Summary
Background
Essential tremor (ET) significantly impacts patients' daily lives and quality of life, presenting a considerable challenge in clinical practice. In recent years, novel therapeutic regimens have been investigated in randomized controlled trials (RCTs). This study aims to investigate and evaluate the relative efficacy and safety of various therapeutic interventions for ET.
Methods
We did a systematic review and Bayesian Model-based Network Meta-analysis (NMA) of RCTs. Following PRISMA-NMA guidelines, a comprehensive database search was conducted up to April 1, 2024 to identify RCTs focused on ET treatments. The Bayesian Markov Chain Monte Carlo (MCMC) method was utilized for the analysis, evaluating the relative efficacy and safety of treatments using standardized mean difference (SMD) and log odds ratios (log ORs), respectively. Additionally, the Surface Under the Cumulative Ranking Curve (SUCRA) was applied to assess the relative efficacy of the treatment modalities. PROSPERO registration: CRD42023415752.
Findings
This study included 33 RCTs involving 1251 patients, covering 19 oral medication treatments and six non-oral medication treatments. NMA showed that deep brain stimulation (DBS) (SMD = −4.93; 95% CI: [−7.73, −2.13]), CX-8998 (SMD = −2.69; 95% CI: [−5.26, −0.14]), atenolol (SMD = −2.36; 95% CI: [−4.70, −0.10]), and propranolol (SMD = −1.59; 95% CI: [−2.25, −0.67]) showed relative efficacy compared to placebo, with DBS demonstrating relative efficacy compared to 15 other treatment methods. However, GRADE assessment indicated that the evidence level for these conclusions was “low” or “very low.” According to SUCRA rankings, DBS (0.97) ranked first in relative efficacy, followed by CX-8998 (0.80), thalamotomy (0.79), atenolol (0.76), metoprolol (0.66), propranolol (0.64), magnetic resonance guided focus ultrasound (MR-FUS) (0.624), ICI-118551 (0.620), nimodipine (0.61) and phenobarbitone (0.59). In terms of safety, as a network graph could not be constructed, DBS and thalamotomy were excluded from the NMA, while other effective treatments showed no significant differences in safety compared to placebo.
Interpretation
Our study results indicate that CX-8998, propranolol, and atenolol demonstrate relative efficacy and safety in treating ET. DBS is effective for medication-resistant ET and ranks first in relative efficacy, though our NMA lacks safety data for DBS. Given the low overall grade of evidence, these results should be applied cautiously in clinical practice. Further large-scale, head-to-head RCTs are needed.
Funding
This work was supported by grants from the National Nature Science Foundation of China (Grant No. 82271459).
Keywords: Essential tremor, Treatment, Efficacy, Safety, Network meta-analysis, RCTs
Research in context.
Evidence before this study
The management of essential tremor (ET) includes a diverse array of treatment modalities, but the optimal treatment approach and the relative efficacy and safety of these options remain uncertain. Most randomized controlled trials (RCTs) and pairwise meta-analysis typically compare a single treatment with a placebo, lacking direct comparisons between different treatment options. Network meta-analysis (NMA) provides an effective strategy to address this issue. We systematically searched MEDLINE, Web of Science, Embase, Scopus, and the Cochrane Central Register of Controlled Trials up to April 1, 2024, using terms “Essential tremor” “random∗” “control∗” “treat∗” “therapy” “Deep Brain Stimulation” “Thalamotomy” “Focused Ultrasound” “Botulinum Toxins” “Propranolol” “Primidone” “Topiramate” “Gabapentin” “Alprazolam” “Clonazepam” “Zonisamide” “Olanzapine” “Clozapine” and “Nimodipine”.
Added value of this study
Our analysis covered 33 RCTs, involving 1251 participants and 25 different treatment methods. The results showed that deep brain stimulation (DBS), CX-8998, atenolol, and propranolol demonstrated relative efficacy compared to placebo, with DBS outperforming 15 other treatment methods. Regarding safety, DBS could not be evaluated due to insufficient data, while the other four effective treatments showed no significant differences compared to placebo.
Implications of all the available evidence
This study conducted a comprehensive comparison of the relative efficacy and safety of various treatment methods for ET through a systematic review and NMA. The findings provide valuable information for clinical guidelines and support the decision-making process between patients and clinicians, helping to select the most appropriate treatment for ET patients.
Introduction
Essential tremor (ET) is a prevalent movement disorder, affecting approximately 1% of the global population, with incidence rates rising to 4%–5% among individuals aged 65 and older.1,2 While most ET patients initially exhibit mild symptoms that progressively worsen, it is estimated that 30%–50% will eventually experience more severe manifestations.3,4 In such cases, the severity of hand and limb tremors escalates markedly, profoundly impairing their ability to perform fine motor tasks like writing and holding objects. This escalation not only diminishes their quality of life but also significantly hinders their work capabilities, leading to broader impacts on their daily living and independence.5
The management of ET includes a diverse array of treatment modalities, broadly categorized into medication treatment, surgical treatment, and non-medication non-surgical treatment.6 Before initiating treatment, patients usually experience a prolonged course of the disease. Once treatment begins, patients typically start with oral medication therapy.7 In medication therapy, according to guidelines established by the American Academy of Neurology (AAN) and the Italian Movement Disorders Association (IMDA), propranolol and primidone are the preferred prescriptions, with medications like gabapentin, topiramate, and benzodiazepines are also frequently utilized.6,8, 9, 10 However, it is important to note that only about half of the patients experience a significant reduction in tremor severity with these medications.11 When pharmacological treatments are ineffective or have significant side effects, patients may gradually transition to invasive treatment methods, such as deep brain stimulation (DBS).7,12 However, due to the lack of Level I evidence, DBS has only received a ‘C' recommendation for the treatment of ET.13,14 Furthermore, recent advancements like magnetic resonance guided focus ultrasound (MR-FUS) have gained traction in research, presenting a novel therapeutic possibility for ET management.14,15
Given the plethora of recent randomized controlled trials (RCTs) concerning the treatment of ET, it is imperative to update existing treatment guidelines. Traditional RCTs typically focus only on comparing a single treatment modality against a placebo, lacking direct comparative analysis among various treatment options. These limitations significantly restrict a comprehensive understanding of the efficacy and safety of interventions for ET. Network Meta-Analysis (NMA) provides an effective strategy to overcome these deficiencies. By integrating data from multiple RCTs, NMA enables a detailed assessment of the relative efficacy and safety of different treatment options. This study aims to establish a more comprehensive and reliable clinical decision-making framework through a systematic review combined with NMA, thereby enhancing the accuracy of evaluations regarding treatment efficacy and safety.
Methods
The NMA was executed in adherence to the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA) guidelines. Additionally, this study was prospectively registered with PROSPERO (CRD42023415752).
Search strategy
A comprehensive literature search was performed across MEDLINE, Web of Science (WOS), Embase, Scopus, and the Cochrane Central Register of Controlled Trials, starting from inception to April 1, 2024, without language restrictions. The search strategy was developed through a comprehensive combination of Medical Subject Headings and free text, focused on “essential tremor” AND (“random∗” OR “control∗”) AND (“treat∗” OR “therapy” OR “Deep Brain Stimulation” OR “Thalamotomy” OR “Focused Ultrasound” OR “Botulinum Toxins” OR “Propranolol” OR “Primidone” among others). The strategy details, including specific terms used, are outlined in Supplementary Table S1. We further ensured comprehensiveness by screening references of relevant reviews.
Selection criteria
Titles and abstracts were screened before the assessment of full texts to determine the eligibility. We restricted inclusion to double-blind RCTs to increase methodological rigor and minimize biases, especially as placebo was part of the NMA.16
Studies were considered eligible if they met the following criteria (a) Population: diagnosed with ET based on clinical history and examination by a neurologist specializing in movement disorders, or according to the diagnostic criteria of the Movement Disorder Society17; (b) Interventions: use of any type of therapy as an intervention; (c) Comparisons: studies should include either a control group without treatment or a comparison group with different treatments; (d) Outcomes: at least one objective or observer-based continuous tremor measurement must be reported to assess the improvement in tremor after treatment (e.g., the Fahn-Tolosa-Marin Tremor Rating Scale [TRS], the Tremor Research Group Essential Tremor Rating Assessment Scale [TERAS], tremor intensity, or other physician assessments); (e) Study design: double-blind RCTs. Exclusion criteria were as follows: (a) study not specifically targeting ET, such as those involving ET and Parkinson's Disease, where ET data cannot be clearly distinguished; (b) missing data or data that could not be extracted; (c) the sample size of the original study experimental group was ≤10. In cases where multiple studies report the same or overlapping patient data, the most recent and comprehensive studies of patient populations are included.
Data extraction
Two reviewers (JZ and YR) independently extracted the required data. From each study, we extracted: (a) study characteristics (author's first name, year of publication, country, sample size, follow-up duration); (b) patient characteristics (mean age, gender ratio, duration of onset); (c) outcome measures (interventions, types and values of outcome measurements, incidence of adverse effects [AEs], and the discontinuation rate due to AEs). For qualitative results reported graphically, we obtained quantitative results through GetData software. If data are not available, we contact the author directly to request more information.
Data synthesis
To ascertain efficacy, we utilized the standardized mean difference (SMD) along with 95% confidence intervals (CIs), quantifying improvements in patients’ tremors or symptoms. For assessing treatment safety, the logarithm of the Odds Ratio (log OR) and its 95% CIs were employed. Our safety endpoints were the proportion of treatment cessation due to AEs and the AEs elicited by the treatment itself. Our analyses predominantly relied on data derived from the intention-to-treat (ITT) sample (subjects as randomized) or the modified intention-to-treat (mITT) sample (subjects who engaged in at least one treatment session), rather than exclusively on the per-protocol set (subjects who completed the treatment).
Our analysis commenced with a conventional meta-analysis, utilizing Stata 14.0 software (STATA Corporation, College Station, Texas) for data processing. A random-effects model was implemented to calculate the SMD and the 95% CIs between various treatments and placebo. We assessed inter-study heterogeneity using the Cochran Q test and the I2 statistic, considering an I2 value exceeding 50% as indicative of significant heterogeneity.18 To evaluate the robustness of our findings, a sensitivity analysis was performed. We also used adjusted funnel plot asymmetry tests to explore potential publication bias and small-study effects, noting that a p-value below 0.10 typically suggests such biases or effects. All tests were bidirectional, with a p-value below 0.05 deemed statistically significant.
In our NMA, we utilized the Bayesian Markov Chain Monte Carlo (MCMC) methodology, implementing it via the Just Another Gibbs Sampler (JAGS) within the R statistical environment, and integrating this process through the GEMTC package. For the analysis of each outcome, a random-effects consistency model was chosen, and its adequacy was evaluated by comparing it with a fixed-effects model, employing the Deviance Information Criterion (DIC) for this purpose. Our approach included the deployment of four independent Markov chains, each consisting of 20,000 burn-in iterations followed by 50,000 simulation iterations, to ensure thorough exploration of the parameter space. To determine the model's fitting accuracy, we generated trajectory density graphs and convergence diagnostic charts. The approach towards a Potential Scale Reduction Factor (PSRF) nearing 1 was indicative of the model achieving satisfactory convergence; in cases where convergence was not achieved, an increase in the number of iterations was warranted. Additionally, in scenarios involving closed loops, we applied the node-splitting method within the GEMTC framework to rigorously evaluate the consistency between direct and indirect comparison outcomes, ensuring robustness in our findings.
To further elucidate the sources of heterogeneity, subgroup analyses were conducted based on the type of treatment. Treatments were categorized into oral medication treatments and non-oral medication treatments (chemodenervation, surgical interventions and non-invasive neuromodulation), with oral medications further subdivided into β-blockers, antipsychotics, and anticonvulsants. Additionally, separate analyses at various time points were carried out to examine the long-term effects of the treatments.
The surface under the cumulative ranking curve (SUCRA) was employed to present the rankings and uncertainties of interventions. SUCRA scores range from 0 to 1, with higher scores indicating a higher probability of the intervention being the most effective. The likelihood of each intervention ranking at various efficacy levels was calculated to assess treatment performance.
To assess result stability, a sensitivity analysis was performed by sequentially omitting each study and recalculating the combined estimates from the remaining studies. Moreover, adjusted funnel plot asymmetry tests were applied to explore the potential for publication bias and small-study effects.
Risk of bias and certainty of evidence
The risk of bias for each included RCT was independently evaluated by two reviewers (JZ, CY) using the revised Cochrane risk-of-bias tool 2.0 (RoB2.0).19 Each study was classified as having a “low,” “some concern,” or “high” risk of bias. Specifically, for studies with a crossover design, the RoB2.0 tool for crossover trials was employed. Any discrepancies were resolved through discussion, and, if necessary, by consulting a third reviewer (TF).
For the NMA, we utilized the Confidence in Network Meta-analysis Internet application (CINeMA) (http://cinema.ispm.ch) to assess the credibility of the evidence.20 Initially, the evidence was considered high-level, but based on the evaluation of evidence quality, it could be maintained or downgraded to moderate, low, or very low. The assessment mainly focused on six domains: (a) within-study bias; (b) across-studies bias (primarily publication bias); (c) indirectness; (d) imprecision; (e) heterogeneity; and (f) inconsistency.
Ethical statement
Relevant data were retrieved from public databases, including MEDLINE, WOS, Embase, Scopus, and the Cochrane Central Register of Controlled Trials. Ethical approval and informed consent was covered in the original studies and was not applicable for this study.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had responsibility for the decision to submit for publication.
Results
Selection of studies and bias risk analysis
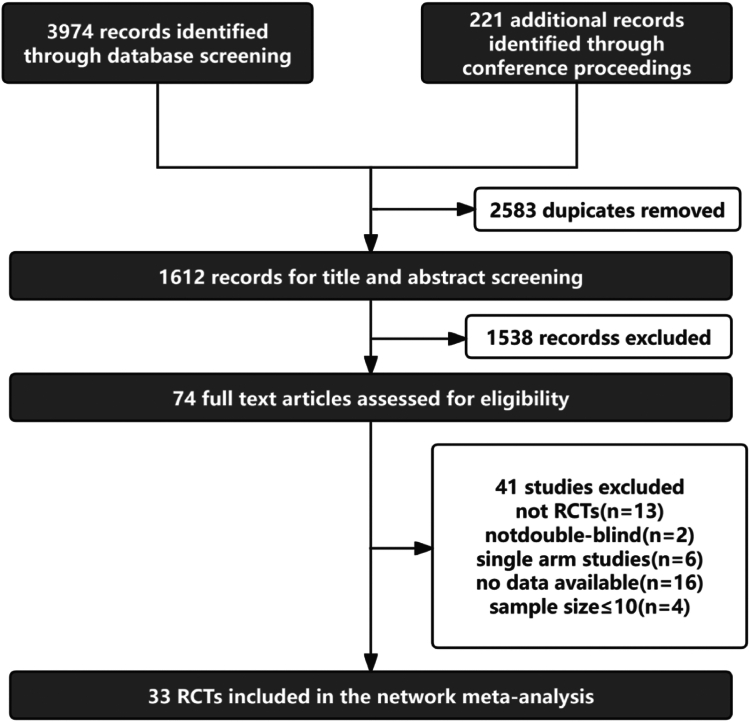
Our initial search of an extensive database retrieved 4195 records. After deduplication 1612 abstracts underwent screening, resulting in 74 full-text articles being thoroughly examined. Of these, 33 studies21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 satisfied our inclusion criteria, as illustrated in Fig. 1. Of these 33 RCTs, 23 were crossover studies and 10 were parallel-group studies. Additionally, 10 studies had no dropouts, making the ITT and mITT populations identical. Among the remaining studies, 16 used the mITT population and seven used the ITT population. The participants had an average age ranging from a minimum of 38.6 years to a maximum of 73.7 years. The duration of the condition varied from 4.6 to 41 years. The main outcomes were measured using various methods, including the TERAS spiral drawing test, the FTM-TRS rating scale (parts A, B, C), subjective assessments, and functional tremor severity assessments. Detailed characteristics of these studies are shown in Table 1.
Fig. 1.
PRISMA flow diagram of study selection. Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA).
Table 1.
Characteristics of included RCTs.
| Study | Subjects (intervention/control) | Age Mean ± SD/Mean (range) | Intervention | Duration (y) Mean ± SD/Mean (range) | Outcome |
|---|---|---|---|---|---|
| Pahwa et al. (2018) | 48/43 | 70.5 ± 11.2/69.8 ± 10.1 | PNS | 29.4 ± 24.9/33.6 ± 22.4 | TERAS upper limb tremor scores |
| Shin et al. (2019) | 12/9 | 68.8 (61–79)/65.2 (25–81) | rTMS | 8.8 ± 6.4/10.6 ± 11.9 | FTM-TRS |
| Lin et al. (2018) | 10/13 | NA | PNS | NA | TERAS spiral drawing |
| Elias et al. (2016) | 56/20 | 70.8 ± 8.7/71.4 ± 7.3 | MR-FUS/Thalamotomy | 28.3 ± 16.4/27.9 ± 14.9 | FTM-TRS |
| schuurman et al. (2000) | 6/7 | 64 ± 7.6/63 ± 17 | Thalamotomy/DBS | NA | Frenchay Activities Index |
| Jonathan et al. (2015) | 14/6 | 68 (48–79) | DBS | NA | Tremor severity |
| Brin et al. (2001) | 45/45 | 68.5 ± 11.2 | BT | 25.5 ± 17.7 | Subjective assessment |
| Jankovic et al. (1996) | 13/12 | 67.4 ± 12.4/65.2 ± 13 | BT | 41.1(20–60)/31.9(12–59) | Functional severity of tremor |
| Jog et al. (2020) | 19/11 | 68.1 ± 10.6/68.2 ± 10.2 | BT | 24.7 ± 19.6/35.3 ± 10.2 | FTM-TRS (A items 5 or 6) FTM-TRS B |
| Mittal et al. (2018) | 28/28 | 66.5 (25–82) | BT | NA | FTM-TRS (B-Drawing) |
| Teravainen et al. (1986) | 18/18 | 44.3 (21–61) | Propranolol/ICI-118551 | NA | Tremor intensity |
| Larsen et al. (1982) | 24/24/24 | 44.7 ± 13.8 | Propranolol/Atenolol | NA | Tremor intensity |
| Calzetti et al. (1983) | 26/26 | 47 (19–72) | Propranolol | NA | Tremor amplitude (%) |
| Leslie et al. (1985) | 11/11 | 53.9 (24–71) | Phenobarbitone | 8.7 (2–70) | Tremor magnitude (%) |
| Papapetropoulos et al. (2022) | 48/47 | 64 ± 9.6/63 ± 10.8 | CX-8998 | 24 ± 16.3/21 ± 15.7 | TETRAS-PS |
| Ondo et al. (2006) | 108/100 | 61 ± 13/64 ± 13 | Topiramate | 24 ± 17/22 ± 18 | FTM-TRS |
| Zesiewicz Th et al. (2007) | 11/11 | 53.91 ± 13.01/60.4 ± 14.83 | Pregabalin | 17.58 ± 19.86/18.33 ± 14.07 | FTM-TRS |
| Zesiewicz et al. (2007) | 10/10 | 57.6 ± 12.8/61.5 ± 17.2 | Zonisamide | 7.4 ± 3.3/4.6 ± 1.6 | FTM-TRS |
| Connor et al. (2008) | 62/62 | 62 ± 15 | Topiramate | NA | FTM-TRS |
| Gironell et al. (2005) | 15/15 | 71.4 (60–79) | Amantadine | 12.2 (4–26) | FTM-TRS A + B |
| Morita et al. (2005) | 14/14 | 68.4 ± 15.6 | Zonisamide/Arotinolol | 8.3 ± 6.5 | FTM-TRS |
| Calzetti et al. (1981) | 23/23/23 | 49.2 (19–72) | Propranolol/Metoprolol | NA | Tremor magnitude |
| Phwa et al. (2003) | 13/13 | 73.7 | Mirtazapine | 24.1 | FTM-TRS |
| Lee et al. (2003) | 71/74 | 54.93 ± 15.59/58.43 ± 15.3 | Arotinolol/Propranolol | 11.3 ± 9.06/11.3 ± 9.2 | Self-reported disability scale |
| Ondo et al. (2000) | 20/20 | 69.9 ± 6.2 | Gabapentin | 29.1 ± 20.9 | ALD |
| Gunal et al. (2000) | 19/19/19/19 | 51.5 (18–83) | Alprazolam/Acetazolamide/Primidone | 24.2 | Functional score |
| Gironell et al. (1999) | 16/16/16 | 67.9 (47–79) | Gabapentin/Propranolol | 12.2 (3–30) | FTM-TRS A + B |
| Pahwa et al. (1998) | 18/18 | 66.5 | Gabapentin | 33.4 | Total tremor score |
| Mally et al. (1995) | 10/10/10 | 68 (23–85) | Propranolol/Theophylline | 1–10 | Tremor scores |
| Biary et al. (1995) | 15/15 | 38.6 (19–66) | Nimodipine | 14.2 | Clinical score |
| Jefferson et al. (1987) | 10/10/10 | 40.7 (22–62) | ICI-118551/Propranolol | NA | Modified postural |
| Elble et al. (2007) | 15/15 | 62.4 ± 14 | Levetiracetam | 35.2 ± 21.7 | FTM-TRS |
| Laesen T et al. (1982) | 24/24 | 44.7 ± 13.8 | Propranolol | NA | Tremor intensity |
Fahn-Tolosa-Marin Tremor Rating Scale (FTM-TRS); The Tremor Research Group Essential Tremor Rating Assessment Scale (TERAS); Activities of Daily Living (ADL); Quality of Upper Extremity Skill Test (QUEST); Total Tremor Scale (TTS); Botulinum Toxin (BT); Deep Brain Stimulation; Magnetic Resonance-guided Focused Ultrasound Surgery (MR-FUS); Peripheral Nerve Stimulation (PNS); repetitive Transcranial Magnetic Stimulation (rTMS).
The studies analyzed treatments for 1251 patients, covering 19 pharmacological options—acetazolamide, alprazolam, amantadine, atenolol, CX-8998, levetiracetam, metoprolol, mirtazapine, nimodipine, phenobarbitone, pregabalin, primidone, theophylline (each with 1 study); arotinolol, ICI-118551, topiramate, zonisamide (2 studies each); gabapentin (3 studies); and propranolol (10 studies)—alongside 6 non-pharmacological therapies: Botulinum Toxin (BT) (4 studies), DBS and Peripheral Nerve Stimulation (PNS) (2 studies each), and repetitive Transcranial Magnetic Stimulation (rTMS), thalamotomy, MR-FUS (1 study each).
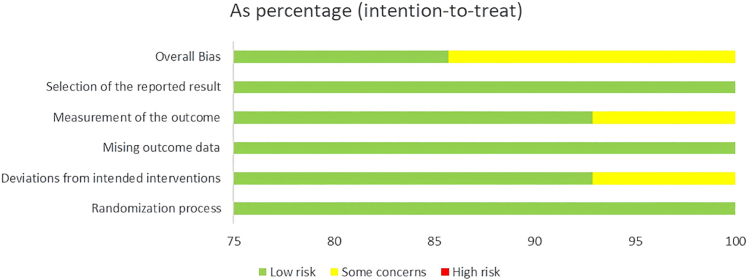
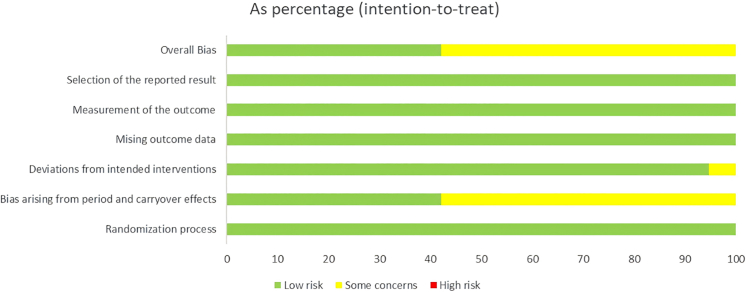
We conducted an evaluation of all potential bias sources utilizing the Cochrane Risk of Bias Tool. Thirteen of the studies fell under the classification of “some concerns,” with the main issue being the inability to verify the identical or nearly identical allocation of participants across the two sequences of the crossover trials. The other twenty studies were deemed to present a low risk of bias. The overall risk of bias characteristics for the included studies is depicted in Fig. 2, Fig. 3. Details on the specific bias situations for each study are provided in Supplementary Figures S2 and S3.
Fig. 2.
Risk of bias plot: RCTs. Randomized controlled trials (RCTs).
Fig. 3.
Risk of bias plot: crossover trials.
Pairwise meta-analysis
We evaluated the efficacy of various treatments: BT (across 4 studies), PNS (2 studies), gabapentin (3 studies), propranolol (7 studies), and topiramate (2 studies). Moreover, we conducted a comparative analysis of propranolol versus ICI-118551 in two distinct investigations. Our findings indicate that both propranolol (SMD = −1.76, 95% CI: [−2.88, −0.64], I2 = 93%) and topiramate (SMD = −0.60, 95% CI: [−0.82, −0.38], I2 = 0) significantly outperform placebo in terms of efficacy (Supplementary Fig. S1 and S2). Regarding AEs, the incidence associated with propranolol (OR = 3.82, 95% CI: [0.56, 25.94], I2 = 0) and PNS (OR = 1.84, 95% CI: [0.42, 8.03], I2 = 58.8%) was not significantly different from placebo. However, the occurrence of AEs in treatments with BT, gabapentin, and topiramate was notably higher compared to placebo (Supplementary Fig. S3–S8). Publication bias revealed by Egger's test was not significant, and sensitivity analysis showed no difference in results.
Network Meta-analysis of all treatments
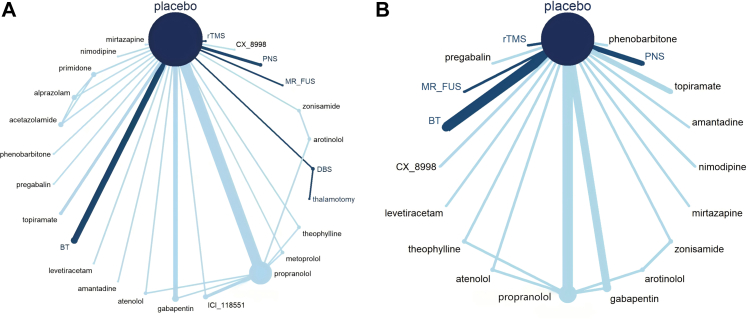
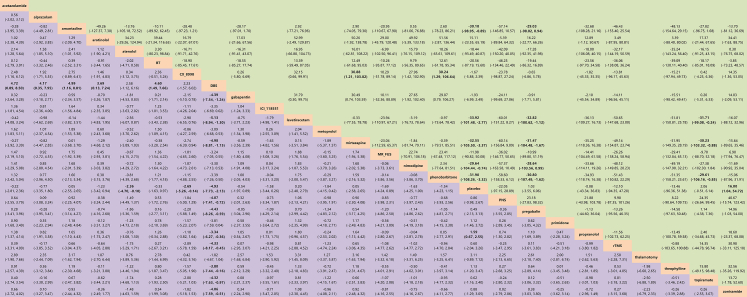
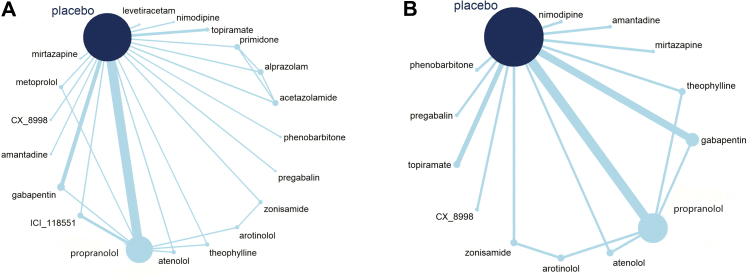
The efficacy of 25 treatments for ET was assessed in an analysis of 33 RCTs involving 1251 patients, as illustrated in the network diagram in Fig. 4 (A). The findings indicate that DBS (SMD = −4.93, 95% CI: [−7.73, −2.13]), CX-8998 (SMD = −2.69, 95% CI: [−5.26, −0.14]), atenolol (SMD = −2.36, 95% CI: [−4.70, −0.10]), and propranolol (SMD = −1.59, 95% CI: [−2.25, −0.67]) demonstrated significantly greater efficacy compared to placebo. Notably, DBS showed superior therapeutic effects over the other 15 treatments, which include levetiracetam, amantadine, mirtazapine, acetazolamide, BT, pregabalin, gabapentin, rTMS, topiramate, alprazolam, PNS, zonisamide, theophylline, arotinolol, and propranolol, with SMDs ranging from −5.13 to −3.34 (Fig. 5).
Fig. 4.
Network diagram. (A) for comparing all treatments efficacy. (B) for comparing all treatments safety. Botulinum Toxin (BT); Deep Brain Stimulation; Magnetic Resonance-guided Focused Ultrasound Surgery (MR-FUS); Peripheral Nerve Stimulation (PNS); repetitive Transcranial Magnetic Stimulation (rTMS).
Fig. 5.
Efficacy (lower triangle in purple)/safety (upper triangle in green) of all treatment methods. Botulinum Toxin (BT); Deep Brain Stimulation (DBS); Magnetic Resonance-guided Focused Ultrasound Surgery (MR-FUS); Peripheral Nerve Stimulation (PNS); repetitive Transcranial Magnetic Stimulation (rTMS). The bold values indicate the value of p < 0.05.
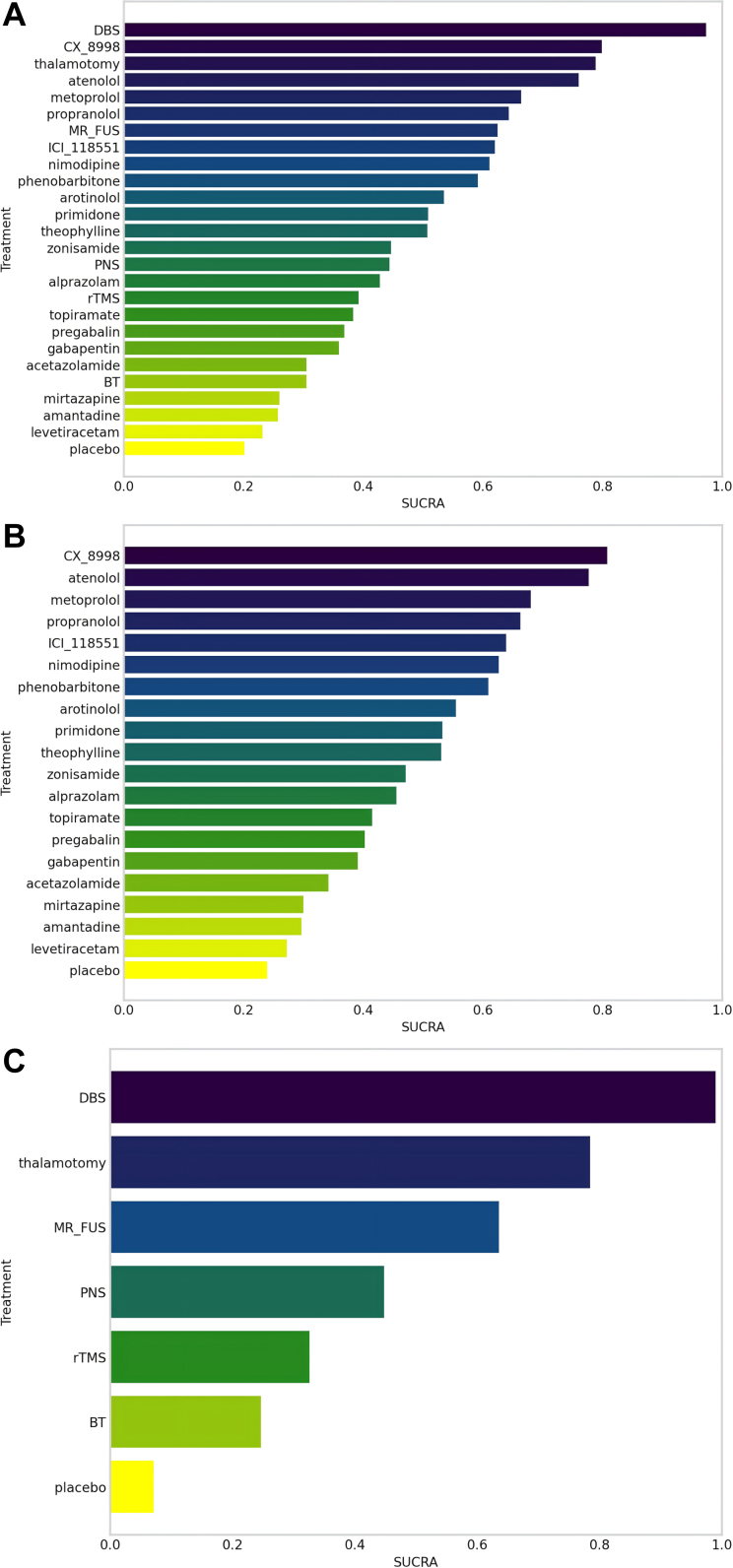
DBS had the highest likelihood of being the most effective treatment, with a SUCRA score of 0.97. In the NMA, DBS had the highest likelihood of being the most effective treatment, with a SUCRA score of 0.97. This was followed by CX-8998 with a SUCRA score of 0.80, thalamotomy closely trailing at 0.79, and atenolol scoring 0.76. Other notable treatments included metoprolol (SUCRA = 0.66), propranolol (SUCRA = 0.64), MR-FUS (SUCRA = 0.624), ICI-118551 (SUCRA = 0.620), nimodipine (SUCRA = 0.61) and phenobarbitone (SUCRA = 0.59). It's noteworthy that the SUCRA rankings for all these treatment methods were above that of placebo, indicating their relative efficacy. The complete SUCRA rankings for all evaluated treatments are detailed in Fig. 6 (A).
Fig. 6.
SUCRA ranking of (A) all treatments, (B) oral medication treatments, (C) non-oral medication treatments. Surface under the cumulative ranking curve (SUCRA), Botulinum Toxin (BT), Deep Brain Stimulation (DBS); Magnetic Resonance-guided Focused Ultrasound Surgery (MR-FUS), Peripheral Nerve Stimulation (PNS), repetitive Transcranial Magnetic Stimulation (rTMS).
We evaluated the safety of various treatment methods for ET by analyzing study withdrawal rates due to AEs, excluding specific treatments such as acetazolamide, alprazolam, DBS, ICI-118551, metoprolol, primidone, and thalamotomy (due to the lack of relevant data in the original studies) (Fig. 4 [B]). Our findings revealed that withdrawal rates were notably higher for levetiracetam (Log OR = −33.92, 95% CI: [−101.48, −2.77]), mirtazapine (Log OR = −32.55, 95% CI: [−105.50, −3.37]), phenobarbitone (Log OR = −31.90, 95% CI: [−108.26, −3.32]), amantadine (Log OR = −30.18, 95% CI: [−98.95, −0.89]), nimodipine (Log OR = −29.64, 95% CI: [−104.44, −0.74]) and zonisamide (Log OR = −16.00, 95% CI: [−54.72, −1.04]) compared to placebo (Fig. 5).
Specifically, topiramate showed a lower withdrawal rate than levetiracetam (Log OR = −31.17, 95% CI: [−99.08, −0.24]), mirtazapine (Log OR = −30.23, 95% CI: [−103.32, −0.88]), and phenobarbitone (Log OR = −29.61, 95% CI: [−106.16, −0.85]). CX-8998's withdrawal rate was significantly lower than that for mirtazapine (Log OR = −30.88, 95% CI: [−103.82, −1.21]) and phenobarbitone (Log OR = −30.24, 95% CI: [−106.64, −1.29]). Furthermore, the withdrawal rate for levetiracetam (Log OR = −32.82, 95% CI: [−100.62, −1.12]), mirtazapine (Log OR = −31.47, 95% CI: [−104.48, −1.67]), phenobarbitone (Log OR = −30.80, 95% CI: [−107.49, −1.62]), amantadine (Log OR = −29.03, 95% CI: [−98.02, 0.94]) and nimodipine (Log OR = −28.64, 95% CI: [−103.56, −0.99]) were significantly higher compared to pregabalin (Fig. 5).
According to the SUCRA rankings, treatments like levetiracetam, phenobarbitone, mirtazapine, amantadine and nimodipine were comparatively less safe. Conversely, PNS and arotinolol were indicated to have a potentially higher safety profile, with lower SUCRA values than placebo (Supplementary Fig. S9).
The comparison-corrected funnel plot demonstrated a predominantly symmetrical distribution of studies around the median line. Nevertheless, a few studies on propranolol exhibited an asymmetric distribution, hinting at possible publication bias (Supplementary Fig. S10). Moreover, two studies, located directly below and adjacent to the funnel plot, suggested the presence of a small sample effect. Sensitivity analyses, conducted by sequentially excluding these studies, showed no significant differences in the findings. For pairwise comparisons between different interventions that were statistically significant, the credibility of the evidence was assessed using CINeMA, indicating that the certainty of the evidence was “low” or “very low. Specific details are provided in Supplementary Fig. S11.
Network Meta-analysis of oral medication treatments
In our analysis of the efficacy of oral medication treatments, we reviewed 23 RCTs encompassing 947 participants and 19 different treatment methods31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 (Fig. 7 [A]). This analysis revealed that propranolol (SMD = −1.61, 95% CI: [−2.76, −0.48]) exhibited significantly higher efficacy compared to placebo (Supplementary Fig. S12). The SUCRA rankings further demonstrated that all the drug treatments evaluated were more effective than placebo. The top five treatments, based on SUCRA values, were CX-8998 (SUCRA = 0.81), atenolol (SUCRA = 0.78), metoprolol (SUCRA = 0.70), propranolol (SUCRA = 0.66) and ICI-118551 (SUCRA = 0.64). The comprehensive SUCRA rankings for all the oral medication treatments assessed are presented in Fig. 6 (B).
Fig. 7.
Network diagram. (A) for comparing oral medication treatments efficacy. (B) for comparing oral medication treatments safety.
In our study, we compared the safety of 13 different oral medication treatments (the other seven treatments lacked data related to AEs) by examining their rates of AEs (Fig. 7 [B]). Our analysis showed that the incidence of AEs for amantadine and gabapentin was significantly higher than that of placebo, with amantadine also having a higher AE incidence compared to the other 12 treatment methods. In contrast, theophylline had a lower AE incidence than both placebo and the other 12 treatments (Supplementary Fig. S12). A complete ranking of the incidence of AEs based on the SUCRA methodology is detailed in Supplementary Fig. S13.
We further categorized the drugs into three distinct groups for separate analysis: β-blockers and anticonvulsants. In the β-blockers group, propranolol was more effective than placebo (SMD = −1.72, 95% CI: [−3.35, −0.13]), with SUCRA rankings as follows: atenolol, metoprolol, propranolol, alprenolol, ICI-118551, and placebo. Among the anticonvulsants, phenobarbitone (SMD = −1.54, 95% CI: [−2.76, −0.33]), primidone (SMD = −1.12, 95% CI: [−2.12, −0.11]), and topiramate (SMD = −0.60, 95% CI: [−1.20, −0.01]) were more effective than placebo, with SUCRA rankings as follows: phenobarbitone, primidone, topiramate, pregabalin, zonisamide, gabapentin, placebo, and levetiracetam.
Network Meta-analysis of non-oral medication treatments
Our study also incorporated an analysis of ten RCTs focusing on non-oral medication treatments,21, 22, 23, 24, 25, 26, 27, 28, 29, 30 which covered 6 distinct treatment methods and involved 304 participants (Supplementary Fig. S14). The NMA revealed that DBS (SMD = −4.93, 95% CI: [−6.92, −2.94]), thalamotomy (SMD = −3.11, 95% CI: [−5.97, −0.25]), and MR-FUS (SMD = −1.68, 95% CI: [−3.35, −0.001]) demonstrated significantly superior treatment effects compared to placebo. Notably, DBS outperformed the other non-oral medication methods (SMDs range from −4.93 to −3.24), with the sole exception of thalamotomy (Supplementary Fig. S15). SUCRA rankings indicate that all non-oral medication treatment methods surpassed placebo in terms of effectiveness. The efficacy hierarchy, according to SUCRA, is as follows: DBS, thalamotomy, MR-FUS, PNS, rTMS, and BT (Fig. 6[C]).
Comparative efficacy over time: SUCRA rankings
Our study broadened to examine the SUCRA rankings at several key intervals, focusing on immediate outcomes post-treatment, within one month after treatment, at the one-month milestone, and during the period from one month to six months post-treatment. In evaluating immediate outcomes post-treatment, the SUCRA value rankings placed DBS, propranolol, metoprolol, PNS, and rTMS ahead, with placebo ranking last (Supplementary Fig. S16). Within one-month post-treatment, the SUCRA value rankings were as follows: CX-8998, atenolol, propranolol, ICI-118551, nimodipine, topiramate, gabapentin, mirtazapine, amantadine, and placebo (Supplementary Fig. S17). At the one-month mark, all treatments, with the exception of levetiracetam, had higher SUCRA values than placebo. The order of SUCRA value was CX-8998, MR-FUS, primidone, alprazolam, rTMS, arotinolol, theophylline, propranolol, zonisamide, BT and acetazolamide (Supplementary Fig. S18). In the time frame extending from one month to six months post-treatment, the treatments ranked from highest to lowest in SUCRA rankings are MR-FUS, phenobarbitone, topiramate, gabapentin, pregabalin, and BT, with all these treatments ranking higher than placebo (Supplementary Fig. S19).
Reliability assessment: consistency and convergence analysis
We employed node-splitting analysis to evaluate potential inconsistencies by comparing the discrepancies between direct and indirect evidence. Upon implementing the node-splitting model, our findings revealed an absence of significant inconsistency or qualitative discrepancies within the study. This outcome suggests that the consistency model adopted in our analysis is reliable and robust. Furthermore, we meticulously controlled the PSRF to remain below 1. This measure indicates a high efficiency of convergence in our study, ensuring that our results are both stable and reliable.
Discussion
In our study, we integrated both direct and indirect evidence from 33 RCTs, encompassing 1251 patients with ET across 25 different treatment modalities, to evaluate the relative efficacy and safety of all available treatment strategies. Our analysis also provides a rank order through NMA to determine the best intervention measures. The NMA results indicate that DBS significantly outperforms placebo and 15 other treatment regimens in alleviating symptoms of ET. In addition, compared to placebo, treatments such as CX-8998, propranolol, and atenolol have shown significant symptom improvement. However, the level of evidence for these findings is “low” or “very low”. Among all treatment methods, DBS, CX-8998, thalamotomy, atenolol, propranolol, and MR-FUS are ranked at the forefront in terms of relative efficacy. In terms of safety assessment, due to the original studies only providing comparative data between DBS and thalamotomy, which cannot be indirectly compared with other treatments to form a network, we did not include them in our NMA. Apart from this, the aforementioned treatments that showed relative efficacy did not exhibit significant differences compared to placebo, either in terms of discontinuation rates due to AEs or the incidence of AEs. Consequently, our conclusions should be applied with caution in clinical practice, and further high-quality RCTs are required in the future.
The main strategies for treating ET currently include the use of oral medications such as β-blockers, antipsychotics, and anticonvulsants.6 Propranolol, a non-selective β-blocker, is the only drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of ET and is recommended as a first-line treatment in both the AAN guidelines and the IMDA guidelines.8, 9, 10 This aligns with our research findings, where propranolol demonstrated significant efficacy in our NMA, supporting its status as a first-line medication. In both our NMA and pairwise meta-analyses, propranolol showed no significant safety concerns compared to placebo. However, bradycardia and bronchospasm were the most common AEs reported in the studies included,31,32,35,40 leading to contraindications for propranolol in patients with bronchial asthma and severe bradycardia or cardiac conduction abnormalities.54,55
Atenolol, a selective β-blocker, emerges as a valuable second-line treatment option for patients with specific cardiac conditions or those who experience AEs from non-selective β-blockers, due to its cardiovascular safety.6,9,56,57 It also demonstrated relative efficacy in our NMA without significant AEs. In addition, our NMA included two other selective β-blockers, metoprolol and ICI 118,551. ICI 118,551 is primarily used in laboratory research rather than as a clinical drug.33,36 These two drugs are recommended as second-line treatments in the IMDA guidelines, but the evidence level is “very low”.9 However, our NMA results showed that neither drug demonstrated significant relative efficacy. For patients who cannot use propranolol due to contraindications but can tolerate other non-selective β-blockers, three alternative non-selective beta-blockers—arololol, sotalol, and nadolol—are available as second-line treatments.8, 9, 10 In our NMA, we studied arololol but found it lacked significant efficacy.
Primidone, an anticonvulsant drug, is recommended as a first-line medication, and topiramate has also been elevated to first-line treatment status in the guidelines of the IMDA.8, 9, 10 However, neither treatment demonstrated significant efficacy in our NMA, possibly due to the limited number of studies and the small sample sizes included. Due to restrictions related to RCT studies and analyzable data, we included only one RCT of primidone conducted over twenty years ago.48 In the original study, the effectiveness of primidone was comparable to another anticonvulsant, alprazolam, but it was slightly inferior in improving tremor scores, suggesting that its efficacy might be relatively limited. Therefore, further research is necessary to more comprehensively verify and understand the efficacy of primidone. Additionally, although topiramate did not show significant efficacy in our NMA, it performed better than placebo in our pairwise meta-analyses, which might be attributed to differences in analytical methods and the studies included. Likewise, other second-line treatment drugs like alprazolam, gabapentin, and zonisamide, also did not show significant efficacy in our NMA.
Clozapine is an atypical neuroleptic recommended as a third-line treatment by the AAN and IMDA guidelines,8, 9, 10 but it is associated with a rare risk of agranulocytosis, which can lead to fatal infections.58 Olanzapine, another atypical neuroleptic, was recommended as a second-line treatment in the 2013 IMDA guidelines, with fewer side effects, particularly in terms of extrapyramidal symptoms and agranulocytosis.56 However, there is a lack of RCT investigating the use of clozapine for treating ET, and only one substandard RCT study has been conducted using olanzapine for this purpose. Therefore, it is not possible to evaluate the efficacy and safety of these two drugs in the treatment of ET using the NMA method based on RCT.
In addition, nimodipine and amantadine did not show significant efficacy in our NMA. However, CX-8998, a T-type voltage-gated calcium channel modulator still in the research phase and not yet approved for clinical use, has shown therapeutic potential in Phase I and II clinical trials involving patients with moderate to severe ET,45,59 and it also demonstrated significant relative efficacy in our NMA.
When patients develop resistance to medications or cannot tolerate the side effects, neurosurgical interventions often become the alternative solution. The primary therapeutic target is the ventral intermediate nucleus of the thalamus, which links the cerebellum to the cortical motor pathways. DBS has been established as the standard surgical approach for treating medication-resistant ET and has been proven safe for treating bilateral and axial symptoms since its FDA approval in 1997.60 However, due to the lack of placebo-controlled trials, these findings are rated as evidence level C. Our NMA indicates that DBS significantly outperforms placebo and most other interventions in treating ET. This holds true both in our comprehensive NMA covering all treatment modalities and in NMA focusing solely on non-pharmacological interventions. Although the efficacy of DBS is generally significant, the choice of specific targets may influence treatment outcomes. In the two studies we included, one focused on thalamic stimulation, while the other covered stimulation of the thalamus or an unspecified area. Due to the challenges of conducting RCTs for surgical treatments of ET, there is a scarcity of studies, and we were unable to thoroughly explore differences in efficacy between different brain regions.
Since the 1950s, thalamotomy has been used to treat ET and other movement disorders. Although this method provides long-term effects, due to its potential side effects and irreversibility, it has been gradually replaced by DBS. In the studies we included that compared thalamotomy with DBS, the incidence of AEs was significantly higher for thalamotomy, including cognitive impairment, mild dysarthria, and gait disturbances. Notably, AEs in the DBS group disappeared six months after the pulse generator was turned off, indicating that DBS's AEs are reversible. In contrast, MR-FUS, approved by the FDA in 2016 as a non-invasive surgical option, demonstrated lower surgical risks and shorter recovery times.61, 62, 63 Our NMA shows that the dropout rate due to AEs from MR-FUS treatment is comparable to that of placebo and other treatments. However, MR-FUS may also lead to permanent neurological AEs. Our included RCTs show that AEs caused by MR-FUS mainly include gait disturbances, sensory abnormalities or numbness, with some patients still experiencing similar AEs after 12 months.
According to guidelines from the AAN guideline, the use of thalamotomy is recommended on a “case-based decision”. The AAN and the IMDA guidelines recommend MR-FUS for medically refractory cases.8, 9, 10 In our comprehensive NMA covering all ET treatments, MR-FUS and traditional thalamotomy did not show significant advantages over placebo. However, in NMAs focused on non-oral medication treatments, both interventions were significantly more effective than placebo. This disparity may be attributed to the inclusion of various ET treatment modalities in the comprehensive NMA, which could have masked the effects of MR-FUS and thalamotomy. Conversely, in the NMA focused solely on non-oral medication treatments, only non-oral medication treatments were included, reducing heterogeneity between treatments and making the advantages of these interventions over placebo more pronounced. The relative efficacy rankings indicate that DBS, thalamotomy, and MR-FUS are the three most effective non-pharmacological treatments according to SUCRA rankings.
In exploring the diversity of treatments for ET, we evaluated the efficacy of non-oral medication treatments such as PNS, rTMS, and BT.6 BT injections are recommended for medically refractory cases, with the most common AEs being weakness.21, 22, 23, 24 In the case of cervical injections, this may also lead to difficulties with swallowing or breathing.6,64,65 The other two treatments are not mentioned in treatment guidelines. In our NMA, these methods did not significantly improve ET symptoms, and the discontinuation rate due to AEs was not significantly different from that of placebo and other treatments. Furthermore, in traditional meta-analyses, BT did not demonstrate treatment efficacy and had a significantly higher incidence of AEs compared to placebo.
To the best of our knowledge, this is the first comprehensive assessment of all ET treatment modalities, encompassing both oral medication and non-oral medication treatments. Our study integrates a considerable number of RCTs, offering a thorough comparison across a wide range of treatment methods. However, our study is not without limitations. The most notable is that 16 out of 25 treatment modalities were supported by only a single RCT, often with small sample sizes. To address this, we conducted sensitivity analyses by excluding studies with small sample sizes, which showed no significant changes in the results. Additionally, the limited number of studies prevented us from performing a comprehensive subgroup analysis based on factors such as outcome variables, age, disease duration, and diagnostic criteria. Furthermore, the credibility of the evidence indicates that our results have a “low” or “very low” level of evidence, underscoring the necessity of applying these results cautiously in clinical practice. Additionally, the predominant comparison of treatments against placebo rather than direct comparisons among different treatments means that many of our effect size estimates are derived from indirect comparisons and should be interpreted with caution. The need for more direct, head-to-head evidence is clear. Lastly, the RCTs often included narrowly selected patient populations, which may not fully reflect the broader community of ET patients, indicating that our conclusions might not fully extend to real-world effectiveness.
Notwithstanding these limitations, this comprehensive analysis holds significant clinical importance, particularly highlighting the superior efficacy of DBS in alleviating ET symptoms compared to other treatments, thus underscoring its vital role, especially for patients unresponsive to drug therapies. Notably, our analysis also emphasizes the efficacy of medications like CX-8998, atenolol, and propranolol. Propranolol, in particular, has a long-standing validation for ET treatment, reaffirming its crucial role in pharmacological strategies. Additionally, our study indicates that emerging therapies like CX-8998 show promising potential, advocating for the continued exploration of new treatment avenues. Given these findings, there is an urgent need for further high-quality RCTs in the future to solidify and expand the current evidence base.
Contributors
JZ conceived the idea, designed the study, and drafted the initial manuscript. JZ and RY did the literature searches. JZ and RY designed the data-extraction form. JZ and YC extracted the data. JZ did the statistical analyses. YR supervised the statistical analyses. JZ, RY and DS cross checked the data and results. TF and JZ revised it critically for important intellectual content. All authors reviewed the drafted manuscript for critical content and approved the final version. JZ and RY accessed and verified the data. All authors had full access to all the raw or generated data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The data and analytic code that support the findings of this study are available on request from the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
This study was funded by the National Nature Science Foundation of China (grant number 82271459).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102889.
Appendix A. Supplementary data
References
- 1.Louis E.D., Ferreira J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Louis E.D., Broussolle E., Goetz C.G., Krack P., Kaufmann P., Mazzoni P. Historical underpinnings of the term essential tremor in the late 19th century. Neurology. 2008;71(11):856–859. doi: 10.1212/01.wnl.0000325564.38165.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis E.D., Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (N Y) 2014;4:259. doi: 10.7916/D8TT4P4B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz D., Schwieger D., Moises H., Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21(8):1114–1118. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 5.Gerbasi M.E., Nambiar S., Reed S., et al. Essential tremor patients experience significant burden beyond tremor: a systematic literature review. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.891446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanker V. Essential tremor: diagnosis and management. BMJ. 2019;366 doi: 10.1136/bmj.l4485. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G., Raethjen J., Hellriegel H., Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10(2):148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 8.Zesiewicz T.A., Elble R.J., Louis E.D., et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;77(19):1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zappia M., Albanese A., Bruno E., et al. Treatment of essential tremor: a systematic review of evidence and recommendations from the Italian Movement Disorders Association. J Neurol. 2013;260(3):714–740. doi: 10.1007/s00415-012-6628-x. [DOI] [PubMed] [Google Scholar]
- 10.Zesiewicz T.A., Elble R., Louis E.D., et al. Practice parameter: therapies for essential tremor: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2005;64(12):2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 11.Louis E.D., Rios E., Henchcliffe C. How are we doing with the treatment of essential tremor (ET)?: persistence of patients with ET on medication: data from 528 patients in three settings. Eur J Neurol. 2010;17(6):882–884. doi: 10.1111/j.1468-1331.2009.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahwa R., Lyons K.E., Wilkinson S.B., et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104(4):506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 13.Flora E.D., Perera C.L., Cameron A.L., Maddern G.J. Deep brain stimulation for essential tremor: a systematic review. Mov Disord. 2010;25(11):1550–1559. doi: 10.1002/mds.23195. [DOI] [PubMed] [Google Scholar]
- 14.Elble R.J., Shih L., Cozzens J.W. Surgical treatments for essential tremor. Expert Rev Neurother. 2018;18(4):303–321. doi: 10.1080/14737175.2018.1445526. [DOI] [PubMed] [Google Scholar]
- 15.Kondapavulur S., Silva A.B., Molinaro A.M., Wang D.D. A systematic review comparing focused ultrasound surgery with radiosurgery for essential tremor. Neurosurgery. 2023;93(3):524–538. doi: 10.1227/neu.0000000000002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hróbjartsson A., Thomsen A.S.S., Emanuelsson F., et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ. 2013;185(4):E201–E211. doi: 10.1503/cmaj.120744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deuschl G., Bain P., Brin M. Consensus statement of the movement disorder society on tremor. Ad hoc scientific committee. Mov Disord. 1998;13(Suppl 3) doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brin M.F., Lyons K.E., Doucette J., et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology. 2001;56(11):1523–1528. doi: 10.1212/wnl.56.11.1523. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J., Schwartz K., Clemence W., Aswad A., Mordaunt J. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov Disord. 1996;11(3):250–256. doi: 10.1002/mds.870110306. [DOI] [PubMed] [Google Scholar]
- 23.Jog M., Lee J., Scheschonka A., et al. Tolerability and efficacy of customized IncobotulinumtoxinA injections for essential tremor: a randomized, double-blind, placebo-controlled study. Toxins. 2020;12(12) doi: 10.3390/toxins12120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal S.O., Jog M., Lee J., Jabbari B. Novel botulinum toxin injection protocols for Parkinson tremor and essential tremor - the yale technique and sensor-based kinematics procedure for safe and effective treatment. Tremor Other Hyperkinet Mov. 2020;10 doi: 10.5334/tohm.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyam J.A., Pereira E.A., McCulloch P., et al. Implementing novel trial methods to evaluate surgery for essential tremor. Br J Neurosurg. 2015;29(3):334–339. doi: 10.3109/02688697.2014.997670. [DOI] [PubMed] [Google Scholar]
- 26.Schuurman P.R., Bosch D.A., Bossuyt P.M., et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 27.Elias W.J., Lipsman N., Ondo W.G., et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 28.Pahwa R., Dhall R., Ostrem J., et al. An acute randomized controlled trial of noninvasive peripheral Nerve stimulation in essential tremor. Neuromodulation. 2019;22(5):537–545. doi: 10.1111/ner.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin P.T., Ross E.K., Chidester P., et al. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham-controlled pilot trial. Mov Disord. 2018;33(7):1182–1183. doi: 10.1002/mds.27350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H.W., Hallett M., Sohn Y.H. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor. Park Relat Disord. 2019;64:304–307. doi: 10.1016/j.parkreldis.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Larsen T.A., Teräväinen H. Propranolol and essential tremor: Rôle of the membrane effect. Acta Neurol Scand. 1982;66(3):289–294. doi: 10.1111/j.1600-0404.1982.tb06847.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen T.A., Teräväinen H., Calne D.B. Atenolol vs. propranolol in essential tremor a controlled, quantitative study. Acta Neurol Scand. 1982;66(5):547–554. doi: 10.1111/j.1600-0404.1982.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 33.Teravainen H., Huttunen J., Larsen T.A. Selective adrenergic beta-2-receptor blocking drug, ICI-118.551, is effective in essential tremor. Acta Neurol Scand. 1986;74(1):34–37. doi: 10.1111/j.1600-0404.1986.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 34.Calzetti S., Findley L.J., Gresty M.A., Perucca E., Richens A. Effect of a single oral dose of propranolol on essential tremor: a double-blind controlled study. Ann Neurol. 1983;13(2):165–171. doi: 10.1002/ana.410130210. [DOI] [PubMed] [Google Scholar]
- 35.Gironell A., Kuliscvsky J., Barbanoj M., Lopcz-Villegas D., Heniández G., Pascual-Sedano B. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol. 1999;56(4):475–480. doi: 10.1001/archneur.56.4.475. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson D., Wharrad H., Birmingham A., Patrick J. The comparative effects of ICI 118551 and propranolol on essential tremor. Br J Clin Pharmacol. 1987;24(6):729–734. doi: 10.1111/j.1365-2125.1987.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connor G.S., Edwards K., Tarsy D. Topiramate in essential tremor: findings from double-blind, placebo-controlled, crossover trials. Clin Neuropharmacol. 2008;31(2):97–103. doi: 10.1097/WNF.0b013e3180d09969. [DOI] [PubMed] [Google Scholar]
- 38.Elble R.J., Lyons K.E., Pahwa R. Levetiracetam is not effective for essential tremor. Clin Neuropharmacol. 2007;30(6):350–356. doi: 10.1097/WNF.0b013E31807A32C6. [DOI] [PubMed] [Google Scholar]
- 39.Calzetti S., Findley L.J., Gresty M.A., Perucca E., Richens A. Metoprolol and propranolol in essential tremor: a double-blind, controlled study. J Neurol Neurosurg Psychiatry. 1981;44(9):814–819. doi: 10.1136/jnnp.44.9.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mally J., Stone T.W. Efficacy of an adenosine antagonist, theophylline, in essential tremor: comparison with placebo and propranolol. J Neurol Sci. 1995;132(2):129–132. doi: 10.1016/0022-510x(95)00128-o. [DOI] [PubMed] [Google Scholar]
- 41.Gironell A., Kulisevsky J., Pascual-Sedano B., Flamarich D. Effect of amantadine in essential tremor: a randomized, placebo-controlled trial. Mov Disord. 2006;21(4):441–445. doi: 10.1002/mds.20676. [DOI] [PubMed] [Google Scholar]
- 42.Ondo W., Hunter C., Vuong K.D., Schwartz K., Jankovic J. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov Disord. 2000;15(4):678–682. doi: 10.1002/1531-8257(200007)15:4<678::aid-mds1012>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Pahwa R., Lyons K., Hubble J.P., et al. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord. 1998;13(3):465–467. doi: 10.1002/mds.870130315. [DOI] [PubMed] [Google Scholar]
- 44.Pahwa R., Lyons K.E. Mirtazapine in essential tremor: a double-blind, placebo-controlled pilot study. Mov Disord. 2003;18(5):584–587. doi: 10.1002/mds.10371. [DOI] [PubMed] [Google Scholar]
- 45.Papapetropoulos S., Lee M.S., Versavel S., et al. A phase 2 proof-of-concept, randomized, placebo-controlled trial of CX-8998 in essential tremor. Mov Disord. 2021;36(8):1944–1949. doi: 10.1002/mds.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zesiewicz T.A., Ward C.L., Hauser R.A., et al. A pilot, double-blind, placebo-controlled trial of pregabalin (Lyrica) in the treatment of essential tremor. Mov Disord. 2007;22(11):1660–1663. doi: 10.1002/mds.21629. [DOI] [PubMed] [Google Scholar]
- 47.Zesiewicz T.A., Ward C.L., Hauser R.A., Sanchez-Ramos J., Staffetti J.F., Sullivan K.L. A double-blind placebo-controlled trial of zonisamide (Zonegran) in the treatment of essential tremor. Mov Disord. 2007;22(2):279–282. doi: 10.1002/mds.21282. [DOI] [PubMed] [Google Scholar]
- 48.Gunal D.I., Afsar N., Bekiroglu N., Aktan S. New alternative agents in essential tremor therapy: double-blind placebo-controlled study of alprazolam and acetazolamide. Neurol Sci. 2000;21(5):315–317. doi: 10.1007/s100720070069. [DOI] [PubMed] [Google Scholar]
- 49.Biary N., Bahou Y., Sofi M.A., Thomas W., Al Deeb S.M. The effect of nimodipine on essential tremor. Neurology. 1995;45(8):1523–1525. doi: 10.1212/wnl.45.8.1523. [DOI] [PubMed] [Google Scholar]
- 50.Findley L.J., Cleeves L. Phenobarbitone in essential tremor. Neurology. 1985;35(12):1784–1787. doi: 10.1212/wnl.35.12.1784. [DOI] [PubMed] [Google Scholar]
- 51.Ondo W.G., Jankovic J., Connor G.S., et al. Topiramate in essential tremor - a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672–677. doi: 10.1212/01.wnl.0000200779.03748.0f. [DOI] [PubMed] [Google Scholar]
- 52.Lee K.S., Kim J.S., Kim J.W., Lee W.Y., Jeon B.S., Kim D. A multicenter randomized crossover multiple-dose comparison study of arotinolol and propranolol in essential tremor. Park Relat Disord. 2003;9(6):341–347. doi: 10.1016/s1353-8020(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 53.Morita S., Miwa H., Kondo T. Effect of zonisamide on essential tremor: a pilot crossover study in comparison with arotinolol. Park Relat Disord. 2005;11(2):101–103. doi: 10.1016/j.parkreldis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Koller W.C., Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology. 1989;39(12):1587–1588. doi: 10.1212/wnl.39.12.1587. [DOI] [PubMed] [Google Scholar]
- 55.Calzetti S., Sasso E., Baratti M., Fava R. Clinical and computer-based assessment of long-term therapeutic efficacy of propranolol in essential tremor. Acta Neurol Scand. 1990;81(5):392–396. doi: 10.1111/j.1600-0404.1990.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 56.Chyou T.-Y., Nishtala R., Nishtala P.S. Comparative risk of Parkinsonism associated with olanzapine, risperidone and quetiapine in older adults-a propensity score matched cohort study. Pharmacoepidemiol Drug Saf. 2020;29(6):692–700. doi: 10.1002/pds.5007. [DOI] [PubMed] [Google Scholar]
- 57.Heel R.C., Brogden R.N., Speight T.M., Avery G.S. Atenolol: a review of its pharmacological properties and therapeutic efficacy in angina pectoris and hypertension. Drugs. 1979;17(6):425–460. doi: 10.2165/00003495-197917060-00001. [DOI] [PubMed] [Google Scholar]
- 58.Meltzer H.Y. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(Suppl B):47–52. [PubMed] [Google Scholar]
- 59.Papapetropoulos S., Lee M.S., Boyer S., Newbold E.J. A phase 2, randomized, double-blind, placebo-controlled trial of CX-8998, a selective modulator of the T-type calcium channel in inadequately treated moderate to severe essential tremor: T-CALM study design and methodology for efficacy endpoint and digital biomarker selection. Front Neurol. 2019;10:597. doi: 10.3389/fneur.2019.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benabid A.L., Pollak P., Gervason C., et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet (London, England) 1991;337(8738):403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 61.Barbe M.T., Reker P., Hamacher S., et al. DBS of the PSA and the VIM in essential tremor: a randomized, double-blind, crossover trial. Neurology. 2018;91(6):e543–e550. doi: 10.1212/WNL.0000000000005956. [DOI] [PubMed] [Google Scholar]
- 62.Agrawal M., Garg K., Samala R., Rajan R., Naik V., Singh M. Outcome and complications of mr guided focused ultrasound for essential tremor: a systematic review and meta-analysis. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.654711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammed N., Patra D., Nanda A. A meta-analysis of outcomes and complications of magnetic resonance-guided focused ultrasound in the treatment of essential tremor. Neurosurg Focus. 2018;44(2) doi: 10.3171/2017.11.FOCUS17628. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira J.J., Mestre T.A., Lyons K.E., et al. MDS evidence-based review of treatments for essential tremor. Mov Disord. 2019;34(7):950–958. doi: 10.1002/mds.27700. [DOI] [PubMed] [Google Scholar]
- 65.Haubenberger D., Hallett M. Essential tremor. N Engl J Med. 2018;378(19):1802–1810. doi: 10.1056/NEJMcp1707928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.