Abstract
Background: There is a need to explore pharmacological options for syndrome (FMS), such as medical cannabis. The aim of this systematic review was to synthesize and analyze the available information about the effectiveness/efficacy and safety of cannabis-based products for medical use (CBPMs) and cannabis-based medicines (CBMs), in patients with FMS. Methods: Interventional or observational studies, systematic reviews and meta-analysis regarding the effectiveness/efficacy and safety of CBPMs and CBMs in patients with FMS were retrieved from the PubMed/Medline database until April 2024. Then, the information was summarized in tables, with the type of CBPM and CBM, the method used in the study and the effective-ness/efficacy and safety outcomes. Results: 19 publications were selected from the search or form the relevant references. Different CBPM and CBM were used across the studies. Also, different instruments for measuring the effectiveness were used. In general, the use of CBPMs and CBM showed an important improvement in pain, quality of life, and sleep habits. There were no serious adverse events. Conclusions: The results show that CBMPs and CBMs could be effective and safe in patients with FMS; however, the evidence is limited and there is a need for high-quality clinical studies conducted with improved methodological design.
Keywords: Medical cannabis, Fibromyalgia syndrome, Efficacy, Effectiveness, Safety, Systematic review
Highlights
-
•
Cannabis-based products for medicinal use (CBPMs) and cannabis-based medicines (CBMs) improve the fibromyalgia symptoms.
-
•
Both CBPMs and CBM can decrease the pain measured trough instruments such as Visual analogue scale and Numeric rating scale.
-
•
CBPMs and CBM can also improve the score in specific questionnaires for fibromyalgia, like Fibromyalgia impact questionnaire.
-
•
The use of CBPMs and CBM is a safety alternative. The most common adverse events are not serious.
1. Introduction
Fibromyalgia syndrome (FMS) is a condition characterized by chronic and widespread musculoskeletal pain of uncertain etiology,1, 2, 3 usually accompanied by fatigue, cognitive disturbance, and symptoms related to depression.1,3 It is supposed that the FMS heighten sensitivity to pain, affecting the painful and nonpainful stimuli processed through the brain and spinal cord.4,5
The prevalence of FMS ranges from 2 to 5 % in the worldwide population,6,7 being more frequent in women between 30 and 50 years old.6,8 A large study from 2013 reported a mean worldwide prevalence of 2,7 %.9 In some publications, the prevalence ranges from 0.5 % to 12 %, depending on the population studied (e.g., different ages, sexes) and the diagnostic criteria used.10
Currently, there is no effective pharmacotherapy for FMS3,11; however, some available drugs are supported by clinical trial data showing their effectiveness in decreasing pain and other symptom domains. For instance, the Food and Drug Administration (FDA) has approved the gabapentinoid pregabalin (approved in 2007),12 and the serotonin and norepinephrine reuptake inhibitors (SNRIs) duloxetine (approved in 2008)13 and milnacipran (approved in 2009).14 Also, amitriptyline is commonly used off-label for FMS.15 Pharmacotherapy should be used in conjunction with non-pharmacologic interventions, such as cognitive behavioral therapy and exercise.2 Nevertheless, current pharmacological treatment options for FMS afford only modest benefits for most patients.3,11 Therefore, there is a need for exploring other pharmacological options, with different mechanisms of action.16
Current expert reviews on the treatment of FMS emphasize the need for research in pharmacotherapy focused on developing more effective and targeted therapeutic interventions. This includes exploring new perspectives, such as drugs that target neuroinflammation, immunomodulation, and the endocannabinoid system. In this sense, cannabis and cannabinoids are pharmacotherapy interventions that are under study and need more data regarding their efficacy and safety in patients with FMS, which could lead the availability of effective and safety drugs.17
Medicinal products containing cannabinoids have emerged as a potential treatment option for various conditions, including chronic pain, which is the primary symptom of FMS. As a result, the medical use of cannabis in FMS patients has increased. Namely, in Canada, a cohort study involving 117 patients with FMS reported that 28 (23.9 %; 95 % CI: 16.5 %–32.7 %) has used cannabis after its legalization18; however, current evidence is limited. Thus, cannabis and its derivatives are being continually investigated for managing pain and others associated symptoms as well as its and impact on quality of life in patients with FMS.
The effects of cannabinoids, for instance delta-9-tetrahydrocannabinol (THC) or cannabidiol (CBD) are explained by their capacity to bind and to modulate CB1 and CB2 receptors, which belonging to the G-protein-coupled receptor family. In detail, THC causes a psychoactive effect mainly acting through CB1 receptor and modifies both the pain and emotions. CBD has analgesic and anti-inflammatory effects also through CB1 receptor. Thus, the THC:CBD ratio defines the global effect cause by the medicinal product with cannabinoids. In this sense, CB1 cannabinoid receptors are mainly found in the central nervous and peripheral nervous systems, and therefore, substances that can act as CB1 receptor agonists can modulate the pain along sensory pathways19.
Overall, drugs inhibiting spinal synaptic transmission can act on the nociceptor or the spinothalamic neuron. At the nociceptor, drugs act by inhibiting release of the neurotransmitters associate to pain, for instance glutamate, through the direct or indirect inhibition of calcium channels. In this sense, at the nociceptor both the endogenous (e.g., anandamide and 2-arachidonoylglycerol) and the exogenous cannabinoids (e.g., cannabis-based products containing CBD or THC), by inhibiting calcium channels transporter through CB1 receptors, reduce glutamate release and control pain perception.17
Medical cannabis includes cannabis-based products for medicinal use (CBPMs) and cannabis-based medicines (CBMs).20 Overall, the first corresponds to all preparations or products containing cannabis, cannabis resin, or cannabis derivative used for a medical condition; between them, the CBMs are medicinal products, or substances or preparations for use as an ingredient of a medicinal product, similar to conventional drugs. For instance, synthetic compounds such as nabilone or dronabinol, which have similar structure to naturally occurring THC (structural isomers) are CBMs.20
Although some results denote that cannabinoids may offer pain relief and improve sleep in patients with FMS, mainly through endocannabinoid system modulation; overall, the evidence on the effectiveness and safety of CBPMs, including CBMs for improving symptoms in patients with FMS is limited. Global, few clinical trials have studied the efficacy/effectiveness and safety of cannabinoid-modulating products for the treatment of patients with FMS. Nevertheless, information collected from patients with FMS using a variety of CBPMs shows improving in some symptoms without serious adverse effects.21 In this way, the National Institute for Health and Care Excellence-NICE (United Kingdom) in 2019 (updated in 2023) recommended conducting research regarding the clinical and cost-effectiveness of CBD, whether containing traces of THC or not, as an add-on to the standard treatment in adults with FMS.20
Overall, we consider that the evidence provides by clinical studies and some systematic reviews focused to assess the effectiveness and safety of cannabis-based products for medical use in patients with FMS leaves a gap, may be due to methodology limitations and insufficiency of longitudinal studies to assess clinical outcomes. For instance, some systematic reviews addressed CBPMs and CBMs for chronic pain in general or they included specific articles focused regarding only one product, or they did not address details regarding methods for assessing effectiveness and safety results. Also, the studies included are characterized by small sample sizes and short duration, thus results precluded unbiased conclusions.17 Therefore, there is a need for identifying and synthetizing high-quality evidence to better inform prescribers and patients, which may be generate by more recent systematic reviews. Thus, this systematic review aimed to synthesize and analyze the available information about the effectiveness and safety of CBPMs or CBMs in patients with FMS.
2. Materials and methods
A systematic review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.22 PubMed/Medline database was comprehensively searched for all kind of studies (observational or interventional studies), systematic reviews or meta-analyses related to the effectiveness/efficacy or safety of medical cannabis in patients with FMS published until April 2024.
2.1. Inclusion and exclusion criteria
Inclusion criteria: Observational or interventional studies, systematic reviews or meta-analyses in patients with FMS using medicinal cannabis (CBPMs and CBM), without restrictions by sex, age, or disease stage.
Exclusion criteria: 1) articles about perceptions or opinions regarding medical cannabis in FMS; 2) articles not including CBPMs as treatment for patients with FMS; and 3) clinical trial protocols; and 4) Studies with conclusion only for primary chronic pain (without specific conclusions regarding patients with FMS).
2.2. Search strategy
The search strategy used was: “(fibromyalgia) AND (cannabis)” in all fields, with the filters “Clinical Study, Clinical Trial, Clinical Trial Phase II, Clinical Trial Phase I, Clinical Trial Phase III, Clinical Trial Phase IV, Controlled Clinical Trial, Systematic Review, Meta-Analysis, Multicenter Study, Observational Study, and Randomized Controlled. Articles published in English or Spanish and with full-text access were identified. Also, relevant references of the included articles that matched the inclusion criteria were included. The studies identified were reviewed by two researchers (V.L, JC.R); according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA). Thus, titles and abstracts of the identified articles were screened independently for eligibility and the findings were then compared. Any discrepancies were referred to a third researcher (P.A) and they were resolved by consensus.
2.3. Quality assessment
The quality of interventional studies was analyzed using Grading of Recommendations, Assessment, Development and Evaluation (GRADE)23 and patient, intervention, comparison, outcome (PICO).24
2.4. Data synthesis
The information identified in the articles of this systematic review is presented following a narrative synthesis both in text and tables. Thus, the information was extracted in a database according to the following items: article title, the aim of the study, cannabis product evaluated (drug or product), dose, route of administration, population, efficacy/effectiveness measure, safety measure, main efficacy results, and main safety results.
2.5. Heterogeneity managing
To improve the synthesis of the results a subgroup analyses based on: a) the kind of study (observational or interventional clinical studies); b) the kind of CBPM used; and c) the methods used for assessing the efficacy/effectiveness, mainly Fibromyalgia Impact Questionnaire (FIQ), Visual analog scale (VAS) or Numeric Rating Scale (NRS).
3. Results
3.1. Results of the search
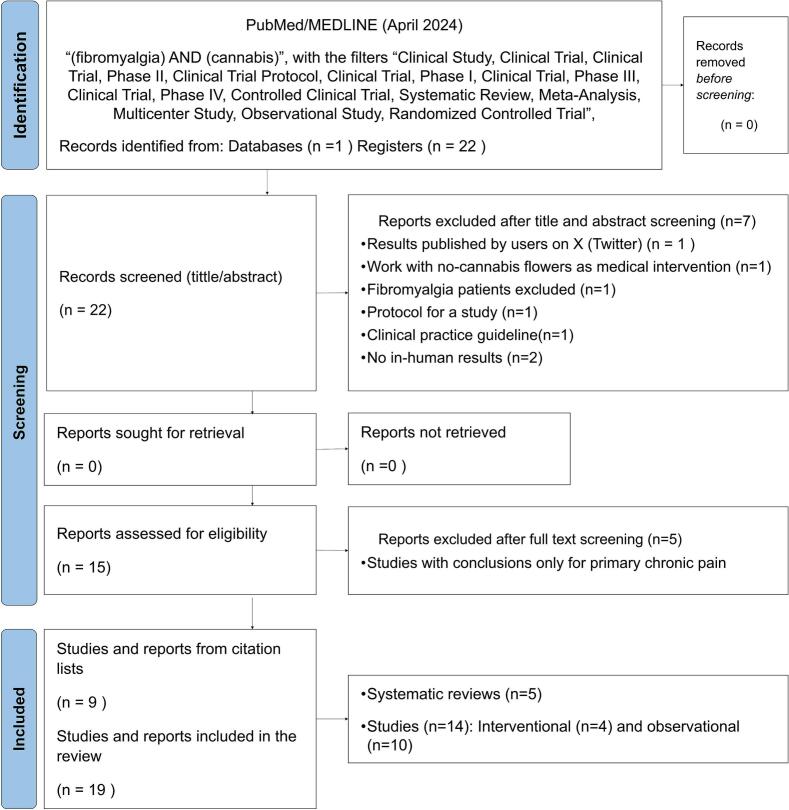
In the PubMed/Medline database, 22 articles were identified. Among them, 5 corresponded to clinical studies,25, 26, 27, 28, 29 10 to reviews or systematic reviews,16,30, 31, 32, 33, 34, 35, 36, 37, 38 and 7 articles did not meet the inclusion criteria.39, 40, 41, 42, 43, 44, 45 Also, 9 articles were identified and included from the reference list46, 47, 48, 49, 50, 51., 52, 53, 54 After full-text reading, 5 articles were excluded for lack of specifics results regarding efficacy or safety in patients with FMS.30,31,35,37,38 (Fig. 1).
Fig. 1.
Preferred reporting items for systematic review and meta-analysis22: flow diagram for the systematic review of cannabis use in Fibromyalgia.
Among the 19 articles included, 5 were systematic reviews,16,32, 33, 34,36 4 were interventional clinical studies,25,26,29,47 and 10 observational clinical studies27,28,46,48, 49, 50, 51., 52, 53, 54 (Fig. 1). Information for assessing the efficacy/effectiveness in observational (Table 2) and interventional (Table 3) clinical studies were summarized. Also, key information for assessing the efficacy/effectiveness and safety in systematic reviews are presented in Table 4. A meta-analysis was not considered due to high degree of heterogeneity between the instruments used in each one, the kind of CBPMs and CBM product used, and the patients included.
Table 2.
Key information regarding observational clinical studies assessing the efficacy/effectiveness of CBPMs in patients with fibromyalgia syndrome.
| Reference | Objective | Cannabis-based medicinal products (route of administration) | Dose | Efficacy/effectiveness measure | Efficacy/effectiveness results |
|---|---|---|---|---|---|
| 27 | To assess any clinical improvement with the addition of CBPMs to standard analgesic treatment in patients with FMS | Bedrocan, (22 % THC and less than 1 % CBD), and Bediol, (6.3 % THC and 8 % CBD), prepared in olive oil (1 g cannabis, 10 g of olive oil) (Oral) | Bedrocan at night, Bediol at the morning 10–30 drops, not exceeding 120 drops/day |
|
|
| 28 | To evaluate the possible role of CBPMs as add-on therapy in the management of Low back pain in FMS patients. | The recommended one was 1:4 THC to CBD, with THC levels less than 5 % (Smoked or vaporized) | 20 g of cannabis for a month. It could be increased to 30 g/month |
|
|
| 46 | To evaluate the efficacy of Cannabis flos 19 % on pain, fatigue, sleep disturbances, anxiety, and depression in FMS patients. | Cannabis flowering tops marketed as Cannabis flos 19 % for oral decoction (Oral) |
30 mg twice a day for the first month, 60 mg twice a day for the second month |
|
|
| 48 | To describe the patterns of CBPMs use and the associated benefits reported by patients with FMS | The cannabis derivative used in every case was cannabis whole plant (Smoked and oral) | The most frequent doses were between 1 and 2 cigarettes each time when patients smoked and 1 full spoonful each time when eating |
|
|
| 49 | To investigate the safety and effectiveness of FMS patients receiving CBPMs | Not specified: product contains CBD/THC | The median cannabis approved dosage was 670 mg/day at initiation and 1000 mg/day at 6 months. The median of THC was 140 mg/day and for CBD was 39 mg/day at 6 months |
|
|
| 50 | To examine the effects of CBPMs on patients with FMS | Not specified: product contains whole cannabis plant (Smoked or inhaled) | The mean cannabis dose was 26 g/month |
|
|
| 51 | To assess the effects of CBPMs on pain intensity, disability, widespread pain, disease severity, and mood disorders | Cannabis with the same proportion of THC and CBD as the first option, or THC-dominant (Oral as decoction, vaporized as oil, and sublingual as oil) | The starting dose of the milled flowers in the sachet was 50 or 100 mg twice per day. In the case of cannabis olive oil extract, one drop every 3–4 days and a subsequent increase |
|
|
| 52 | To explore the efficacy of THC on electrically induced pain, axon reflex flare, and psychometric variables | THC (Oral) | FMS patients received a daily dose of 2.5–15 mg of THC. The dosage was increased weekly by 2.5 mg THC, as long as no severe side effects were reported |
|
|
| 53 | To assess the effects of CBPMs on pain intensity, disability, widespread pain, disease severity, and mood disorders” | Dronabinol THC in liquid or capsules (Oral) |
The mean dose administered of THC concentration was 7.5 mg/day (interquartile range 5–12.5 mg). It could increase by 2.5 mg of THC weakly as long as no adverse effects were reported |
|
|
| 54 | To assess the outcomes of patients prescribed with CBPMs on fibromyalgia-specific symptoms, health-related quality of life, anxiety, and sleep | CBD or THC (Oral, sublingual, or vaporized) | The median dose of THC was 100.00 (IQR: 20.00–195.00) mg/day and the median dose of CBD was 20.00 (IQR: 20.00–35.00) mg/day. |
|
|
NS* Not significant; CBD: Cannabidiol; THC: Tetrahydrocannabinol; CBPMs: cannabis-based products for medicinal use; FMS: Fibromyalgia syndrome; SD: standard deviation; CI95 %: Confidence interval 95 %; IQR: interquartile range.
Table 3.
Key information regarding interventional clinical studies assessing the efficacy/effectiveness of CBPMs in patients with fibromyalgia syndrome.
| Reference | Objective | Cannabis-based medicinal products (route of administration) | Dose | Efficacy/effectiveness measure | Efficacy/effectiveness results |
|---|---|---|---|---|---|
| 25 | To evaluate the impact of CBPMs (oil) on symptoms and quality of life of individuals with FMS | 30-mL green glass dropper bottle containing cannabis oil with 24.44-mg/mL concentration of THC and 0.51 mg/mL of CBD (Sublingual) | The initial dose in both groups was one drop (1.2 mg of THC and 0.02 mg of CBD) a day |
|
|
| 26 | To evaluate the analgesic effects of inhaled CBPMs using the cannabis plant with all its natural components | Bedrocan with 22 % THC and less than 1 % CBD (Vaporized) Bedrolite with 9 % CBD and less than 1 % THC Bediol with 6.3 % THC and 8 % CBD (Vaporized) Placebo variety without any THC or CBD content (Vaporized) |
Not specified |
|
|
| 29 | To determine whether nabilone is equivalent to amitriptyline in improving the quality of sleep in patients with FMS, and the improvement in other outcomes | Nabilone (Oral) | Doses of nabilone 0.5 mg, with a possible dose increase to 1 mg |
|
|
| 47 | To evaluate if nabilone will significantly reduce the pain and improve quality of life in FMS patients compared with placebo | Nabilone (Oral) | 0.5 mg nabilone at bedtime for 1 week. Increase to 0.5 mg/12 h after 7 days. Finally increasing to 1 mg/12 h |
|
|
NS* Not significant. CBD: Cannabidiol; THC: Tetrahydrocannabinol; CBPMs: cannabis-based products for medicinal use; FMS: Fibromyalgia syndrome; SD: standard deviation; CI95%: Confidence interval 95%.
Table 4.
Key information regarding systematic reviews assessing the efficacy/effectiveness of CBPMs in patients with fibromyalgia syndrome.
| Reference (year) | Objective; articles, and number of participants (n) included | Cannabis-based medicinal products (dose) | Main efficacy/effectiveness results | Main conclusions |
|---|---|---|---|---|
| 16 (2016) | To assess the efficacy, tolerability and safety of cannabinoids for fibromyalgia symptoms in adults; 2 (72) | Any formulation of cannabis products; however, only Nabilone was identified (1 mg/day) |
|
There is no convincing, unbiased, high-quality evidence suggesting that nabilone is of value in treating people with fibromyalgia. |
| 32 (2021) | To analyze the role of the cannabinoid system in fibromyalgia syndrome (FMS); 22 (1326) | Nabilone (0.5–1 mg/day); Dronabinol (7.5 mg/day); Bedrocan (22.4 mg THC, <1 mg CBD), Bediol (13.4 mg THC, 17.8 mg CBD), and Bedrolite (18.4 mg CBD, <1 mg THC) |
|
Data suggest that medical cannabis is a safe and effective treatment for fibromyalgia pain; however, several limitations regarding dosage, length of treatment, adverse effects, long-term follow-up, and dependence needs further investigation. |
| 33 (2023) | To examine and discuss current clinical evidence regarding the use of cannabis for the treatment of fibromyalgia; 9 (564) | Nabilone (0.5–1 mg/day) and cannabis in various forms, administered as a pill, oil smoke, or vapor (no specified) |
|
The use of cannabis in fibromyalgia treatment is still an area of ongoing study. Although, some studies show promising results effective in reducing pain and improving sleep) others have been inconclusive. Therefore, the effectiveness of these cannabinoids in treating fibromyalgia remains uncertain and more research is needed to verify the efficacy. |
| 34 (2021) | To assess current evidence on medicinal cannabis for FMS to evaluate safety and efficacy in patients with fibromyalgia syndrome (FMS); 10 (1136) | Nabilone (0.5–1 mg/day); Dronabinol (2.5–15 mg/day); Bedrocan (22.4 mg THC, <1 mg CBD), Bediol (13.4 mg THC, 17.8 mg CBD), and Bedrolite (18.4 mg CBD, <1 mg THC) |
|
Medical cannabis may be beneficial for some patients with FMS; however, more studies are required to confirm the possible impact of cannabis on pain. Also, it is important to identify what chemovar types, THC to CBD ratios, dosage regimen, or form of administration are appropriate for various symptomology, and what assessment tools are required to quantify and interpret outcomes |
| 35 (2022) | To evaluate the efficacy and safety of cannabinoid administration in chronic primary pain (CPP); 8 (Total = 240, of them 115 with FMS). | Sublingual cannabis THC-rich oil, dronabinol oral capsules, oral nabilone, CBD gums, inhaled vaporized pharmaceutical grade medicinal cannabis (Bedrocan, Bediol, Bediol), different doses of delta-9-THC pharmaceutical-grade medicinal cannabis smoked cigarettes |
|
Cannabinoids in chronic primary pain has limited benefit in pain reduction, but cannabinoids might improve pain and FIQ in FMS with long-term administration |
CBD: Cannabidiol; THC: Tetrahydrocannabinol, FMS: fibromyalgia syndrome.
3.2. Quality assessment
In the 4 interventional studies, an assessment was conducted on: outcome criteria, the number of patients enrolled and quality. Thus, the quality was determined by Grading of Recommendations, Assessment, Development and Evaluation (GRADE),23 and for the intervention's impact on outcomes, and comparative risk the patient, intervention, comparison, outcome; GRADE - PICO24 was used. This evaluation aided in gauging the significance of these studies concerning the effectiveness, efficacy, and safety of cannabinoid utilization in FMS. It is worth noting the diversity in outcomes, especially regarding sleep quality (including insomnia), pain levels (measured by VAS), and overall quality of life. Notably, the impact on quality emerged as a consistent factor across the studies. The results of the quality assessment are presented in Table 1.
Table 1.
Quality assessment of interventional studies following GRADE and PICO criteria.
| Reference | Outcome of interest | Number of participants | Quality of evidence (GRADE) | Relative effect | Comparative risk (PICO) |
|
|---|---|---|---|---|---|---|
| Placebo group | Intervention group | |||||
| 25 | To determine the benefit of a tetrahydrocannabinol (THC)-rich cannabis oil on symptoms and quality of life of fibromyalgia patients | 17 (8 in the treatment group and 9 in the placebo group) | Moderate to low (a) | Fibromyalgia Impact Questionnaire (FIQ) mean scores: 75.5 to 30.5 points (p < 0.001) | Moderate to high-risk patients | Moderate to high-risk patients |
| 26 | Analgesic effects of inhaled pharmaceutical grade cannabis | 20 allocated to intervention, without placebo group | Moderate (b) | N/A | N/A | Moderate risk |
| 29 | Nabilone vs Amitriptyline. The primary outcome was sleeping quality, measured by the Insomnia Severity Index and the Leeds Sleep Evaluation Questionnaire. Secondary outcomes included pain, mood, quality of life, and adverse events | 32 (29 completed the study) | High (c) | Insomnia Severity Index difference = 3.2 (IC 95% = 1.2–5.3) Leeds Sleep Evaluation Questionnaire difference = 0.5 (0.0–1.0) (wakefulness) difference = 0.3 (−0.2–0.8) |
Moderate risk |
N/A |
| 47 | Nabilone in Fibromyalgia. Determine the benefit of nabilone in pain management and quality of life improvement | 40 and finished 33. (15 in the treatment group and 18 in the control group) | High | Visual Analog Scale (VAS) = −2.04, p < 0.02. Fibromyalgia Impact Questionnaire (FIQ) = −12.07, p < 0.02. Anxiety = −1.67, p < 0.02 |
Moderate risk | Moderate risk |
GRADE: Grading of Recommendations, Assessment, Development and Evaluation; PICO: patient, intervention, comparison, outcome; N/A (No applicable).
(a) It is a study with a small number of patients, all with high risk of FM and/or comorbidities, which makes it prone to selection and execution or information biases.
(b) There is randomization of patients for each visit and use of each treatment, but there is no clear masking and although they describe the use of placebo there is no clarity about the randomization in this group.
(c) It is a study with good randomization, blinded, crossover. 2 patients did not complete the study due to adverse effects and there was a large refusal of patients to enter the study, which possibly allowed the outcomes to be determined in a better way with clear inclusion and exclusion criteria. Although a CI contains 1, the study design and expected outcomes allow it to be of high quality.
3.3. Efficacy/effectiveness
Among the 14 clinical studies, 5 evaluated the whole cannabis plant, with different proportions of Cannabidiol (CBD) and THC,26, 27, 28,46,48,51 one study evaluated THC,52 two evaluated THC or CBD25,54 and 3 evaluated the CBM nabilone29,47 or dronabinol53; one study used more than one type of CBPMs, for instance, cigarettes and oil,49 and one study only mention the use of licensed medical cannabis.50 Specific information about the CBPMs, CBM, or the treatment used in the studies is presented in Table 2, Table 3.
Most of the studies evaluated the effectiveness of the CBPMs in patients with FMS, but some of them assessed specifically patients not responding to standard analgesic therapy,27 resistant patients48 or resistant or intolerant to these medications.51 Other studies evaluated only one specific symptom of FMS: low back pain,28 chronic insomnia,29 or central neuropathic pain.53
Instruments for assessing some symptom domains regarding the effectiveness of CBPMs can be identified in clinical studies. Among these tools, the most common are questionnaires, which assess all the dimensions of the FMS, for example, the Fibromyalgia Impact Questionnaire (FIQ). Also, there are some that measure the effectiveness only in one dimension of symptoms, for example, The Pittsburgh Sleep Quality Index (PSQI) for sleep dimension. Threshold perception of electrical stimulation or threshold perception of pain with an algometer are tools to get measures less subjective. These instruments assess the immediate effect of pain perception after CBPMs use. The Visual Analogue Scale (VAS) and the Numeric Rating Scale (NRS) are instrument widely used for pain assess. The instruments used in each study are shown in Table 2, Table 3.
3.3.1. Observational studies
The key information regarding observational clinical studies assessing the effectiveness of CBPMs in patients with FMS is shown in Table 2, including the objective, kind of CBPMs (or CBMs) dose, route of administration, and effectiveness measure.
3.3.2. Interventional studies
The key information regarding interventional clinical studies assessing the effectiveness of CBPMs in patients with FMS is shown in Table 3, including objective, kind of CBPMs (or CBMs) dose, route of administration, and effectiveness measure.
3.3.3. Systematic reviews
We identified and included 5 systematic reviews16,32, 33, 34,36 regarding effects of medical cannabis in patients with SFM. The most relevant data from these reviews are showed in Table 4.
The systematic reviews included for their analysis 2,16 8,36 9,33 10,34 and 22 studies.32 One review was centered in assessing the effect of nabilone. Results showed no convincing evidence of the value of this drug in treating FMS.16 Overall, the other reviews conclude that CBPMs have potential benefits in the treating of patients with this syndrome32, 33, 34,36; however, all of them conclude that further investigation is needed in order to determine the effectiveness of medical cannabis in FMS.16,32, 33, 34,36 Finally, one review identified and reported a significant effect of CBPMs when the follow-up was made after more than 4 weeks of treatment.36
In the case where the results were not significant at the last assessment visit, the data in the table corresponds to the result of the follow up when the outcome was significant.
3.4. Safety
The safety assessment of the CBPMs in patients with FMS was based on identifying and recording the adverse events using different instruments and classifying them according to their seriousness. One study used two questionnaires to measure the CBPMs safety26: the Bowdle questionnaire, which evaluates 3 psychedelic effects (drug high, alterations in internal perception, and alterations in external perception); and the Bond and Lader questionnaire, which score yields 3 main factors of alertness (alert, strong, clear-headed, coordinated, energetic, quick-witted, attentive, proficient, and interested), contentment (contented, happy, amicable, gregarious, and tranquil), and calmness (calm and relaxed). A high score indicates impairment. The use of the Bowdle questionnaire showed that Bedrocan (22 % THC and less than 1 % CBD) and Bediol (6.3 % THC and 8.0 % CBD) caused moderate drug high responses. Bedrolite (9 % CBD and less than 1 % THC) caused less drug high compared to Bedrocan and Bediol. The results obtained with the Bond and Lader questionnaire indicate mild deterioration in mood with Bediol and mild deterioration in alertness with Bedrocan.26
Overall, Table 5 summarized the most common adverse effects identified and reported by the different clinical studies, specifying their frequencies.
Table 5.
Most common adverse effects reported and their percentage by the included studies.
| Reference | Number of patients (n) | Somnolence | Dizziness / Vertigo | Dry mouth/ Sore-throat | Nausea/ vomiting | Red eyes | Fatigue | Increase in appetite- Hunger |
|---|---|---|---|---|---|---|---|---|
| 25 | 9 | 88 % | 25 % | 25 % | NR | NR | NR | NR |
| 26 | 20 | NR | 15 % to 20 %⁎⁎ | 25 %–35 %⁎⁎ | 5 % to 30 %⁎⁎ | NR | NR | NR |
| 27 | 102 | 16 % (sleepiness) | 21 % | NR | 9 % | NR | NR | 5% |
| 28 | 31 | NR | NR | 10 %⁎ | NR | 90 % ⁎ | NR | 16 % |
| 29 | 32 | 19 % (Drowsiness)⁎ | 31 %⁎ | 22 %⁎ | 28 %⁎ | NR | 6 %⁎ | NR |
| 46 | 15 | NR | NR | NR | NR | NR | NR | NR |
| 47 | 15 | 47 % (Drowsiness)⁎ | 27 %⁎ | 33 %⁎ | NR | NR | NR | NR |
| 48 | 28 | 64 %⁎ | 36 %⁎ | 61 %⁎ | NR | 25 % (Conjunctival irritation) ⁎ | NR | NR |
| 49 | 239 | 4.2 % (Drowsiness) | 7.9 % | 6.7 % | 5.4 % | NR | NR | 3.8 % |
| 50 | 26 | NR | NR | 27 % | NR | 27 % | NR | 15 % |
| 51 | 35 | 11 % | 14 % | 5 % | 14 % | NR | 2 % | 2 % |
| 52 | 4 | NR | NR | NR | NR | NR | NR | NR |
| 53 | 124 | NR | 3 %⁎ | NR | NR | NR | 2 % (tiredness)⁎ | NR |
| 54 | 306 | 19 % | 16 % | 23 % | 12 % | NR | 25 % | NR |
NR: no reported.
The percentages were calculated with data contained in the articles.
Depending on the variety of cannabis used.
The systematic reviews reported no serious adverse events and adequate tolerance to the treatment.16,32, 33, 34,36 However, one systematic review reported more adverse events for nabilone than for placebo or other treatments.16 These adverse events were: dizziness, nausea, dry mouth and drowsiness,16 similar to the most common adverse events reports in the others systematic reviews. Also, drug high was a frequent adverse event.33,34 Finally, one systematic review concluded that there were no significant difference in adverse events between cannabinoids and placebo.36
4. Discussion
In this systematic review, information regarding the effectiveness/efficacy and safety of CBPMs (or CBMs) was systematically searched, synthesized, and analyzed. Despite the different CBPMs used, results showed an improvement in different domains of FMS in patients with this condition.
Measuring the effectiveness of FMS therapies is still a challenge. Some biomarkers have been explored to have a possible association with FMS. For instance, IL-6, IL-8, BDNF, CRP, and IFN-γ have been found higher in serum, blood, or plasma samples in patients with FMS.55 Despite this, none of those biomarkers are currently used in clinical practice. The Outcome Measures in Rheumatology (OMERACT) initiative has suggested various core sets of outcome measures with the aim of getting a better comparison across clinical trial results in Rheumatology. The core set for FMS was introduced in 2009 and includes pain, tenderness, fatigue, patient global health, multidimensional function, and sleep disturbance.56 The results of this review show that the use of questionnaires to measure the effectiveness in different domains is the more frequent method. The use of this kind of instruments represents a subjective measure. However, this limitation is ameliorated with the use of validated questionnaires and the use of more than one questionnaire. These 2 characteristics were used in most of the studies, except in Chaves C, et al.24 and Crestani F, et al.,49 studies, which only used The Fibromyalgia Impact Questionnaire (FIQ) or their revised version (FIQR).
Among 14 clinical studies included (Fig. 1) in 6 (43 %), the FIQ and their revised version (the Revised Fibromyalgia Impact Questionnaire -FIQR) was used as instruments to assess the drug therapy effectiveness in patients with FMS. On these instruments, the total score ranges from 0 to 100, and higher scores mean a greater impact (more negative) on a patient's quality of life.25,57 However, in one study the FIQR construal was 0: total disability and 100: no disability.28 In this context, the identified effectiveness results assessed with FIQ are shown in Table 6.
Table 6.
Fibromyalgia Impact Questionnaire (FIQ) results after and before the use of cannabis.
| Reference | Before Cannabis- Baseline | After cannabis | Significance |
|---|---|---|---|
| 25 | 75.50 ± 12.93 | 30.50 ± 16.18 | p < 0.001 |
| 27 | 69.00 ± 19.18⁎ | 62.25 ± 22.75⁎ | p < 0.001⁎ |
| 28 | 45.30 ± 10.2⁎⁎ | 80.50 ± 12.2⁎⁎ | p < 0.0001⁎⁎ |
| 46 | 74.40 ± 17.2⁎ | 60.30 ± 24.3⁎ | p = 0.061⁎ |
| 47 | 66.45 ± 12.76 | 54.38 | p < 0.02 |
| 52 | 52.0 ± 20.0 | 35 ± 15.0 | Not Significant |
The result corresponds to FIQR.
In the FIQR used in this study 0 was total disability and 100 no disability.
Other studies did not show the data at baseline and follow-up. Fiz et al.48 reported lack of significant effects using the FIQ: mean score of CBPMs users (65.56 SD = 11.9) versus non-users (65.56 SD = 12.8, p = 0.36). Results showed by Habib et al.50 with the FIQR analyzed each item of this questionnaire, rather than giving a global score. There was one study comparing the effect of amitriptyline and cannabis. In this study, the FIQ was used to assess the outcomes of each treatment. No differences were noted between treatments (amitriptyline and cannabis), with a mean difference of −0.7; 95 % CI95 % = −7.3 to 5.8.29
According to FIQ and FIQR scores, the use of CBPMs showed some improvement in the impact of the FMS on the quality of life of the patients.25,27, 28, 29,46, 47, 48,50,52 This self-administered instrument measures physical functioning, work status, depression, anxiety, sleep, pain, stiffness, fatigue, and well-being (Table 6).
Specific domains of FMS were accessed through other instruments. Pain was one of the specific dimensions improved with the use of CBPMs. VAS scale was used in,26,28,46, 47, 48,52,54 and NRS in49,51., 52, 53 studies (tales 2 and 3). In all cases, a reduction of both scales was reported. In detail, reductions of 2 cm,46 3.7 cm,48 and 5.3 cm52 on the VAS scale, and 449 and 4.551 on the NRS scale. Systematic reviews also used VAS as an outcome and reported a reduction in this outcome32,33 (Table 4).
The different methods used for assessing effectiveness/efficacy in the included article limits to compare results between studies (Table 2, Table 3). However, it is possible regarding to the CBPMs used in the same study. In a clinical trial, formulations with high THC content, similar THC and CBD, and high CBD content were evaluated.26 Bediol (6.3 % THC and 8 % CBD) and Bedrocan (22 % THC and less than 1 % CBD) had significant greater effect in spontaneous pain scores and tolerance to pressure pain compared to Bedrolite (9 % CBD and less than 1 % THC). Another observational study evaluating THC showed the potential effect of delta-9-THC to alleviate chronic and experimentally induced pain.52 Regarding the different types of cannabinoids used in future researches, it is important to denote that they should be focused on CBPMs with CBD, whether containing traces of THC or not, according to the NICE recommendation.20 However, it is important to denote that the THC:CBD proportion defines the global effect cause by CBPMs in patients with FMS; although, substances that act as CB1 receptor agonists can modulate and ameliorate the pain in this group of patients.19
The effects of cannabinoids, for instance THC or CBD are explained by their capacity to bind and to modulate CB1 and CB2 receptors, which belonging to the G-protein-coupled receptor family.19 In detail, THC causes a psychoactive effect mainly acting through CB1 receptor and modifies both the pain and emotions.19 CBD has analgesic and anti-inflammatory effects also through CB1 and CB2 receptors.19,58 Multi-targeted properties of CBD allow to have therapeutic potential in these conditions without psychotropic adverse events. CBD interacts with around 56 different molecular targets, including enzymes, ion channels, ionotropic, and metabotropic receptors in the nervous system acting as an agonist, inverse agonist, antagonist, or allosteric modulator on different targets. These interactions contribute to CBD's diverse pharmacological effects on various conditions such as epilepsy, pain, neuropsychiatric disorders, Alzheimer's disease, and inflammatory diseases. Understanding these molecular targets is crucial for utilizing CBD safely and effectively as a therapeutic agent and could have a safer profile and be preferred for treating pain in in patients with FMS.59,60
Regarding the safety of CBPMs in patients with FMS, the adverse effects identified were heterogeneous and the frequency ranged from 5 to 48 %, including dropout rates (Table 5). However, all adverse events were categorized from mild to moderate; therefore, no serious adverse events were identified in the studies included26, 27, 28, 29,47,47–49,51., 52, 53, 54 in this systematic review. Similar, in the systematic reviews identified no serious adverse events were reported16,32, 33, 34,36 (Table 4). Related to the frequency of adverse events, the most commonly reported were ‘sometimes’ for somnolence, sedation, dizziness, high, tachycardia, and conjunctival irritation, and ‘always’ for dry mouth, sedation, and hypotension in one study.48 It is important to note the relation between one adverse effect with the effectiveness of medicinal cannabis. In this sense, spontaneous pain scores were strongly correlated with the magnitude of drug high for Bedrocan and Bediol. In this case, drug high was measured with the Bowdle questionnaire.26 Nevertheless, current findings and systematic reviews support that the use of cannabis and cannabinoids is a tolerable treatment in patients with FMS.
Regardless of the common observed adverse effects reported across the studies, it would be important for comparing studies and treatments to have a standardized list of the most common adverse events. In this sense, in one protocol of study42 the safety will be measured by rating 10 common adverse effects: dizziness (when getting up), sleepiness, insomnia, headache, nausea, vomiting, constipation, drug high, hallucinations, and paranoia.
The dose titration could be a factor contributing to the safety and tolerability of CBPMs. In some studies,25,27, 28, 29,46,47,50,52,53 the dose was increased as long as no important side effects were detected; or if the clinician considered some improvement of the patient health. However, the maximum dose was not used in any study.
Related to patients withdrawn due to adverse events, in one study adverse effects leading to an interruption of cannabis therapy occurred in 17 patients (48.6 %). All of these side effects were reversed after cannabis cessation, but any of these were serious according to the FDA definition.51 This was the study with the highest percentage of patients retired from the study. Other percentages of withdrawn due to adverse effects were: 5 %,27 15 %,47 14 %,51 26 %,52 and 17 %.53 Regarding to other treatment, the comparison between amitriptyline and nabilone triggered 3 severe adverse effects. Headache and insomnia during amitriptyline and drowsiness with Nabilone (CBM).29
Drug interactions with cannabis is another aspect related to CMPS safety. When introducing CBPMs to a patient, is important to consider possible drug interactions between the pharmacological treatment for FMS or other comorbidities. In this sense, warfarin, tacrolimus, and buprenorphine may lead to clinically relevant interactions with cannabis.61 The safety use of CBPMs with concomitant opioids has to be addressed. Opioids are a common pharmacological group of drugs used as pain relievers. Their concomitant use with cannabis could be associated with serious psychological distress.62 However, many states of the EEUU have officially authorized cannabis and cannabinoids to “replace prescription opioid medications”, including pharmacotherapies for “all conditions for which opioids could be prescribed to treat”; and as “alternatives to opioid treatment”.63 Indeed, there is a protocol for an interventional study aiming to determine whether self-titration with CBPMs adjunct to self-titration with opioids reduces the adverse effects of both therapies.42
Concerning CBPMs rational use, initial prescription of type of products must be made by a specialist medical practitioner and approved by a multidisciplinary group; also, effectiveness and safety should be monitored and continuous evaluated.20 Dose should be tritiated by introducing a specialist prescriber as part of the shared care agreement with the patient. Overall, the evidence regarding the effectiveness and safety of CBMPs in patients with FMS is limited, therefore, additional interventional clinical trials and observation clinical studies are needed to establish the potential risks and benefits of CBPMs in this group of patients.11 Despite the effectiveness identified across the studies, there is still lack of certainty about the specific CBPMs and doses used. The evidence of better quality (interventional studies) has been made with nabilone, dronabinol, and CBPMs. There is a recently published protocol for interventional studies that will use the cannabis variety Bediol (6.3 % THC and 8 % CBD). The route of administration will be inhaled.42 There is also other protocol found in clinicaltrials.com evaluating drops with 1 mg of THC and 0.45 mg of CBD per drop.64
For this systematic review, five similar publications were identified16,32, 33, 34,36; however, in addition to date until search was conducted (April 2024), there are other relevant differences, for instance: a) the information regarding to effectiveness and safety was analyzed, synthesized, and presented in a customized form; b) efficacy/effectiveness data was extracted, analyzed, and synthesized in summary tables, according to type of study (observational or clinical, Table 2 and Table 3, respectively); and c) the safety data was presented in a specify table (Table 5) with the frequency (%) of the most common adverse events reported in the studies included. Therefore, there are some advantages of the current systematic review that are important to remark: a) the presentation of the outcome measure and its respective value for all efficacy measures reported in each study; b) the table with adverse events presenting the percentages of incidence in each study, allowing to the reader to know a range of incidence of each adverse event and the number of patients of each study; and 3) The summary of relevant information reported by the other 5 systematic reviews16,32, 33, 34,36 (Table 4).
Overall, findings reported by the other 5 systematic reviews are similar to those presented in this review; mainly there is evidence that supports that CBPMs could be effective and safe in patients with FMS and may improve their health condition. However, a previous Cochrane review16 reported controversial findings. It is important to denote that the Cochrane review include only 2 articles with nabilone; and main conclusion suggests that there is no evidence suggesting that nabilone is efficacy in treating people with FMS.
Although, the current systematic review produces additional evidence regarding effectiveness and safety of CBPMs in patients with FMS, including detailed information relate to effectiveness and safety in a customized form, the need to identifying and synthetizing high-quality evidence to better inform prescribers and patients continues. Therefore, considering the quality of available evidence, we recommend to continue making clinical studies, using a CBPMs with specific THC and CBD dose comparing with placebo, and selecting the efficacy outcomes more used in the published clinical studies (Visual Analog Scale -VAS-, Fibromyalgia Impact Questionnaire -FIQ-, FIQ-anxiety, FIQ-depression, Numeric Rating Scale -NRS-, and Verbal Rating Scale -VRS-), which may facility the evidence extraction, analysis, and synthesis for further systematic review and metanalysis.
Limitations. This review has some limitations; therefore, the results should be interpreted and used with caution. First, the search only in the PubMed/Medline database may be the main limitation, which may not have identified other clinically relevant publications. However, PubMed covers more than 37 million citations for biomedical literature from MEDLINE, life science journals. Also, in PubMed, in addition to Medline articles, there is access to PubMedCentral papers (full text articles deposited to promote open access, and articles that are “in process”). Also, we include relevant refences from the original retrieved articles to include more evidence. Therefore, this situation could be minimized with the inclusion of 9 publications identified as relevant in the reference list of the included articles. Second, in the included studies a high degree of heterogeneity in the study design was identified, resulting in difficulty in assessing the accurate effectiveness and safety of the CBMPs interventions. This limitation was also identified in the systematic reviews included.16,32, 33, 34,36 This could imply different beneficial effect under “real world” clinical settings with the therapeutic use of CBMPs. Third, due to a lack of known clinical and molecular biomarkers for the assessment of efficacy/effectiveness, it was mainly achieved by questionnaire; therefore, the subjective assessments and the inter-subject variability may lead to inaccurate clinical outcomes. This could imply that a change in the effectiveness methods or instruments used, can lead to different results. Finally, the use of the whole plant from a cultivation of cannabis without standardized amount of cannabinoids, such as the cannabis flowers 19 %,46 can lead to efficacy/effectiveness or safety imprecise assessments due to the possible variation between the percentage of the cannabinoids.65 This situation limits the external validity of these intervention in patients with FMS.
5. Conclusions
There is information that supports that cannabis-based products for medicinal use (CBPMs) could be effective and safe in patients with fibromyalgia syndrome (FMS); thereby, these products can improve musculoskeletal, somatic, and psychiatric symptoms in patients with FMS, mainly pain, fatigue, and depression; also, these products could be considered as safe.
Additional large-scale, high-quality, and multi-center randomized controlled trials are required to verify the efficacy of CBPMs, focused on medicinal products with CBD, whether containing traces of THC or not, according to current recommendations. There is a need to conduct more comprehensive studies and clinical trials to establish the real efficacy/effectiveness in terms of pain management, quality of life, and improvement of associated symptoms, as well as the effect on the use of other medications for managing chronic pain and safety concern possible. Therefore, the efficacy/effectiveness and safety of CBPMs in patients with FMS need clinical studies conducted with improved methodological design, mainly, large-scale, high-quality, and multi-center randomized controlled trials.
Funding source
The Pharmaceutical Promotion and Prevention Group received financial support from the Committee for Development Research (CODI) and sustainability program, Universidad de Antioquia.
CRediT authorship contribution statement
Valentina Lopera: Writing – original draft, Resources, Formal analysis, Data curation. Juan Carlos Restrepo: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Pedro Amariles: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
Juan-Carlos Restrepo is chief officer scientific of “El dorado botanical” If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the members of the Research Group on Pharmaceutical Promotion and Prevention of the University of Antioquia for their comments about the manuscript.
Conflicts of Interest: V.L and P.A: The authors declare no conflict of interest. JC.R is the chief officer scientific of “El dorado botanical”.
Contributor Information
Valentina Lopera, Email: valentina.loperag@udea.edu.co.
Juan Carlos Restrepo, Email: jcstat@jcrrlresearch.com.
Pedro Amariles, Email: pedro.amariles@udea.edu.co.
References
- 1.Bhargava J., Hurley J.A. StatPearls. StatPearls Publishing; 2024. Fibromyalgia.http://www.ncbi.nlm.nih.gov/books/NBK540974/ Accessed March 22, 2024. [Google Scholar]
- 2.Giorgi V., Bazzichi L., Batticciotto A., et al. Fibromyalgia: one year in review 2023. Clin Exp Rheumatol. 2023;41(6):1205–1213. doi: 10.55563/clinexprheumatol/257e99. [DOI] [PubMed] [Google Scholar]
- 3.Sarzi-Puttini P., Giorgi V., Marotto D., Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16(11):645–660. doi: 10.1038/s41584-020-00506-w. [DOI] [PubMed] [Google Scholar]
- 4.Berwick R., Barker C., Goebel A., Guideline Development Group The diagnosis of fibromyalgia syndrome. Clin Med Lond Engl. 2022;22(6):570–574. doi: 10.7861/clinmed.2022-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo clinic . Mayo Clinic; 2024. Fibromyalgia.https://www.mayoclinic.org/es/diseases-conditions/fibromyalgia/symptoms-causes/syc-20354780 Accessed July 3, 2024. [Google Scholar]
- 6.Tzadok R. In: Fibromyalgia Syndrome. Ablin J.N., Shoenfeld Y., editors. Springer International Publishing; 2021. Fibromyalgia: Classification, criteria, and diagnosis—What is fibromyalgia? pp. 83–89. [DOI] [Google Scholar]
- 7.Fitzcharles M.A., Perrot S., Häuser W. Comorbid fibromyalgia: a qualitative review of prevalence and importance. Eur J Pain Lond Engl. 2018;22(9):1565–1576. doi: 10.1002/ejp.1252. [DOI] [PubMed] [Google Scholar]
- 8.Bhargava J., Hurley J.A., Bhargava J., Hurley J.A. StatPearls. StatPearls Publishing; Treasure Island (FL): 2024. Fibromyalgia.https://www.ncbi.nlm.nih.gov/books/NBK540974/ Updated 2023 Jun 11. Available from: [Google Scholar]
- 9.Queiroz L.P. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. doi: 10.1007/s11916-013-0356-5. [DOI] [PubMed] [Google Scholar]
- 10.Flynn D. Chronic pain syndromes: fibromyalgia. FP Essent. 2023;533:7–15. [PubMed] [Google Scholar]
- 11.David P., Mohsen A., Amital H. Is medical Cannabis a solution for controlling fibromyalgia symptoms? Mayo Clin Proc. 2024;99(4):524–526. doi: 10.1016/j.mayocp.2024.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Pfizer's Lyrica Receives FDA Approval for Fibromyalgia Based on Expedited Review | Pfizer. 2007. https://www.pfizer.com/und/news/press-release/press-release-detail/pfizer_s_lyrica_receives_fda_approval_for_fibromyalgia_based_on_expedited_review Accessed May 3, 2024.
- 13.FDA Approves Cymbalta(R) for the Management of Fibromyalgia | Eli Lilly and Company. 2008. https://investor.lilly.com/news-releases/news-release-details/fda-approves-cymbaltar-management-fibromyalgia Accessed May 3, 2024.
- 14.SAVELLA® (milnacipran HCl) tablets. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/022256Orig1s024lbl.pdf Accessed May 3, 2024.
- 15.Farag H.M., Yunusa I., Goswami H., Sultan I., Doucette J.A., Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network Meta-analysis. JAMA Netw Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walitt B., Klose P., Fitzcharles M.A., Phillips T., Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694. doi: 10.1002/14651858.CD011694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarzi-Puttini P., Giorgi V., Sirotti S., et al. Pharmacotherapeutic advances in fibromyalgia: what’s new on the horizon? Expert Opin Pharmacother. 2024;25(8):999–1017. doi: 10.1080/14656566.2024.2365326. [DOI] [PubMed] [Google Scholar]
- 18.Fitzcharles M.A., Rampakakis E., Sampalis J.S., et al. Use of medical cannabis by patients with fibromyalgia in Canada after cannabis legalisation: a cross-sectional study. Clin Exp Rheumatol. 2021;39(suppl 130):115–119. doi: 10.55563/clinexprheumatol/qcyet7. 3. [DOI] [PubMed] [Google Scholar]
- 19.Cohen-Biton L., Buskila D., Nissanholtz-Gannot R. Review of fibromyalgia (FM) syndrome treatments. Int J Environ Res Public Health. 2022;19(19):12106. doi: 10.3390/ijerph191912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence . 2021. National Institute for Health and Care Excellence. NICE. Cannabis-based medicinal products NICE guideline 144. 2023.https://www.nice.org.uk/guidance/ng144 Available at. Accessed January 15 of 2024. [PubMed] [Google Scholar]
- 21.Bourke S.L., Schlag A.K., O’Sullivan S.E., Nutt D.J., Finn D.P. Cannabinoids and the endocannabinoid system in fibromyalgia: a review of preclinical and clinical research. Pharmacol Ther. 2022;240 doi: 10.1016/j.pharmthera.2022.108216. [DOI] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schünemann H., Brożek J., Guyatt G., Oxman A. 1st ed. P.A Orrego & M.X; Rojas: 2013. MANUAL GRADE Grading of Recommendations, Assessment, Development and Evaluation Versión En Español 2017. [Google Scholar]
- 24.Miller S.A., Forrest J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract. 2001;1(2):136–141. doi: 10.1016/S1532-3382(01)70024-3. [DOI] [Google Scholar]
- 25.Chaves C., Bittencourt P.C.T., Pelegrini A. Ingestion of a THC-rich Cannabis oil in people with fibromyalgia: a randomized, double-blind, placebo-controlled clinical trial. Pain Med Malden Mass. 2020;21(10):2212–2218. doi: 10.1093/pm/pnaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Donk T., Niesters M., Kowal M.A., Olofsen E., Dahan A., van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–869. doi: 10.1097/j.pain.0000000000001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgi V., Bongiovanni S., Atzeni F., Marotto D., Salaffi F., Sarzi-Puttini P. Adding medical cannabis to standard analgesic treatment for fibromyalgia: a prospective observational study. Clin Exp Rheumatol. 2020;38(suppl 123):53–59. 1. [PubMed] [Google Scholar]
- 28.Yassin M., Oron A., Robinson D. Effect of adding medical cannabis to analgesic treatment in patients with low back pain related to fibromyalgia: an observational cross-over single Centre study. Clin Exp Rheumatol. 2019;37(suppl 116):13–20. 1. [PubMed] [Google Scholar]
- 29.Ware M.A., Fitzcharles M.A., Joseph L., Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):604–610. doi: 10.1213/ANE.0b013e3181c76f70. [DOI] [PubMed] [Google Scholar]
- 30.Stockings E., Campbell G., Hall W.D., et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932–1954. doi: 10.1097/j.pain.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 31.Guillouard M., Authier N., Pereira B., Soubrier M., Mathieu S. Cannabis use assessment and its impact on pain in rheumatologic diseases: a systematic review and meta-analysis. Rheumatol Oxf Engl. 2021;60(2):549–556. doi: 10.1093/rheumatology/keaa534. [DOI] [PubMed] [Google Scholar]
- 32.Khurshid H., Qureshi I.A., Jahan N., et al. A systematic review of fibromyalgia and recent advancements in treatment: is medicinal Cannabis a new Hope? Cureus. 2021;13(8) doi: 10.7759/cureus.17332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strand N.H., Maloney J., Kraus M., et al. Cannabis for the treatment of fibromyalgia: a systematic review. Biomedicines. 2023;11(6):1621. doi: 10.3390/biomedicines11061621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurlyandchik I., Tiralongo E., Schloss J. Safety and efficacy of medicinal Cannabis in the treatment of fibromyalgia: a systematic review. J Altern Complement Med N Y N. 2021;27(3):198–213. doi: 10.1089/acm.2020.0331. [DOI] [PubMed] [Google Scholar]
- 35.Fitzcharles M.A., Baerwald C., Ablin J., Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz Berl Ger. 2016;30(1):47–61. doi: 10.1007/s00482-015-0084-3. [DOI] [PubMed] [Google Scholar]
- 36.Giossi R., Carrara F., Padroni M., et al. Systematic review and Meta-analysis seem to indicate that cannabinoids for chronic primary pain treatment have limited benefit. Pain Ther. 2022;11(4):1341–1358. doi: 10.1007/s40122-022-00434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzcharles M.A., Ste-Marie P.A., Häuser W., et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Care Res. 2016;68(5):681–688. doi: 10.1002/acr.22727. [DOI] [PubMed] [Google Scholar]
- 38.Lynch M.E., Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–744. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mücke M., Phillips T., Radbruch L., Petzke F., Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182. doi: 10.1002/14651858.CD012182.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yavne Y., Kabaha A., Rosen T., et al. The powers of flowers: evaluating the impact of floral therapy on pain and psychiatric symptoms in fibromyalgia. Isr Med Assoc J IMAJ. 2019;21(7):449–453. [PubMed] [Google Scholar]
- 41.Mullins C.F., Ffrench-O’Carroll R., Lane J., O’Connor T. Sharing the pain: an observational analysis of twitter and pain in Ireland. Reg Anesth Pain Med. 2020;45(8):597–602. doi: 10.1136/rapm-2020-101547. [DOI] [PubMed] [Google Scholar]
- 42.van Dam C.J., van Velzen M., Kramers C., et al. Cannabis-opioid interaction in the treatment of fibromyalgia pain: an open-label, proof of concept study with randomization between treatment groups: cannabis, oxycodone or cannabis/oxycodone combination-the SPIRAL study. Trials. 2023;24(1):64. doi: 10.1186/s13063-023-07078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell A.D., MacCallum C., Margolese S., et al. Clinical practice guidelines for Cannabis and cannabinoid-based medicines in the Management of Chronic Pain and co-Occurring Conditions. Cannabis Cannabinoid Res. 2024;9(2):669–687. doi: 10.1089/can.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurlyandchik I., Lauche R., Tiralongo E., Warne L.N., Schloss J. Plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with chronic widespread pain and fibromyalgia: a systematic review and meta-analysis. Pain Rep. 2022;7(6) doi: 10.1097/PR9.0000000000001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McPartland J.M., Guy G.W., Di Marzo V. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0089566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiara Gerardi M., Batticciotto A., Talotta R., Chiara Ditto M., Atzeni F., Sarzi-Puttini P. Efficacy of Cannabis Flos in patients with fibromyalgia: a monocentric observational study. ACR Meeting Abstracts September. 2016;28 https://acrabstracts.org/abstract/efficacy-of-cannabis-flos-in-patients-with-fibromyalgia-a-monocentric-observational-study/ Accessed March 12, 2024. [Google Scholar]
- 47.Skrabek R.Q., Galimova L., Ethans K., Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9(2):164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Fiz J., Durán M., Capellà D., Carbonell J., Farré M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and health-related quality of life. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagy I., Bar-Lev Schleider L., Abu-Shakra M., Novack V. Safety and efficacy of medical Cannabis in fibromyalgia. J Clin Med. 2019;8(6):807. doi: 10.3390/jcm8060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crestani F. Medical Cannabis for the treatment of fibromyalgia. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2018;24(5):281. doi: 10.1097/RHU.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 51.Mazza M. Medical cannabis for the treatment of fibromyalgia syndrome: a retrospective, open-label case series. J Cannabis Res. 2021;3(1):4. doi: 10.1186/s42238-021-00060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schley M., Legler A., Skopp G., Schmelz M., Konrad C., Rukwied R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr Med Res Opin. 2006;22(7):1269–1276. doi: 10.1185/030079906x112651. [DOI] [PubMed] [Google Scholar]
- 53.Weber J., Schley M., Casutt M., et al. Tetrahydrocannabinol (Delta 9-THC) treatment in chronic central neuropathic pain and fibromyalgia patients: results of a multicenter survey. Anesthesiol Res Pract. 2009;2009 doi: 10.1155/2009/827290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Erridge S., Holvey C., et al. Assessment of clinical outcomes in patients with fibromyalgia: analysis from the UK medical Cannabis registry. Brain Behav. 2023;13(7) doi: 10.1002/brb3.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumbhare D., Hassan S., Diep D., et al. Potential role of blood biomarkers in patients with fibromyalgia: a systematic review with meta-analysis. PAIN. 2022;163(7):1232. doi: 10.1097/j.pain.0000000000002510. [DOI] [PubMed] [Google Scholar]
- 56.Döhmen A., Kock M., Fischer F., Rose M., Obbarius A., Klapproth C.P. Are OMERACT recommendations followed in clinical trials on fibromyalgia? A systematic review of patient-reported outcomes and their measures. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2023;32(6):1521–1536. doi: 10.1007/s11136-022-03261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burckhardt C.S., Clark S.R., Bennett R.M. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 58.Peng J., Fan M., An C., Ni F., Huang W., Luo J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD) Basic Clin Pharmacol Toxicol. 2022;130(4):439–456. doi: 10.1111/bcpt.13710. [DOI] [PubMed] [Google Scholar]
- 59.Castillo-Arellano J., Canseco-Alba A., Cutler S.J., León F. The Polypharmacological effects of Cannabidiol. Molecules. 2023;28(7):3271. doi: 10.3390/molecules28073271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mlost J., Bryk M., Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21(22) doi: 10.3390/ijms21228870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopera V., Rodríguez A., Amariles P. Clinical relevance of drug interactions with Cannabis: a systematic review. J Clin Med. 2022;11(5):1154. doi: 10.3390/jcm11051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nigatu Y.T., Elton-Marshall T., Mann R.E., Hamilton H.A. Associations of cannabis use, opioid use, and their combination with serious psychological distress among Ontario adults. Stress Health J Int Soc Investig Stress. 2022;38(1):38–46. doi: 10.1002/smi.3071. [DOI] [PubMed] [Google Scholar]
- 63.Voelker R. States move to substitute opioids with medical marijuana to quell epidemic. JAMA. 2018;320(23):2408–2410. doi: 10.1001/jama.2018.17329. [DOI] [PubMed] [Google Scholar]
- 64.Knop Laboratorios . 2020. Phase II clinical trial, use of KL16-012 in women with fibromyalgia Refractary to conventional treatment.clinicaltrials.govhttps://clinicaltrials.gov/study/NCT04239469 Accessed December 31, 2023. [Google Scholar]
- 65.Geweda M.M., Majumdar C.G., Moore M.N., et al. Evaluation of dispensaries’ cannabis flowers for accuracy of labeling of cannabinoids content. J Cannabis Res. 2024;6(1):11. doi: 10.1186/s42238-024-00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]