Abstract
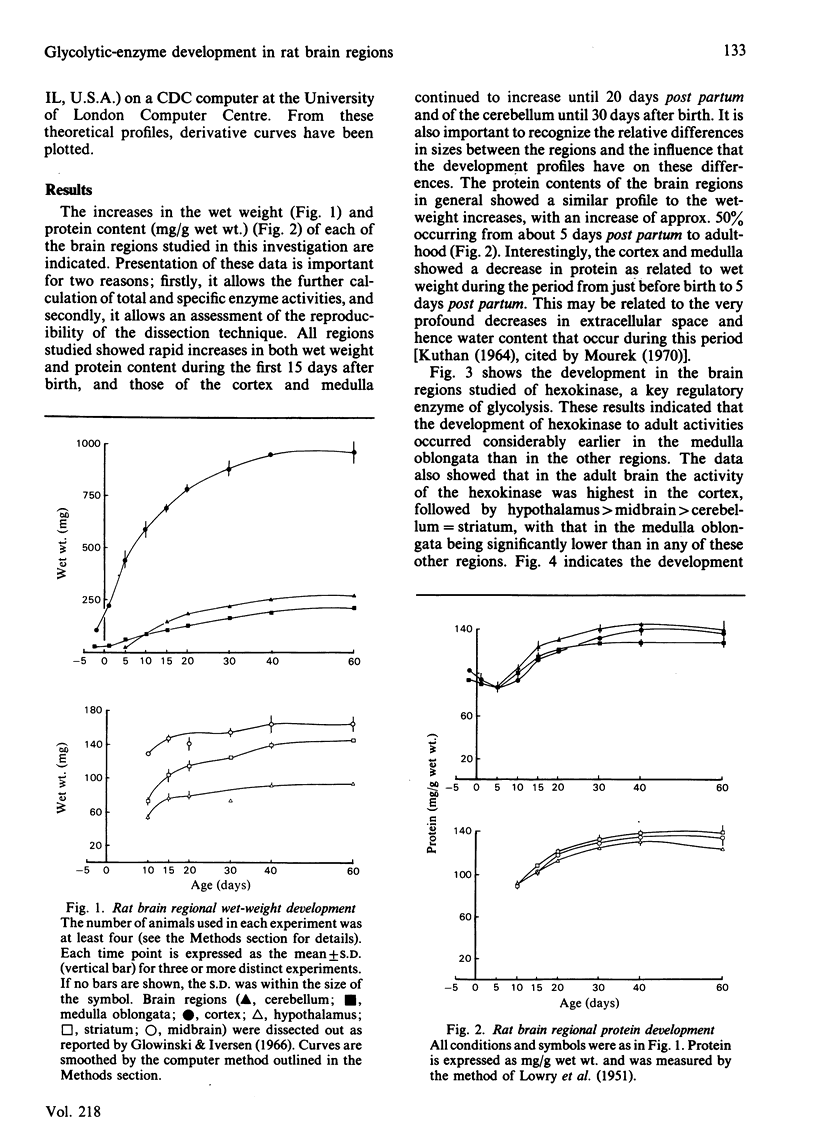
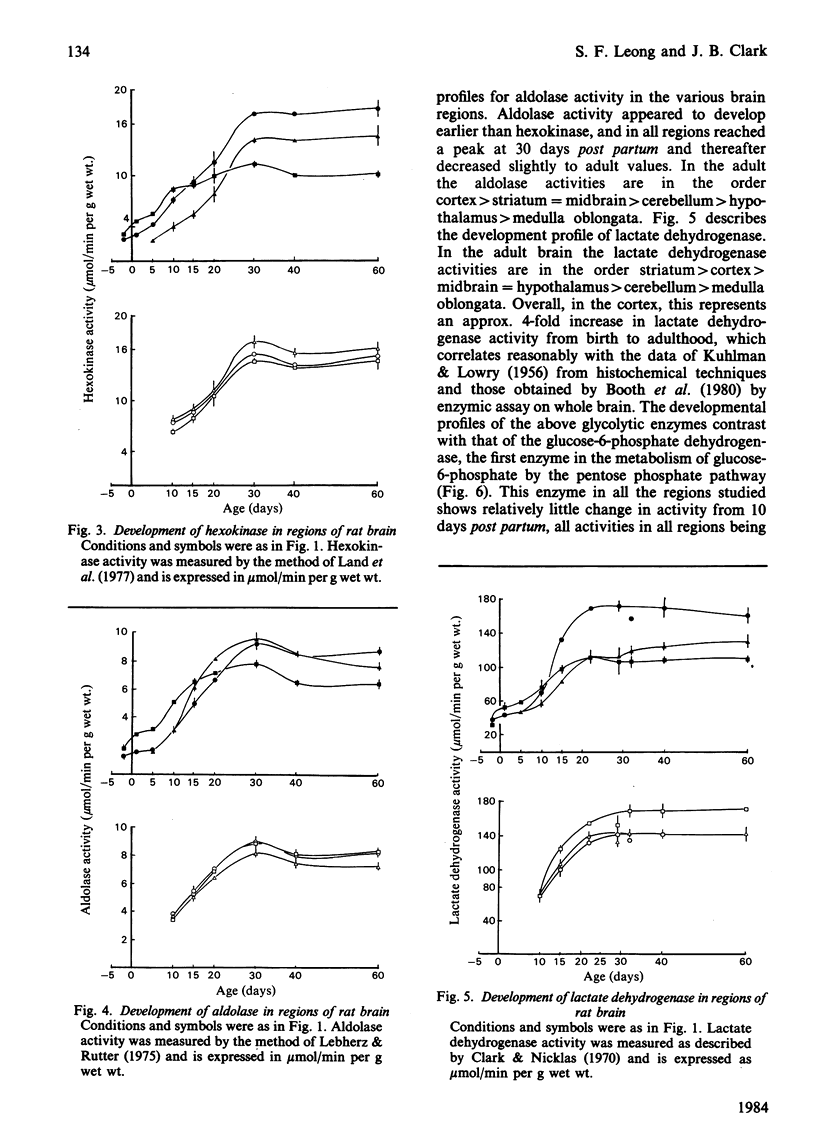
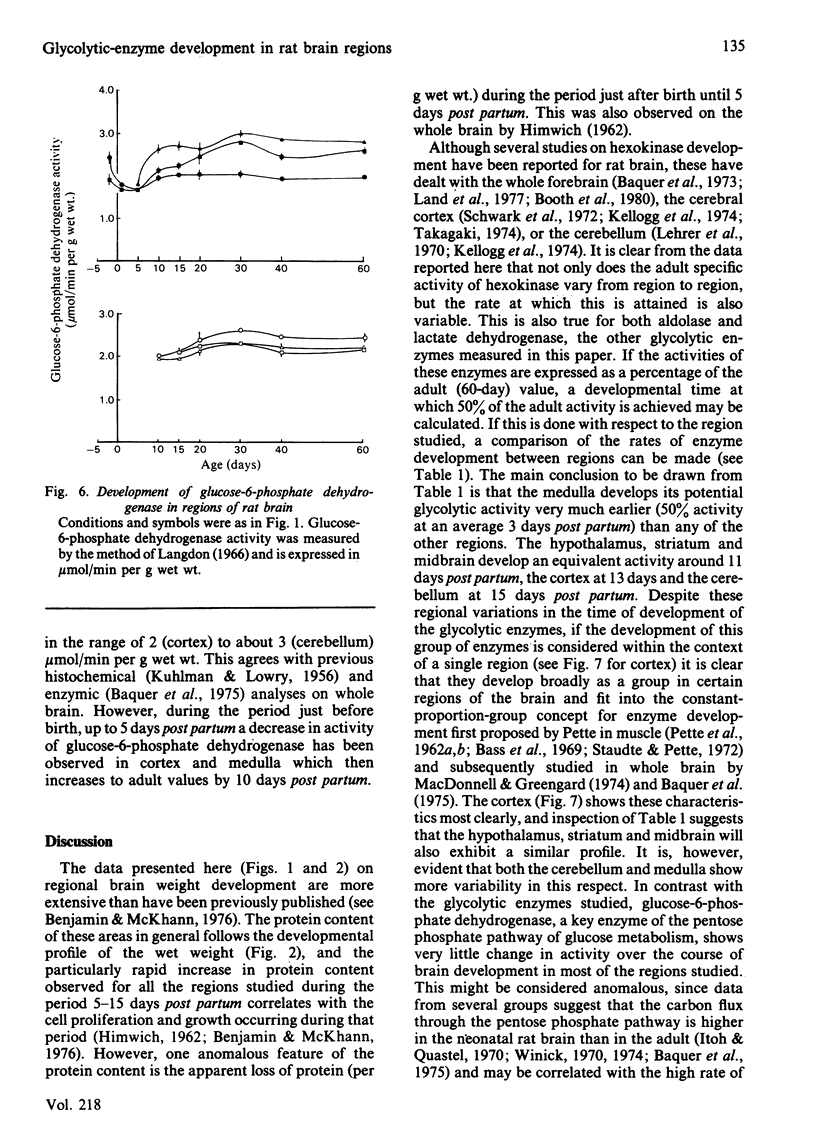
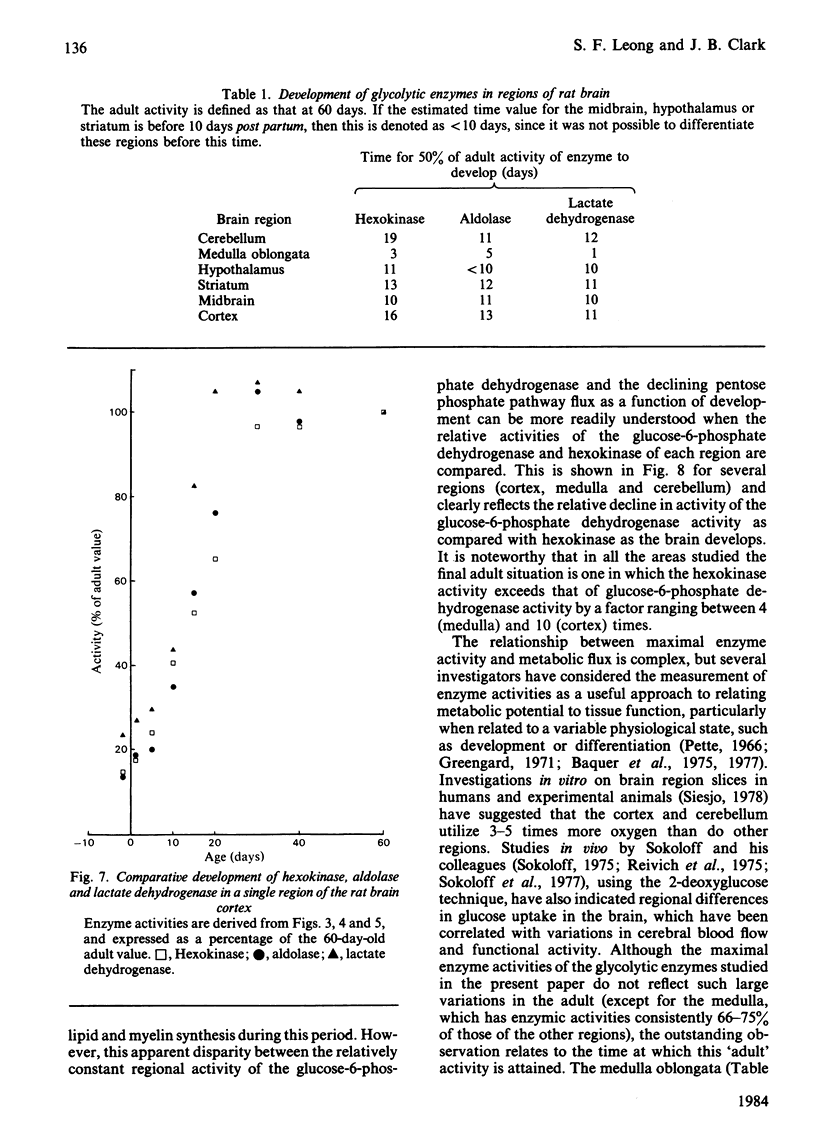
The development of key enzyme activities concerned with glucose metabolism was studied in six regions of the rat brain in animals from just before birth (-2 days) through the neonatal and suckling period until adulthood (60 days old). The brain regions studied were the cerebellum, medulla oblongata and pons, hypothalamus, striatum, mid-brain and cortex. The enzymes whose developmental patterns were investigated were hexokinase (EC 2.7.1.1), aldolase (EC 4.1.2.13), lactate dehydrogenase (EC 1.1.1.27) and glucose-6-phosphate dehydrogenase (EC 1.1.1.49). Hexokinase, aldolase and lactate dehydrogenase activities develop as a single cluster in all the regions studied, although the timing of this development varies from region to region. Glucose-6-phosphate dehydrogenase activity, however, declines relative to glycolytic enzyme activity as the brain matures. When the different brain regions are compared, it is clear that the medulla develops its glycolytic potential, as indicated by its potential enzyme activity, considerably earlier than the other regions (hypothalamus, striatum and mid-brain), with the cortex and cerebellar activities developing even later. This enzyme developmental sequence correlates well with the neurophylogenetic development of the brain and adds support to the hypothesis that the development of the potential for glycolysis in the brain is a necessary prerequisite for the development of neurological competence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquer N. Z., Hothersall J. S., McLean P., Greenbaum A. L. Aspects of carbohydrate metabolism in developing brain. Dev Med Child Neurol. 1977 Feb;19(1):81–104. doi: 10.1111/j.1469-8749.1977.tb08027.x. [DOI] [PubMed] [Google Scholar]
- Baquer N. Z., McLean P., Greenbaum A. L. Enzymic differentiation in pathways of carbohydrate metabolism in developing brain. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1282–1288. doi: 10.1016/0006-291x(73)90604-9. [DOI] [PubMed] [Google Scholar]
- Bass A., Brdiczka D., Eyer P., Hofer S., Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969 Sep;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Booth R. F., Patel T. B., Clark J. B. The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem. 1980 Jan;34(1):17–25. doi: 10.1111/j.1471-4159.1980.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Cremer J. E., Heath D. F. The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem J. 1974 Sep;142(3):527–544. doi: 10.1042/bj1420527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian tissues. Essays Biochem. 1971;7:159–205. [PubMed] [Google Scholar]
- Hawkins R. A., Biebuyck J. F. Ketone bodies are selectively used by individual brain regions. Science. 1979 Jul 20;205(4403):325–327. doi: 10.1126/science.451608. [DOI] [PubMed] [Google Scholar]
- Ito T., Quastel J. H. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J. 1970 Feb;116(4):641–655. doi: 10.1042/bj1160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHLMAN R. E., LOWRY O. H. Quantitative histochemical changes during the development of the rat cerebral cortex. J Neurochem. 1956 Dec;1(2):173–180. doi: 10.1111/j.1471-4159.1956.tb12070.x. [DOI] [PubMed] [Google Scholar]
- KUTHAN V. FUNK CN'I VZTAH NERVOV'YCH A GLIOV'YCH STRUKTUR. NOV'E POHLEDY NA FYSIOLOGII NERVOV'E SOUSTAVY. Cesk Fysiol. 1964 Jan;13:108–125. [PubMed] [Google Scholar]
- Kellogg E. W., Knull H. R., Wilson J. E. Soluble and particulate hexokinase in developing neural systems. J Neurochem. 1974 Mar;22(3):461–463. doi: 10.1111/j.1471-4159.1974.tb07615.x. [DOI] [PubMed] [Google Scholar]
- Kraus H., Schlenker S., Schwedesky D. Developmental changes of cerebral ketone body utilization in human infants. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):164–170. doi: 10.1515/bchm2.1974.355.1.164. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land J. M., Booth R. F., Berger R., Clark J. B. Development of mitochondrial energy metabolism in rat brain. Biochem J. 1977 May 15;164(2):339–348. doi: 10.1042/bj1640339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz H. G., Rutter W. J. The class I (Schiff base) fructose-diphosphate aldolase of Peptococcus aerogenes. Methods Enzymol. 1975;42:249–258. doi: 10.1016/0076-6879(75)42122-x. [DOI] [PubMed] [Google Scholar]
- Lehrer G. M., Bornstein M. B., Weiss C., Furman M., Lichtman C. Enzymes of carbohydrate metabolism in the rat cerebellum developing in situ and in vitro. Exp Neurol. 1970 Jun;27(3):410–425. doi: 10.1016/0014-4886(70)90104-4. [DOI] [PubMed] [Google Scholar]
- MacDonnell P. C., Greengard O. Enzymes in intracellular organelles of adult and developing rat brain. Arch Biochem Biophys. 1974 Aug;163(2):644–655. doi: 10.1016/0003-9861(74)90525-6. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B., Settergren G., Dahlquist G. Cerebral arterio-venous difference of acetoacetate and D- -hydroxybutyrate in children. Acta Paediatr Scand. 1972 May;61(3):273–278. doi: 10.1111/j.1651-2227.1972.tb16098.x. [DOI] [PubMed] [Google Scholar]
- Schwark W. S., Singhal R. L., Ling G. M. Metabolic control mechanisms in mammalian systems. Regulation of key glycolytic enzymes in developing brain during experimental cretinism. J Neurochem. 1972 Apr;19(4):1171–1182. doi: 10.1111/j.1471-4159.1972.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Staudte H. W., Pette D. Correlations between enzymes of energy-supplying metabolism as a basic pattern of organization in muscle. Comp Biochem Physiol B. 1972 Mar 15;41(3):533–540. doi: 10.1016/0305-0491(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Takagaki G. Developmental changes in glycolysis in rat cerebral cortex. J Neurochem. 1974 Sep;23(3):479–487. doi: 10.1111/j.1471-4159.1974.tb06049.x. [DOI] [PubMed] [Google Scholar]
- Winick M. Nutrition and nerve cell growth. Fed Proc. 1970 Jul-Aug;29(4):1510–1515. [PubMed] [Google Scholar]


