point, a previous report had similar results to ours and in their cohort type A anatomy and an RHD length of 13 mm were risk factors. 7 Although in our cohort patients with type A anatomy (n = 9) also dominantly had risk anatomy, some patients with type B (n = 2) and D (n = 1) had biliary stricture. Different from the previous report, we measured all‐grade severity of biliary stricture, so there may be a slight difference in the results. When limiting to severity of biliary stricture grade 3 or more (n = 8), seven patients had the type A anatomy and it might be said that the results are similar to those in the previous report.
In addition to this evidence, we found significantly high correlation between incidence or RPHD stricture and bile leakage, and we consider it to be a risk factor for postoperative bile leakage.
Considering causes of postoperative bile leakage, three patterns of bile leakage have been reported: peripheral type, completely disconnected type, and partially disconnected type. 16 Although it was difficult to identify the site of bile leakage in all cases in this cohort, we interpreted that any injury of the root of the RPHD might increase internal pressure of the peripheral RPHD site and result in peripheral bile leakage from the right posterior sectional surface. A significantly high incidence of bile leakage was therefore observed in patients with RPHD stricture in our cohort.
Considering three‐dimensional anatomy in patients with longer RHD and type A RPHD anatomy, the bifurcation of the right posterior and anterior hepatic duct would locate more distally in the Glissonean sheath. When ligating the right anterior Glissonean sheath, RPHD might be entrapped in the ligation and possibly lead to postoperative RPBD stricture, bile leakage, and formation of biloma.
We therefore supposed that dissecting procedures of the right anterior Glissonean pedicle might have influence and we entered these procedural factors into risk analysis (mass ligation at second‐order branch/individual ligation at third‐order branches). Although this was not selected as an independent risk factor, it was shown to be a marginal hazard in univariate analysis, especially when a patient had RHD > 10 mm.
Moreover, we assessed the influence of the use of a stapler device on postoperative biliary complications. Although there was no significant difference in the incidence of total biliary complications, the occurrence of biliary stricture was significantly high when using a stapler device, especially in patients with RHD > 10 mm. A stapler device may create much more compression of the surrounding tissue compared with conventional ligation and cutting procedures, and this might result in biliary stricture. 17
When performing right lobectomy, Katagiri et al recommended that the anterior and posterior sectional Glissonean branches should be dissected and ligated individually (rather than dissecting and ligating the right main Glissonean pedicle) due to concerns about injuries to the left main bile duct branch. 18 This would be the same in our study. The technical ease would depend on the tumor proximity to the right anterior Glissonean pedicle. However, if there is a sufficient tumor margin between the root of the right anterior Glissonean pedicle, it would be better to perform peripheral dissection.
Moreover, if there is a risky situation for biliary complications, preventative measures must be taken against it. Before cutting the right anterior Glissonean pedicle, there are two such measures. One is the intraoperative cholangiography. Although it needs a fluoroscopy system and might be troublesome, it will be a reliable measure to assess biliary injuries. The air leak test is another preventive measure, which will usually be used for detection of bile leaks. At the same time, by using ultrasonography, the communication of the air bubble to the RPHD can be checked and it is possible to assess the biliary stricture. In this cohort, we did not routinely perform intraoperative cholangiography, but we did an air bubble test and/or dye injection test to check for bile leaks. Ultrasonography was not routinely used for checking the communication of the air bubble to the RPHD in this cohort and we did not perform these measures during laparoscopic procedures. Laparoscopic insertion of the contrast injection tube to the biliary duct may be troublesome, so preoperative tube insertion to the RPHD under endoscopic retrograde cholangiography may be to some extent helpful in a risk situation for biliary complications.
This study has some limitations. First, it was a single‐institutional study on a comparatively small scale. Our results might therefore be biased, so confirmation of our results in a prospective multi‐institutional study with a larger sample size is required. Second, although there was no association between postoperative biliary complications and prognostic outcomes, sufficient sample size and a follow‐up period are required to confirm the results.
In conclusion, anatomical factors of RPHD branching type had an influence on postoperative biliary complications in patients who underwent H58 or H458. The supraportal with RHD type was the most common anatomy and also was deemed to be hazardous if the RHD length was >10 mm. Preoperative evaluation of RPHD anatomy is essential for performance of right anterior sectionectomy or central bisectionectomy.
AUTHOR CONTRIBUTIONS
Masaki Ueno: conception and design of the work, analysis, and drafting the work. Shinya Hayami: data acquisition, and revising. Atsushi Miyamoto: data analysis, and revising. Ken‐ichi Okada: interpretation of data for the work, and revising. Yuji Kitahata: data acquisition, and revising. Atsushi Shimizu: data acquisition, and revising. Hideki Motobayashi: data acquisition, and revising. Kyohei Matsumoto: data acquisition, and revising. Manabu Kawai: conception of the work, and revising. All authors approved the version to be published. Also, all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FUNDING INFORMATION
There is no funding or financial support to declare in relation to this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENTS
Approval of the research protocol: This study protocol was reviewed and approved by the Wakayama Medical University Research Ethics Committee, approval number 3612. It conforms to the provisions of the Declaration of Helsinki.
Informed Consent: As this was a retrospective study Wakayama Medical University Research Ethics Committee waived the requirement for informed patient consent. An opt‐out approach was used in lieu of consent to participate.
Registry and Registration Number: N/A.
Animal Studies: N/A.
ACKNOWLEDGMENTS
We thank Benjamin Phillis from the Clinical Study Support Center at Wakayama Medical University for proofreading and editing this article.
Ueno M, Hayami S, Miyamoto A, Okada K‐i, Kitahata Y, Shimizu A, et al. Relationship between postoperative biliary complications and biliary anatomical aspects in performing right anterior‐ or central bisectionectomy: Single‐center retrospective observational study. Ann Gastroenterol Surg. 2024;8:1076–1083. 10.1002/ags3.12805
REFERENCES
- 1. Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakano R, Ohira M, Kobayashi T, Imaoka Y, Mashima H, Yamaguchi M, et al. Independent risk factors that predict bile leakage after hepatectomy for hepatocellular carcinoma: cohort study. Int J Surg. 2018;57:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Gorgec B, Cacciaguerra AB, Aldrighetti LA, Ferrero A, Cillo U, Edwin B, et al. Incidence and clinical impact of bile leakage after laparoscopic and open liver resection: an international multicenter propensity score‐matched study of 13,379 patients. J Am Coll Surg. 2022;234(2):99–112. [DOI] [PubMed] [Google Scholar]
- 4. Riediger C, Hoffmann R, Lock S, Giehl‐Brown E, Dennler S, Kahlert C, et al. Novel personalized score predicts risk for postoperative biliary leak in liver surgery‐a retrospective database analysis. J Gastrointest Surg. 2022;26(10):2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamashita YI, Yamamoto H, Miyata H, Kakeji Y, Kitagawa Y, Yamaue H, et al. Risk factors for bile leakage: latest analysis of 10 102 hepatectomies for hepatocellular carcinoma from the Japanese national clinical database. J Hepatobiliary Pancreat Sci. 2021;28(7):556–562. [DOI] [PubMed] [Google Scholar]
- 6. Nakagawa K, Tanaka K, Nojiri K, Sawada Y, Kumamoto T, Ueda M, et al. Predictive factors for bile leakage after hepatectomy for hepatic tumors: a retrospective multicenter study with 631 cases at Yokohama clinical oncology group (YCOG). J Hepatobiliary Pancreat Sci. 2017;24(1):33–41. [DOI] [PubMed] [Google Scholar]
- 7. Yoon KC, Yu YD, Kang WH, Jo HS, Kim DS. Right posterior bile duct stricture after central bisectionectomy or anterior sectionectomy. Langenbeck's Arch Surg. 2022;407(7):2873–2880. [DOI] [PubMed] [Google Scholar]
- 8. Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, Arai T, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239(1):82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagino M, DeMatteo R, Lang H, Cherqui D, Malago M, Kawakatsu S, et al. Proposal of a new comprehensive notation for hepatectomy: the "New World" terminology. Ann Surg. 2021;274(1):1–3. [DOI] [PubMed] [Google Scholar]
- 10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the international study Group of Liver Surgery. Surgery. 2011;149(5):680–688. [DOI] [PubMed] [Google Scholar]
- 12. Ueno M, Kawai M, Hayami S, Hirono S, Okada KI, Uchiyama K, et al. Partial clamping of the infrahepatic inferior vena cava for blood loss reduction during anatomic liver resection: a prospective, randomized, controlled trial. Surgery. 2017;161(6):1502–1513. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi H, Shimizu A, Kubota K, Notake T, Masuo H, Yoshizawa T, et al. Impact of sarcopenic obesity on post‐hepatectomy bile leakage for hepatocellular carcinoma. PLoS One. 2023;18(10):e0286353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shehta A, Farouk A, Said R, Nakeeb AE, Aboelenin A, Elshobary M, et al. Bile leakage after hepatic resection for hepatocellular carcinoma: does it impact the short‐ and long‐term outcomes? J Gastrointest Surg. 2022;26(10):2070–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadamori H, Yagi T, Shinoura S, Umeda Y, Yoshida R, Satoh D, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg. 2013;100(1):122–129. [DOI] [PubMed] [Google Scholar]
- 16. Murata J, Shigekawa M, Ishii S, Suda T, Ikezawa K, Hirao M, et al. Efficacy and associated factors of endoscopic transpapillary drainage for postoperative biliary leakage. DEN Open. 2024;4(1):e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hori Y, Ueno M, Miyamoto A, Hayami S, Okada KI, Kitahata Y, et al. Right posterior bile duct stricture after laparoscopic anterior sectionectomy with long right bile duct: a case report. Asian J Endosc Surg. 2023;16(3):636–639. [DOI] [PubMed] [Google Scholar]
- 18. Katagiri S, Ariizumi S, Kotera Y, Takahashi Y, Yamamoto M. Right hepatectomy using Glissonean pedicle transection method with anterior approach (with video). J Hepatobiliary Pancreat Sci. 2012;19(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


