Abstract
Background
Emergence agitation (EA) is a state of psychomotor hyperactivity following general anesthesia and is associated with postoperative complications. Patients undergoing ear, nose, and throat (ENT) surgery are at a high risk for EA. We aim to assess whether preoperative dexmedetomidine nasal spray reduces the occurrence of EA for patients undergoing ENT surgery.
Methods
This is a protocol for a randomized, double-blind, controlled trial that will include 160 adults scheduled for ENT surgery. Patients will be randomly assigned, in a 1:1 ratio, to receive dexmedetomidine nasal spray (100 μg) or a same volume of normal saline approximately 30 min before general anesthesia induction. The primary endpoint is the incidence of EA, defined as a Riker sedation agitation scale (RSAS) score ≥5 from discontinuation of sevoflurane until 5 min after tracheal extubation. Secondary endpoints include (1) the maximal RSAS score during emergence; (2) the incidence of agitation in the postoperative care unit (PACU); (3) pain at rest and while coughing in the PACU and at 24 h postoperatively; (4) postoperative sleep disturbance on the first night after surgery; (5) anxiety within 24 h postoperatively; and (6) postoperative delirium during the first 24 h after surgery. All analyses will be performed on a modified intention-to-treat basis. For the primary endpoint, subgroup analysis will be conducted on sex, age, and type of surgery.
Discussion
We expect that preoperative dexmedetomidine nasal spray would reduce the incidence of EA after ENT surgery. Our results offer clinical evidence for improving anesthetic care for patients undergoing ENT surgery.
Trial Registration
Chinese Clinical Trial Registry (Identifier: ChiCTR2400086731).
Keywords: emergence agitation, dexmedetomidine, intranasal administration, ENT surgery
Introduction
Emergence agitation (EA) is an acute and self-limited state of negative behaviour during early recovery from general anesthesia, manifesting as psychomotor agitation, restlessness, hyperactivity, disorientation, and perceptual disturbance.1 The proposed risk factors of EA include male sex, younger age, type and duration of surgery, use of benzodiazepines, and postoperative pain.1,2 For patients undergoing ear, nose, and throat (ENT) surgery, the incidence of EA is high (ranging from 22% to 74%),3–6 which may lead to clinically significant consequences such as postoperative bleeding, self-extubation, removal of catheters, injury, and increased healthcare costs.7–9
Studies suggested that the use of α2 adrenoreceptor agonist, magnesium sulfate, ketamine, and multimodal analgesia helped to prevent EA.1 Dexmedetomidine is a selective α2 agonist exerting sympatholytic, sedative, anxiolytic, and analgesic effects. Intraoperative continuous dexmedetomidine infusion provided stable hemodynamics and a smooth emergence for patients undergoing nasal surgery.10 As a non-invasive route, intranasal administration achieves high bioavailability and can avoid pain and inconvenience by venipuncture in intravenous administration.11 Dexmedetomidine administration via nasal spray is simple and convenient with a high level of patient comfort and acceptance.12 For children and adults undergoing surgery, intranasal dexmedetomidine provided safe and satisfactory sedation.13–15 However, whether dexmedetomidine nasal spray would reduce EA following ENT surgery is unknown.
In this randomized controlled trial, we aim to determine the effects of dexmedetomidine nasal spray on the occurrence and severity of EA in patients undergoing ENT surgery. In addition, we will compare postoperative pain, sleep quality, anxiety, and delirium between the two groups.
Methods
Ethics and Registration
The trial protocol was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University (Approval No. 2024–200) on June 12, 2024. This trial was registered at the Chinese Clinical Trial Registry (identifier: ChiCTR2400086731) on July 9, 2024. This study will be conducted in accordance with the Declaration of Helsinki. Written informed consent will be obtained from all patients. This protocol follows the guidelines of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement (Table S1).16
Study Design and Status
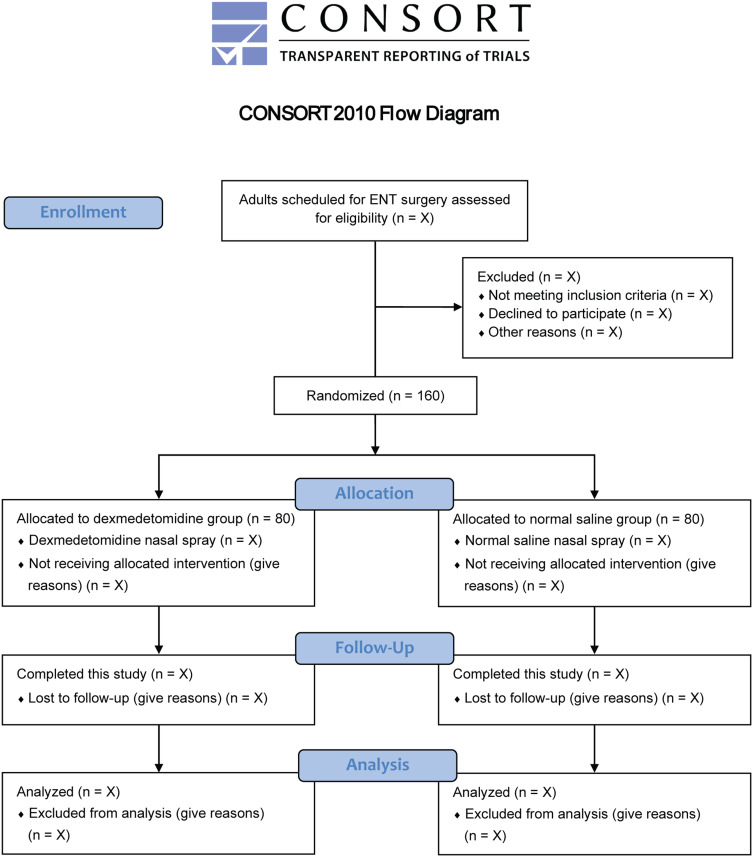
This is an investigator-initiated, single-center, randomized, double-blind, placebo-controlled, parallel-group trial with a superiority design. We will include a total of 160 adult patients undergoing ENT surgery at the First Affiliated Hospital of Soochow University, Suzhou, China. The first subject was enrolled on June 15, 2024. The recruitment is ongoing at the time of this protocol submission. We plan to enroll enough patients by the end of December 2024. Figure 1 shows the study flowchart. Table 1 illustrates the schedule of patient enrollment, study interventions, and measurements in accordance with the SPIRIT statement.
Figure 1.
Study flowchart.
Abbreviation: ENT, ear, nose, and throat.
Table 1.
Schedule of Patient Enrollment, Study Interventions, and Measurements
| Enrollment | Allocation | Post-Allocation | Close-Out | ||||
|---|---|---|---|---|---|---|---|
| Preanesthetic visit |
2h Before Surgery | 30 min Before induction | 5 min After extubation | PACU | 24h After surgery | Hospital Discharge | |
| Enrollment | |||||||
| Eligibility screening | × | ||||||
| Written informed consent | × | ||||||
| Baseline characteristics | × | ||||||
| Randomization | × | ||||||
| Allocation | × | ||||||
| Interventions | |||||||
| Dexmedetomidine nasal spray | × | ||||||
| Normal saline nasal spray | × | ||||||
| Measurements | |||||||
| Emergence agitation | × | ||||||
| The maximal RSAS score | × | × | |||||
| Agitation in PACU | × | ||||||
| Pain scores | × | × | |||||
| Delirium | × | × | |||||
| Sleep quality | × | ||||||
| Anxiety | × | ||||||
| Adverse eventsa | × | × | × | × | × | ||
| Length of PACU stay | × | ||||||
| Postoperative hospital stay | × | ||||||
Notes: According to SPIRIT 2013 statement of defining standard protocol items for clinical trials. a Including hypotension, bradycardia, postoperative nausea and vomiting, dizziness, headache, and sedation.
Abbreviations: RSAS, Riker sedation agitation scale; PACU, post-anesthesia care unit.
Eligibility Criteria
Patients aged ≥18 years with ASA physical status I–III and scheduled for ENT surgery under general anesthesia are eligible. The exclusion criteria include (1) history of neuropsychiatric or mental disorders; (2) left ventricular ejection fraction <40%, second-degree or higher atrioventricular block, sick sinus syndrome, or heart rate <50 beats/min; (3) allergy to any medication used in this study; or (4) refusal for providing the informed consent.
Randomization and Blinding
The randomization sequence will be generated by an independent statistician using an online tool (https://www.sealedenvelope.com/randomisation/) with a 1:1 ratio and block sizes of 2 and 4. The allocation details will be sealed in opaque envelopes to ensure concealment. According to the randomization results, a research nurse not involved in the following study process will randomly assign patients to a dexmedetomidine group or a normal saline group. This nurse will prepare nasal spray medications (dexmedetomidine or normal saline placebo) in identical bottles. One cannot tell the content inside because both medications are clear and colorless fluids. All patients and researchers responsible for data collection and outcome assessment will be blind to group allocation. Because of the sedative effect of dexmedetomidine nasal spray, anesthesia team may be aware of the study groups during anesthesia induction; however, the hypothesis of this study will not be disclosed. At the end of surgery, two investigators who are blind to group allocation will supervise the emergence process and assess the occurrence of EA.
Study Interventions and Anesthesia
In the preoperative waiting room, patients will receive nasal spray with 100 µg dexmedetomidine or normal saline approximately 30 min before anesthesia induction. Patients will be asked to clean their nostrils, sit straight, and tilt their heads slightly forward. A nurse will insert the nozzle into patients’ nasal cavity to administer two sprays into both the left and right nostrils within 2 min. The bispectral index (BIS; Medtronic, Minneapolis, MN, USA) monitoring will be applied from nasal spray administration until discharge from the operating room.
In the operating room, patients will receive peripheral vein cannulation and standard monitoring (cuff blood pressure, electrocardiography, pulse oximetry, end-tidal carbon dioxide, and temperature). During anesthesia induction, patients will receive intravenous dexamethasone 5 mg, sufentanil 0.2–0.4 μg/kg, and propofol 1.5–2 mg/kg. Rocuronium 0.6–0.8 mg/kg will be administered to facilitate tracheal intubation, followed by mechanical ventilation. Anesthesia will be maintained with inhalation of 1–3% sevoflurane, adjusted to BIS values of 40–60. Remifentanil will be infused at a rate of 0.05–0.2 μg/kg/min during surgery. Patients will be given intravenous palonosetron 0.25 mg as prophylactic antiemetics. At the end of surgery, sevoflurane and remifentanil will be stopped, and patients will receive intravenous flurbiprofen axetil 50 mg and sufentanil 0.1 μg/kg for postoperative analgesia. Additional analgesics (tramadol 50–100 mg) could be administered at patient request.
All patients will receive sugammadex 2 mg/kg to reverse neuromuscular blockade. During the emergence period, patients will be aroused using verbal stimuli only at a 30-s interval: “(Patient’s name), please open your eyes!”, with the voice volume at a normal conversation level. Patients will receive no physical stimuli (such as shaking the patients or patting on their shoulder). Tracheal extubation will be performed in the operating room. After extubation, patients will be transferred to the post-anesthesia care unit (PACU). A modified Aldrete score ≥9 indicates the readiness for discharge from the PACU to surgical wards.17 During surgery and in the PACU, hypotension (decrease in mean blood pressure >30% of baseline) and bradycardia (heart rate <50 beats/min) will be treated using intravenous ephedrine 6–10 mg and atropine 0.3–0.5 mg, respectively. Other anesthetic care will follow the institutional clinical standards.
Primary Endpoint
The primary endpoint is the incidence of EA, defined as a Riker sedation agitation scale (RSAS) score ≥5 from discontinuation of sevoflurane until 5 min after tracheal extubation in the operating room.3 The two investigators who are blind to group allocation will confirm the occurrence and severity of EA by reaching an agreement.
The RSAS scores range from 1 to 7, where 1 = unarousable (minimal or no response to noxious stimuli); 2 = very sedated (arousal to physical stimuli but not following commands); 3 = sedated (difficult to arouse, awakening to verbal stimuli or gentle shaking, following simple commands); 4 = calm and cooperate (calm, easy awakening, following commands); 5 = agitated (anxious, attempting to sit up, calming down to verbal instructions); 6 = very agitated (not calming down despite frequent verbal reminding of limits, requiring physical restraints, biting tracheal tube); and 7 = dangerous agitation (attempting to remove tracheal tube or other catheters, thrashing around, trying to climb out of bed rail, lashing out at staff).18
Secondary Endpoints
The secondary endpoints include (1) the maximal RSAS score during emergence; (2) the incidence of agitation in the PACU (defined as RSAS scores ≥ 5); (3) pain intensity at rest and while coughing in the PACU and at 24 h postoperatively; (4) the occurrence of postoperative sleep disturbance on the first night after surgery; (5) the incidence of anxiety within 24 h postoperatively; and (6) the incidence of postoperative delirium during the first 24 h after surgery.
Postoperative pain will be measured using the numerical rating scale (NRS), ranging from 0 (no pain) to 10 (the worst pain imaginable). Sleep quality will be assessed using the Athens Insomnia Scale, with a total score of 0–24 and a score ≥6 indicating postoperative sleep disturbance.19 Postoperative anxiety will be evaluated using the Hospital Anxiety and Depression Scale-Anxiety subscale, with a total score of 0–21 and a score ≥8 indicating anxiety.20 Postoperative delirium will be assessed using the 3-Minute Diagnostic Confusion Assessment Method between 08:00–10:00 and 18:00–20:00 on postoperative day 1.21
Perioperative Non-Endpoint Data
Venipuncture pain before anesthesia induction will be measured using the NRS. The hemodynamic data include mean blood pressure and heart rate at the following time points: baseline, immediately before induction, after tracheal intubation, discontinuation of sevoflurane, response to verbal stimuli, extubation, discharge from the operating room. The depth of anesthesia data are BIS values at those time points. Other perioperative data include time to verbal response, time to extubation, time to operating room discharge, length of PACU stay, length of postoperative hospital stay, need for additional analgesics, adverse events (hypotension, bradycardia, postoperative nausea and vomiting, dizziness, headache, sedation [RSAS score ≤ 3]), and major postoperative complications (Table S2). Patient satisfaction will be rated at 48 h postoperatively using a 5-point Likert scale (5 = very satisfied, 4 = satisfied, 3 = neutral, 2 = dissatisfied, and 1 = very dissatisfied).
Data Collection and Monitoring
A day before the surgery, a research assistant will screen for eligible patients and collect the baseline data. The perioperative non-endpoint data will be extracted from the electronic anesthesia system and via ward visits or by telephone. The data of primary and secondary endpoints will be collected by the blinded investigators. All data will be collected on case report forms and then entered into an electronic database under the supervision of the principal investigator. Once data registration is completed, the database will be locked. The dataset without patients’ personally identifiable information will be sent to an independent statistician for analysis based on the prespecified statistical plan. The whole process of data collection, registration, and statistical analysis will be monitored by a data monitoring committee independent of this research team.
Sample Size Calculation
Previous studies reported that the incidence of EA after ENT surgery was 55.4%.3,4 We expect that dexmedetomidine nasal spray would reduce the EA incidence to 33.2%. To test for this between-group difference, 75 patients in each group are required at an α level of 0.05 and a power of 80%. Considering possible dropouts, a total of 160 patients (n = 80 in each group) will be enrolled. The sample size was calculated using the PASS software (version 15.0.5, NCSS, LCC, Kaysville, UT, USA).
Statistical Analysis
Continuous data will be described as means (standard deviations) or medians (interquartile ranges), depending on data distribution. Categorical data will be shown as numbers (%). Groups will be compared using the independent t test, Mann–Whitney rank sum test, Chi-squared test, or Fisher’s exact test, as appropriate. For the primary and secondary endpoints, between-group differences will be analyzed using the relative risk and difference in means or medians with the 95% confidence intervals. A prespecified subgroup analysis will be conducted for the primary endpoint according to sex (male or female), age (≤30 years old or >30 years old), and type of surgery (ear, nose, or throat). Additionally, we will perform an interaction analysis, and a forest plot will be used for displaying the results of subgroup analysis.
All analyses will be performed on a modified intention-to-treat basis, including all patients who have undergone the randomization and have the main outcome data available. We consider that missing data would be rare in our dataset and there is no imputation plan. The independent statistician will analyze all data using the SPSS software (version 25.0; IBM SPSS, Chicago, IL, USA). A two-sided P value <0.05 denotes a statistically significant difference.
Discussion
This randomized, double-blind, controlled trial will include 160 adult patients undergoing ENT surgery to assess the effects of dexmedetomidine nasal spray compared with normal saline placebo on the incidence of EA. In addition, we will evaluate postoperative pain, quality of sleep, postoperative anxiety, and delirium during the first 24 h after surgery. Our primary hypothesis is that the preoperative use of dexmedetomidine nasal spray would reduce the incidence of EA in patients recovering from ENT surgery. The implementation of this trial and reporting of results will strictly follow the Consolidated Standards of Reporting Trials guidelines.22
Dexmedetomidine has been widely used in clinical anesthesia and intensive care sedation. A previous study conducted by Kim et al reported that intraoperative infusion of dexmedetomidine reduced the incidence of EA and maintained a stable hemodynamic status for patients undergoing nasal surgery.10 Data from a meta-analysis showed that intravenous dexmedetomidine decreased the incidence of EA and postoperative pain in children after sevoflurane anesthesia.23 However, Yang et al found that perioperative dexmedetomidine infusion did not lead to a significant change in the overall incidence of EA in patients undergoing free flap surgery, while the incidence of agitation after PACU admission was reduced.24 When intranasally administered, dexmedetomidine is rapidly and efficiently absorbed with a pharmacological effect similar to that during intravenous administration.25 The bioavailability of intranasal dexmedetomidine was approximately 65%, and peak plasma concentration was reached in 38 min.25 A recent study on healthy volunteers showed that dexmedetomidine nasal spray 100 μg was safe and provided satisfactory sedation.26 In the literature, there is no study investigating the effect of dexmedetomidine nasal spray on EA after ENT surgery.
Postoperative sleep quality on the first night and anxiety during the first 24 h after surgery are among the secondary endpoints. Patients undergoing ENT surgery often experience discomfort and anxiety during the first postoperative night, compromising the quality of sleep. If left unresolved, postoperative sleep disorders could impair patients’ cognitive function, prolong recovery after surgery, and even increase the risks of cardiovascular events.27 Dexmedetomidine is unique compared to traditional sedatives because it induces a state of sedation resembling natural sleep while maintaining respiratory stability.28
Our study has several limitations. First, this is a single-center trial, so the results may not be fully generalizable to patients in other medical centers. Next, a single dose of dexmedetomidine 100 μg will be intranasally administered, and the optimal dose of dexmedetomidine nasal spray requires further research. Last, as patients undergoing ENT surgery are at high risk of EA, we only include these patients in the current trial. Patients undergoing other surgical procedures may also benefit from the preoperative use of dexmedetomidine nasal spray, which needs to be confirmed in future studies.
In conclusion, this randomized clinical trial is designed to determine the effects of dexmedetomidine nasal spray on EA in adults undergoing ENT surgery. We expect that the intranasal administration of dexmedetomidine would lead to a notable reduction in the rate of EA as well as improve the postoperative recovery course following these ENT procedures.
Funding Statement
This work will be supported by National Natural Science Foundation of China (82471290 to KP), Suzhou Medical Health Science and Technology Innovation Project (SKY2022136 to KP), Postgraduate Research & Practice Innovation Program of The First Affiliated Hospital of Soochow University (RSJCX202408 to MYZ), Key Medical Research Projects in Jiangsu Province (ZD2022021 to FHJ), and Suzhou Clinical Medical Center for Anesthesiology (Szlcyxzxj202102 to FHJ).
Data Sharing Statement
Data will be made available on request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
- 1.Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73(6):471–485. doi: 10.4097/kja.20097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson JT. Pharmacologic, physiologic, and psychological characteristics associated with emergence delirium in combat veterans. AANA J. 2014;82(5):355–362. [PubMed] [Google Scholar]
- 3.Tolly B, Waly A, Peterson G, Erbes CR, Prielipp RC, Apostolidou I. Adult emergence agitation: a veteran-focused narrative review. Anesth Analg. 2021;132(2):353–364. doi: 10.1213/ane.0000000000005211 [DOI] [PubMed] [Google Scholar]
- 4.Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2000 patients. Can J Anaesth. 2010;57(9):843–848. doi: 10.1007/s12630-010-9338-9 [DOI] [PubMed] [Google Scholar]
- 5.Elsersy HE, Metyas MC, Elfeky HA, Hassan AA. Intraoperative magnesium sulphate decreases agitation and pain in patients undergoing functional endoscopic surgery: a randomised double-blind study. Eur J Anaesthesiol. 2017;34(10):658–664. doi: 10.1097/EJA.0000000000000642 [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Kim DK, Kim HY, Kim JK, Choi SW. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol. 2015;8(1):46–51. doi: 10.3342/ceo.2015.8.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747–753. doi: 10.1093/bja/ael094 [DOI] [PubMed] [Google Scholar]
- 8.Fields A, Huang J, Schroeder D, Sprung J, Weingarten T. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br J Anaesth. 2018;121(5):1052–1058. doi: 10.1016/j.bja.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–1466. doi: 10.1056/NEJMcp1605501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111(2):222–228. doi: 10.1093/bja/aet056 [DOI] [PubMed] [Google Scholar]
- 11.Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88(1):8–27. doi: 10.1016/j.ejpb.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Yoo H, Iirola T, Vilo S, et al. Mechanism-based population pharmacokinetic and pharmacodynamic modeling of intravenous and intranasal dexmedetomidine in healthy subjects. Eur J Clin Pharmacol. 2015;71(10):1197–1207. doi: 10.1007/s00228-015-1913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen F, Zhang Q, Xu Y, et al. Effect of intranasal dexmedetomidine or midazolam for premedication on the occurrence of respiratory adverse events in children undergoing tonsillectomy and adenoidectomy: a randomized clinical trial. JAMA Network Open. 2022;5(8):e2225473. doi: 10.1001/jamanetworkopen.2022.25473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Zhang LM, Zhang Y, et al. Intranasal dexmedetomidine as a sedative premedication for patients undergoing suspension laryngoscopy: a randomized double-blind study. PLoS One. 2016;11(5):e0154192. doi: 10.1371/journal.pone.0154192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun JH, Kim KN, Kim JY, Song SM. Les effets d’une prémédication intranasale de dexmédétomidine chez l’enfant: revue systématique et méta-analyse [The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis]. Can J Anaesth. 2017;64(9):947–961. doi: 10.1007/s12630-017-0917-x [DOI] [PubMed] [Google Scholar]
- 16.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetta DF, Elgalaly NA, Hetta HF, Fattah Mohammad MA. Preoperative Duloxetine to improve acute pain and quality of recovery in patients undergoing modified radical mastectomy: a dose-ranging randomized controlled trial. J Clin Anesth. 2020;67:110007. doi: 10.1016/j.jclinane.2020.110007 [DOI] [PubMed] [Google Scholar]
- 18.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022 [DOI] [PubMed] [Google Scholar]
- 19.Okajima I, Miyamoto T, Ubara A, et al. Evaluation of severity levels of the athens insomnia scale based on the criterion of insomnia severity index. Int J Environ Res Public Health. 2020;17(23):8789. doi: 10.3390/ijerph17238789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 21.Olbert M, Eckert S, Mörgeli R, Kruppa J, Spies CD. Validation of 3-minute diagnostic interview for CAM-defined Delirium to detect postoperative delirium in the recovery room: a prospective diagnostic study. Eur J Anaesthesiol. 2019;36(9):683–687. doi: 10.1097/eja.0000000000001048 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu M, Wang H, Zhu A, Niu K, Wang G. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLoS One. 2015;10(4):e0123728. doi: 10.1371/journal.pone.0123728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Li Z, Gao C, Liu R. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: a randomized, double-blind, control study. J Oral Maxillofac Surg. 2015;73(6):1065–1072. doi: 10.1016/j.joms.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Iirola T, Vilo S, Manner T, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67(8):825–831. doi: 10.1007/s00228-011-1002-y [DOI] [PubMed] [Google Scholar]
- 26.Kuang Y, Wang SY, Wang MN, et al. Safety, pharmacokinetics/pharmacodynamics, and absolute bioavailability of dexmedetomidine hydrochloride nasal spray in healthy subjects: a randomized, parallel, escalating dose study. Front Pharmacol. 2022;13:871492. doi: 10.3389/fphar.2022.871492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rampes S, Ma K, Divecha YA, Alam A, Ma D. Postoperative sleep disorders and their potential impacts on surgical outcomes. J Biomed Res. 2019;34(4):271–280. doi: 10.7555/jbr.33.20190054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.