Abstract
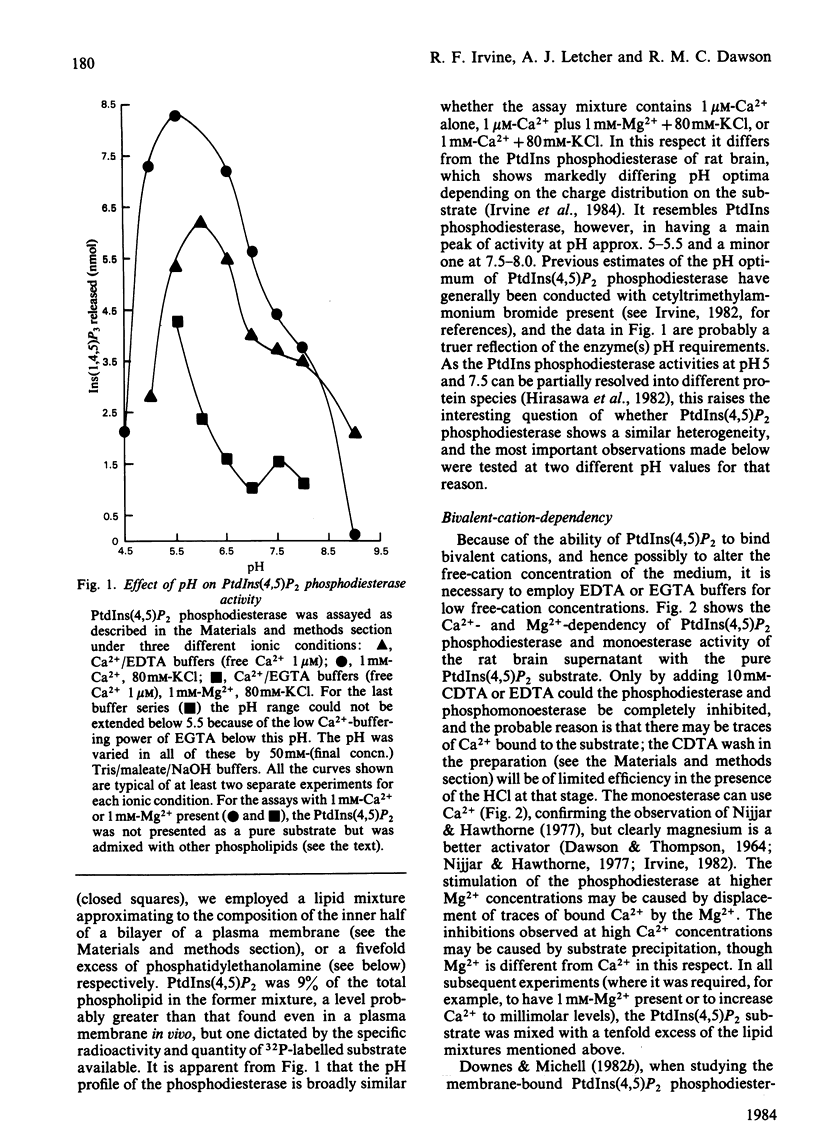
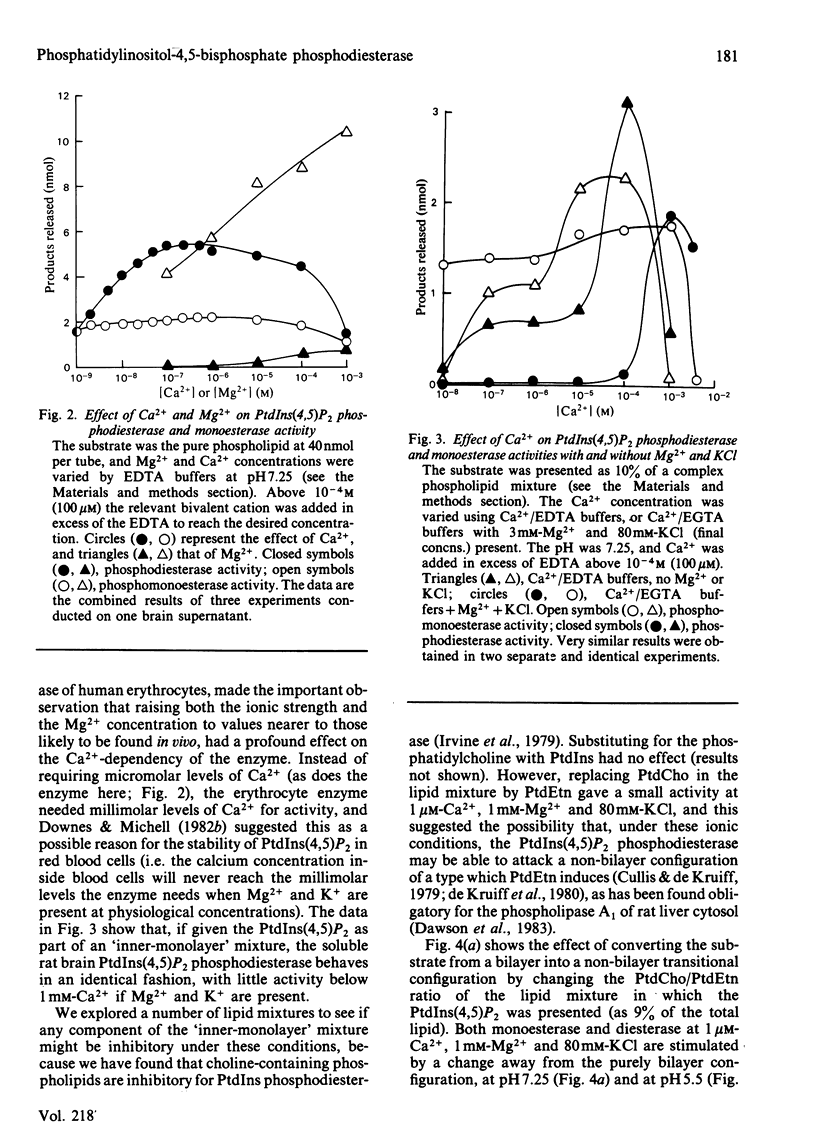
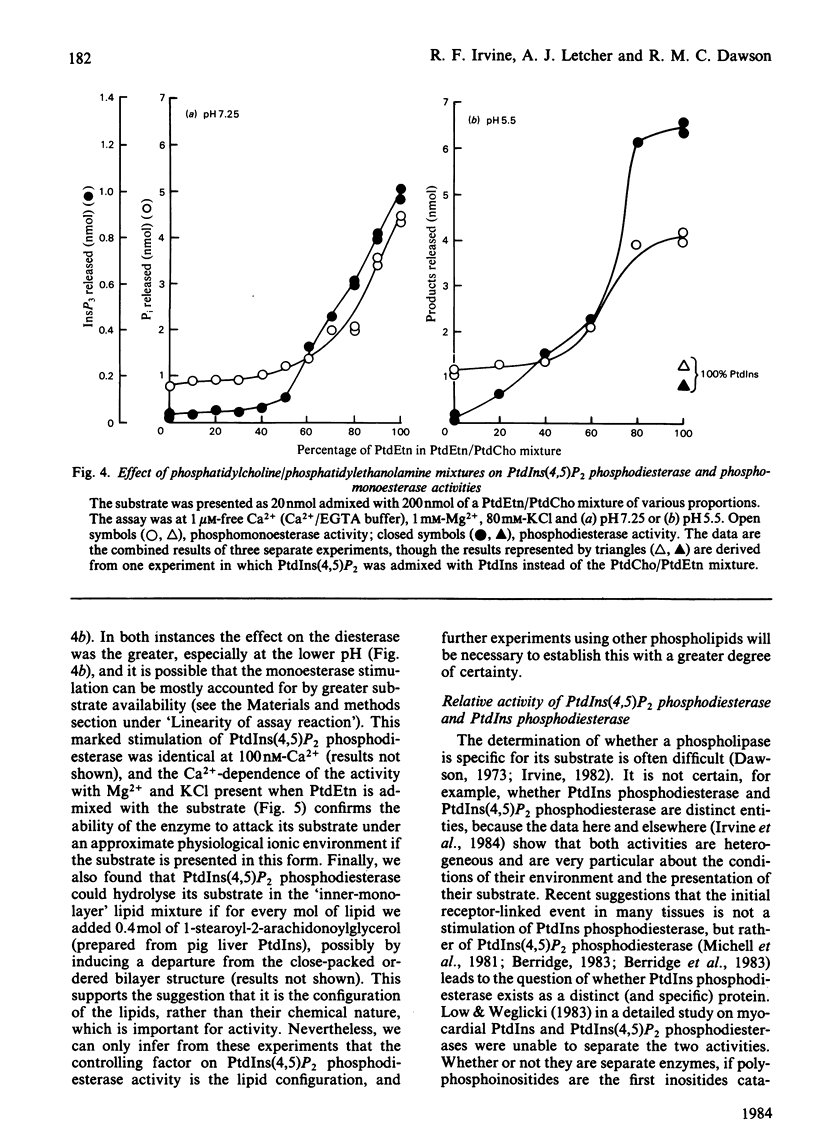
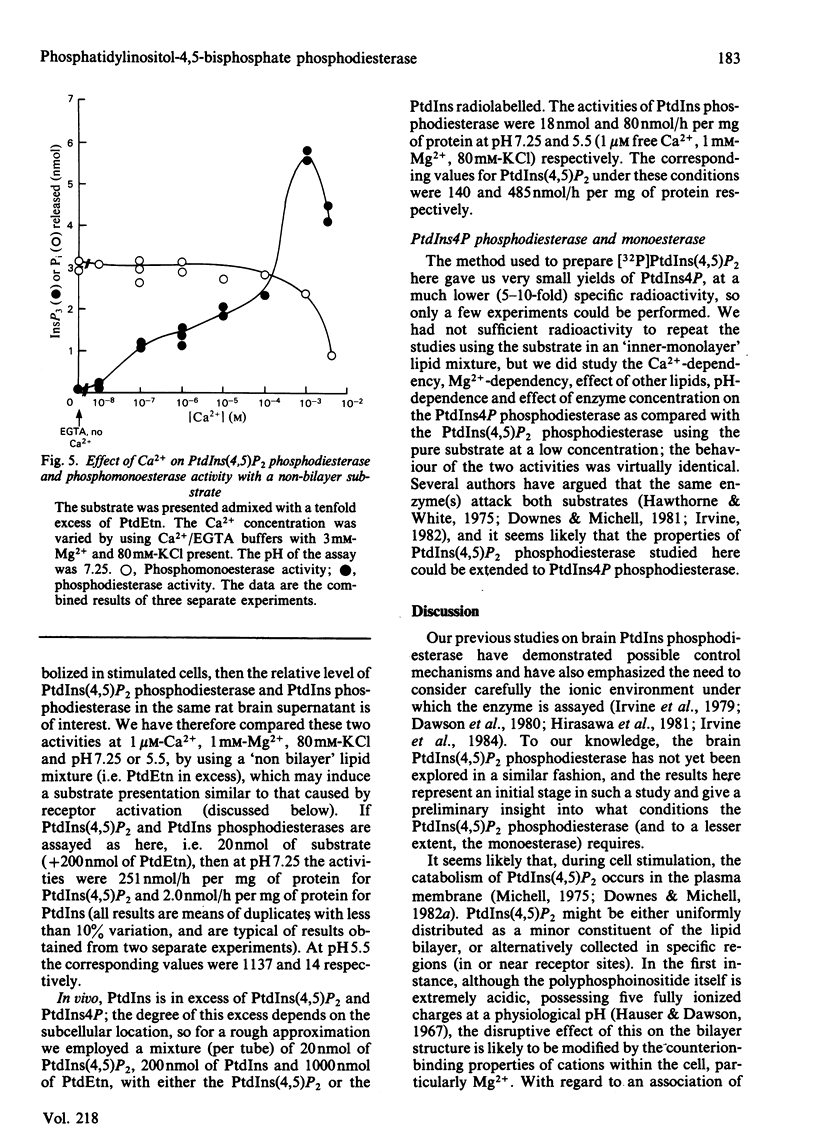
The phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] [and to a lesser extent, the phosphatidylinositol-4-phosphate (PtdIns4P)] phosphodiesterase and monoesterase activities of a rat brain supernatant have been studied by using 32P-labelled substrates prepared from human red blood cells. PtdIns(4,5)P2 monoesterase is maximally stimulated by Mg2+, though some activity is detectable in Ca2+/EDTA (Mg2+-free) buffers. The phosphodiesterase, however, is Ca2+-dependent, and in Ca2+/EDTA buffers with the pure lipid as substrate, shows maximal activity at 100 nM-Ca2+. If PtdIns(4,5)P2 is presented as a component of a lipid mixture of similar composition to that of the inner half of the lipid bilayer of a rat liver plasma membrane, the phosphodiesterase shows considerable activity at 1 microM-Ca2+, and is maximal at 100 microM-Ca2+. However, if it is assayed against the same substrate in Ca2+/EGTA buffers with 3mM-Mg2+ and 80 mM-KCl present (as an approximate parallel with the ionic environment in vivo), it shows no detectable activity below 100 microM-Ca2+, and is maximal at 1 mM-Ca2+. The monoesterase can hydrolyse PtdIns(4,5)P2 in such a lipid mixture at all Ca2+ concentrations with 1 or 3 mM-Mg2+ present. PtdIns(4,5)P2 phosphodiesterase can be induced to attack its substrate under ionic conditions similar to those in vivo (0.1-1 microM-Ca2+; 1 mM-Mg2+; 80 mM-KCl) by the conversion of its substrate into a non-bilayer configuration. If given such a substrate [by mixing PtdIns(4,5)P2 with an excess of phosphatidylethanolamine (PtdEtn)] it shows a shallow Ca2+-dependency curve from 0.1 to 100 microM and then a steep rise to 1 mM-Ca2+. Together these observations lead us to the suggestion that a perturbation in a membrane in vivo equivalent to a non-bilayer configuration would be sufficient to induce phosphodiesterase-catalysed PtdIns(4,5)P2 breakdown. When given substrates mixed with excess PtdEtn at pH 7.25 (or 5.5), 1 microM-Ca2+, 1 mM-Mg2+ and 80 mM-KCl, the rat brain supernatant phosphodiesterase activity hydrolysed PtdIns(4,5)P 50-100-fold faster than it hydrolysed phosphatidylinositol (PtdIns). If the supernatant was presented with such a non-bilayer mixture containing a ten-fold excess of PtdIns over PtdIns(4,5)P2, the latter phospholipid was still hydrolysed by phosphodiesterasic cleavage at nearly ten times the rate of the former. Receptor-stimulated phosphodiesterase cleavage of polyphosphoinositides is an early event in cell activation by many agonists. The properties of PtdIns(4,5)P2 phosphodiesterase in vitro suggest that a change in the presentation of its substrate would be a sensitive and sufficient control on the enzyme's activity in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N., Irvine R. F. The inhibition and activation of Ca2+-dependent phosphatidylinositol phosphodiesterase by phospholipids and blood plasma. Eur J Biochem. 1980 Nov;112(1):33–38. doi: 10.1111/j.1432-1033.1980.tb04983.x. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Hemington N. L., Hirasawa K. The alkaline phospholipase A1 of rat liver cytosol. Biochem J. 1983 Mar 1;209(3):865–872. doi: 10.1042/bj2090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Thompson W. The triphosphoinositide phosphomonoesterase of brain tissue. Biochem J. 1964 May;91(2):244–250. doi: 10.1042/bj0910244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The control by Ca2+ of the polyphosphoinositide phosphodiesterase and the Ca2+-pump ATPase in human erythrocytes. Biochem J. 1982 Jan 15;202(1):53–58. doi: 10.1042/bj2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Evans W. H. Transverse organization of phospholipids across the bilayer of plasma-membrane subfractions of rat hepatocytes. Biochem J. 1978 Aug 15;174(2):563–567. doi: 10.1042/bj1740563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Heterogeneity of the calcium-dependent phosphatidylinositol phosphodiesterase in rat brain. Biochem J. 1982 Aug 1;205(2):437–442. doi: 10.1042/bj2050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. The hydrolysis of phosphatidylinositol monolayers at an air/water interface by the calcium-ion-dependent phosphatidylinositol phosphodiesterase of pig brain. Biochem J. 1981 Feb 1;193(2):607–614. doi: 10.1042/bj1930607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. Phosphatidylinositol-degrading enzymes in liver lysosomes. Biochem J. 1977 Apr 15;164(1):277–280. doi: 10.1042/bj1640277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The calcium-dependent phosphatidylinositol-phosphodiesterase of rat brain. Mechanisms of suppression and stimulation. Eur J Biochem. 1979 Sep;99(3):525–530. doi: 10.1111/j.1432-1033.1979.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Low M. G., Weglicki W. B. Resolution of myocardial phospholipase C into several forms with distinct properties. Biochem J. 1983 Nov 1;215(2):325–334. doi: 10.1042/bj2150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium. 1982 Oct;3(4-5):429–440. doi: 10.1016/0143-4160(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nijjar M. S., Hawthorne J. N. Purification and properties of polyphosphoinositide phosphomonoesterase from rat brain. Biochim Biophys Acta. 1977 Feb 9;480(2):390–402. doi: 10.1016/0005-2744(77)90032-8. [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. Complex-formation between triphosphoinositide and experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):637–646. [PMC free article] [PubMed] [Google Scholar]
- Pieroni G., Verger R. Hydrolysis of mixed monomolecular films of phosphatidylcholine/triacylglycerol by pancreatic phospholipase A2. Eur J Biochem. 1983 May 16;132(3):639–644. doi: 10.1111/j.1432-1033.1983.tb07411.x. [DOI] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- RAAFLAUB J. Applications of metal buffers and metal indicators in biochemistry. Methods Biochem Anal. 1956;3:301–325. doi: 10.1002/9780470110195.ch10. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Reitveld A., Cullis P. R. 31P-NMR studies on membrane phospholipids in microsomes, rat liver slices and intact perfused rat liver. Biochim Biophys Acta. 1980 Aug 4;600(2):343–357. doi: 10.1016/0005-2736(80)90438-1. [DOI] [PubMed] [Google Scholar]



