Abstract
Background:
Crizanlizumab is a novel inhibitor of P-selectin, a key player in multicellular adhesion and inflammatory signaling, that leads to vaso-occlusion in sickle cell disease (SCD).
Objectives:
The SOLACE-adults study evaluated the pharmacokinetics, pharmacodynamics (P-selectin inhibition), safety, and efficacy of crizanlizumab, with or without hydroxyurea/hydroxycarbamide, in patients with SCD.
Design:
Phase II, single-arm, multicenter study.
Methods:
Patients with SCD aged 16–70 years, with ⩾1 vaso-occlusive crisis (VOC) within 12 months before screening, received crizanlizumab 5.0 or 7.5 mg/kg intravenous infusion every 4 weeks; dose groups were enrolled sequentially.
Results:
Of 57 patients enrolled, 45 received crizanlizumab 5.0 mg/kg and 12 received 7.5 mg/kg for a median duration of 206 and 170 weeks, respectively. Crizanlizumab concentrations reached maximum levels after a 30-min infusion and remained steady for 6 h, without significant accumulation. P-selectin inhibition was nearly complete for both doses. The median (interquartile range) absolute change in the annualized rate of VOCs leading to healthcare visit from baseline was −0.79 (−3.04, 2.01) in the 5.0 mg/kg group and −0.98 (−1.11, −0.41) in the 7.5 mg/kg group. All patients experienced at least one adverse event (AE), with no apparent differences between the two doses in the frequency and severity of AEs. Grade ⩾3 AEs occurred in 60% of the 5.0 mg/kg group and 58% of the 7.5 mg/kg group. Two patients in the 5.0 mg/kg group and one in the 7.5 mg/kg group had severe crizanlizumab-related infusion-related reactions, which resolved with treatment. No patients developed antibodies against crizanlizumab.
Conclusion:
Crizanlizumab 5.0 and 7.5 mg/kg demonstrated a dose-proportional increase in exposure, sustained P-selectin inhibition, a tolerable safety profile, and a sustained reduction in VOCs leading to healthcare visit. This suggests that crizanlizumab is a useful treatment option for patients with SCD who have experienced VOCs.
Trial Registration:
Keywords: crizanlizumab, pharmacodynamics, pharmacokinetics, sickle cell disease, vaso-occlusive crises
Plain language summary
Understanding the effects of crizanlizumab in patients with sickle cell disease: Results from the SOLACE-adults study
Crizanlizumab is a drug that inhibits P-selectin, which is involved in the inflammation and blockage of blood vessels that occurs in an inherited blood disorder called sickle cell disease (SCD). The SOLACE-adults study investigated the effects of crizanlizumab on the pharmacokinetics (how the drug moves through the body), pharmacodynamics (its effects on P-selectin), safety, and efficacy in patients with SCD. This was a Phase II study conducted at multiple centers, where all patients received crizanlizumab. Patients aged between 16 to 70 years who had SCD and experienced vaso-occlusive crises (VOCs, painful episodes caused by blocked blood vessels) within the year before the study were given crizanlizumab intravenously every 4 weeks at a dose of either 5.0 mg/kg or 7.5 mg/kg. A total of 57 patients enrolled in the study, of whom 45 received the 5.0 mg/kg dose and 12 received the 7.5 mg/kg dose. Crizanlizumab levels in the blood peaked 30 minutes after the infusion and remained steady for 6 hours. Both doses effectively inhibited P-selectin. The median reduction in the rate of vaso-occlusive crises leading to healthcare visits was -0.79 in the 5.0 mg/kg group and -0.98 in the 7.5 mg/kg group. All patients experienced adverse events, but there were no major differences between the two doses. Grade ⩾3 adverse events occurred in 60% of the 5.0 mg/kg group and 58% of the 7.5 mg/kg group. Some patients had severe reactions to the infusion, but these reactions resolved with treatment. None of the patients developed antibodies against crizanlizumab. In conclusion, crizanlizumab at both doses reached levels of exposure that caused sustained inhibition of P-selectin, had tolerable safety, and reduced VOCs requiring medical visits. This suggests that crizanlizumab is a beneficial treatment option for patients with SCD experiencing these painful crises.
Introduction
Sickle cell disease (SCD) is one of the most common monogenic, autosomal recessive diseases affecting millions of people worldwide. 1 Acute painful vaso-occlusive crisis (VOC), driven by red blood cell sickling and multicellular adhesion, is a very important clinical complication in SCD. Recurrent and unpredictable VOCs are accompanied by pain, chronic organ damage, and life-threatening complications, resulting in decreased quality of life and increased healthcare utilization costs in individuals with SCD.2,3 P-selectin is a cell-adhesion protein that drives multicellular adhesion and inflammatory signaling in SCD, leading to vaso-occlusion.4,5 P-selectin inhibition-based therapies have thus become a target for reducing the frequency of and preventing VOCs in patients with SCD. Crizanlizumab is a first-in-class, humanized monoclonal antibody that selectively binds to and inhibits P-selectin, thereby blocking interactions between activated platelets, sickled red blood cells, leukocytes, and the endothelium.6,7 Based on the SUSTAIN phase II study (NCT01895361) results, crizanlizumab was indicated to reduce the frequency of VOCs in adults and adolescent patients aged ⩾16 years with SCD. 7
The phase II SOLACE-adults study (NCT03264989) was designed to characterize long-term pharmacokinetic (PK)/pharmacodynamic (PD) properties of crizanlizumab and to evaluate the safety and efficacy of crizanlizumab in patients with SCD aged ⩾16 years. The interim analysis of the SOLACE-adults study published previously forecasted promising PK/PD results and demonstrated the long-term safety of crizanlizumab 5.0 and 7.5 mg/kg for a minimum of 12 months in >80% of the patients with SCD. 8 The findings were consistent with the SUSTAIN study results. 6 Herein, we report the final analysis (cutoff date: June 26, 2023) of the SOLACE-adults study including updated information on the PK/PD of crizanlizumab and long-term safety and efficacy outcomes for ~4 years for 5.0 mg/kg and ~3 years for 7.5 mg/kg dose groups.
Methods
Study design, patients, and treatment
The SOLACE-adults study was a phase II, single-arm, open-label study conducted in multiple centers within the United States. 8 The study included male or non-pregnant female patients aged 16–70 years who had a laboratory-confirmed diagnosis of SCD, regardless of their specific genotype. Eligible patients experienced at least 1 VOC episode within the 12 months prior to screening, as determined by medical history. Patients were excluded if they had a history of stem cell transplant, had received blood products within 30 days prior to the first dose of crizanlizumab, and/or were actively participating in a chronic transfusion program. In addition, patients who had experienced an acute VOC event within 7 days prior to the first dose were not eligible to participate.
Patients were sequentially enrolled into two dose groups, the first group treated with 5.0 mg/kg of crizanlizumab and the second group of approximately 10 more patients (to identify six evaluable patients) treated with the exploratory 7.5 mg/kg dose. The objective of the exploratory dose was to analyze the PK and PD in patients with SCD and to facilitate dose escalation in future studies of crizanlizumab in the clinical development program. All patients received crizanlizumab through intravenous infusion over 30-min on day 1, day 15, and subsequently every 4 weeks. Patients who received treatment with hydroxyurea (HU)/hydroxycarbamide (HC) or erythropoietin-stimulating agents for a minimum of 6 months prior to screening on a stable dose were permitted to continue with the same dose and schedule during the study. However, dose escalations were not allowed to ensure that changes in other disease-modifying treatments did not obscure the assessment of the crizanlizumab’s effectiveness. Voxelotor or selectin-targeting agents were not permitted during the study.
Overall, the findings from the SOLACE-adults study reported here include the final data from all patients during the complete study period (last patient last visit: June 26, 2023) in 45 patients who received a dose of 5.0 mg/kg and 12 patients who received a dose of 7.5 mg/kg. At the time of study closure, the patients treated with the crizanlizumab 5.0 mg/kg dose were offered to transition to a commercial supply of the approved 5.0 mg/kg dose of crizanlizumab. The patients treated with the exploratory dose of crizanlizumab 7.5 mg/kg were allowed to join a rollover study (SEG101A2401B; NCT04657822) for continued access to treatment with crizanlizumab.
Endpoints and assessments
The objectives of the study were to characterize the PK/PD of crizanlizumab, particularly its ex vivo P-selectin inhibition, as well as the safety and efficacy of crizanlizumab in patients with SCD who have a history of VOCs. The PK and PD of crizanlizumab 5.0 mg/kg assessed at the starting dose and at steady state were the primary endpoints of the study. PK parameters included the area under the curve (AUC) from time zero to the last measurable concentration sampling time after a single dose (AUCd15), the AUC calculated to the end of a dosing interval at steady state (AUCtau), and the maximum observed serum drug concentration (Cmax). PD parameters included PD-AUCd15 after a single dose and PD-AUCd29 after multiple doses.
PK and PD assessments were conducted in all patients before and after each dose on specific days throughout the study period; on days 1, 2, 4, 8, and 15 after the first dose (week 1 day 1); and subsequently on days 1, 2, 4, 8, 15, 22, and 29 after the fifth dose (week 15 day 1). To evaluate PK before each dose, serum concentrations were collected every 4 weeks from week 3 to 51 and, thereafter, every 24 weeks. PK and PD assessments were also conducted at the onset and resolution of each VOC, fever, or suspected infection whenever possible.
The secondary objectives were to assess the long-term efficacy, safety, and tolerability of crizanlizumab 5.0 and 7.5 mg/kg. Annualized rates of VOC events leading to healthcare visits in clinic, emergency room, or hospital and/or treated at home were assessed as efficacy endpoints. Throughout the study, patients were monitored for VOCs, and medical treatment information was collected and recorded as relevant with the VOC occurrence. Safety and tolerability including frequency of adverse events (AEs) and immunogenicity were assessed in all patients who received ⩾1 dose of crizanlizumab. Exploratory objectives included assessment of PK and PD parameters of crizanlizumab after single and multiple doses of 7.5 mg/kg, including pre-dose concentrations prior to each administration.
Statistical analyses
All quantitative data, including demographic and baseline characteristics, relevant PK and PD measurements, and efficacy and safety measurements, were summarized using descriptive statistics. All patients who received ⩾1 dose of crizanlizumab were analyzed according to the crizanlizumab dose (5.0 or 7.5 mg/kg), which was used for summarizing baseline and demographic characteristics and safety assessments.
The annualized rate of VOCs leading to a healthcare visit for each patient was reported by each year on treatment and was compared descriptively to the rate of VOC-related visits during the 12 months prior to the study screening (baseline). AEs were evaluated based on the Medical Dictionary for Regulatory Activities (MedDRA) version 26, and AE severity was based on the Common Terminology Criteria for Adverse Events version 5.0. Based on the clinical data from the crizanlizumab clinical program, the mechanism of action/type of the drug, and the disease under study, infusion-related reactions (IRRs), infections, effects on hemostasis (e.g., hemorrhage), and immunogenicity were identified as AEs of special interest (AESIs). The potential IRRs, regardless of grade and causality, were evaluated by conducting a search in the Novartis Case Retrieval Strategy database using customized MedDRA queries along with two different search strategies: (1) new combined search (standard, severe reactions, pain events, and complement-mediated IRRs) and (2) severe reactions. See the Supplemental section of Kanter et al. 6 for definitions of the IRR search strategies and the full lists of preferred terms used in each search. Immunogenicity was assessed by monitoring the presence of on-treatment antidrug antibodies specifically targeting crizanlizumab. All analyses were conducted using Novartis SAS version 9.4 software (SAS Institute Inc., Cary, North Carolina).
The study was approved by the institutional review board or independent ethics committee of each participating center (Supplemental Table S1) and was carried out in accordance with the recommendations of the Declaration of Helsinki. All participants gave written informed consent.
Results
Study population
A total of 57 patients participated in the study. There were 45 patients enrolled in the crizanlizumab 5.0 mg/kg group with a median age of 29 years (interquartile range (IQR): 22, 39 years) and 12 patients enrolled in the crizanlizumab 7.5 mg/kg group with a median age of 21 years (IQR: 18, 41 years). Most (98%) patients were Black or African Americans. Hemoglobin SS (HbSS) and hemoglobin SC (HbSC) were the predominant genotypes in the study population (54% and 26%, respectively). In the 5.0 mg/kg group, a higher proportion of patients were using concomitant HU (67% vs 58% in 7.5 mg/kg group) and had experienced ⩾5 VOCs (42% vs 25% in 7.5 mg/kg group) in the 12 months before screening (Table 1).
Table 1.
Baseline characteristics and demographics.
| Characteristic | Crizanlizumab 5.0 mg/kg | Crizanlizumab 7.5 mg/kg | All patients |
|---|---|---|---|
| N = 45 | N = 12 | N = 57 | |
| Age (years) | |||
| Median (IQR) | 29.0 (22.0, 39.0) | 20.5 (18.0, 40.5) | 29.0 (20.0, 39.0) |
| Age category, n (%) | |||
| ⩾16 to <18 years | 1 (2.2) | 2 (16.7) | 3 (5.3) |
| ⩾18 to <70 years | 44 (97.8) | 10 (83.3) | 54 (94.7) |
| Gender, n (%) | |||
| Female | 25 (55.6) | 6 (50.0) | 31 (54.4) |
| Male | 20 (44.4) | 6 (50.0) | 26 (45.6) |
| Race, n (%) | |||
| Black or African American | 44 (97.8) | 12 (100) | 56 (98.2) |
| Multiple (White and Black or African American) | 1 (2.2) | 0 | 1 (1.8) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 33 (73.3) | 8 (66.7) | 41 (71.9) |
| Hispanic or Latino | 3 (6.7) | 0 | 3 (5.3) |
| Unknown | 9 (20.0) | 4 (33.0) | 13 (22.8) |
| Body mass index, kg/m2 | |||
| Median (IQR) | 25.2 (21.9, 29.7) | 23.4 (21.3, 29.4) | 24.5 (21.9, 29.7) |
| ECOG performance status, n (%) | |||
| 0 | 31 (68.9) | 10 (83.3) | 41 (71.9) |
| 1 | 13 (28.9) | 2 (16.7) | 15 (26.3) |
| 2 | 1 (2.2) | 0 | 1 (1.8) |
| Genotype, n (%) | |||
| HbSS | 27 (60.0) | 4 (33.3) | 31 (54.4) |
| HbSC | 10 (22.2) | 5 (41.7) | 15 (26.3) |
| HbSβ0 | 3 (6.7) | 0 | 3 (5.3) |
| HbSβ+ | 3 (6.7) | 0 | 3 (5.3) |
| Other/unknown | 2 (4.4) | 3 (25.0) | 5 (8.8) |
| Concomitant HU use, n (%) | |||
| Yes | 30 (66.7) | 7 (58.3) | 37 (64.9) |
| No | 14 (31.1) | 5 (41.7) | 19 (33.3) |
| Missing | 1 (2) | 0 | 1 (1.8) |
| Number of VOC events in 12 months prior to screening | |||
| Median (IQR) | 4.0 (1.0, 7.0) | 2.0 (1.0, 4.5) | 3.0 (1.0, 6.0) |
| Categories, n (%) | |||
| <5 | 26 (57.8) | 9 (75.0) | 35 (61.4) |
| ⩾5 | 19 (42.2) | 3 (25.0) | 22 (38.6) |
| VOC type in 12 months prior to screening, n (%) | |||
| Uncomplicated sickle cell VOC | 44 (97.8) | 11 (91.7) | 55 (96.5) |
| Acute chest syndrome | 4 (8.9) | 2 (16.7) | 6 (10.5) |
| Priapism | 2 (4.4) | 0 | 2 (3.5) |
ECOG, Eastern Cooperative Oncology Group; HbSβ, hemoglobin S beta-thalassemia; HbSC, hemoglobin SC; HbSS, hemoglobin SS; HU, hydroxyurea; IQR, interquartile range; VOC, vaso-occlusive crisis.
At the time of the decision to close the study, there were 24 patients (42%) who were still receiving treatment with crizanlizumab. There were several reasons for treatment discontinuation prior to the study closure including patient decision (13 patients (29%) in the 5.0 mg/kg group, none in the 7.5 mg/kg group) and physician decision (6 patients (13%) in the 5.0 mg/kg group, 4 patients (33%) in the 7.5 mg/kg group). The specific reasons cited by physicians included initiation of commercial crizanlizumab (n = 3), AEs, medical monitoring, and switch to commercial crizanlizumab (n = 1, each) in the 5.0 mg/kg group, and initiation of commercial crizanlizumab (n = 2), denial of commercial crizanlizumab (n = 1), and disease progression (n = 1) in the 7.5 mg/kg group. Other reasons for discontinuation included AEs (2 (4%) in 5.0 mg/kg group, 1 (8%) in the 7.5 mg/kg group); on-treatment pregnancy (2 (4%) in 5.0 mg/kg group); lost to follow-up, switch to new therapy, or protocol deviation (1 (8%) in the 7.5 mg/kg group); and death (1 (2%) in the 5.0 mg/kg group, 1 (8%) in the 7.5 mg/kg group). At the time of study closure, treatment was discontinued for the remaining 24 patients (19 patients (42%) in the 5.0 mg/kg group, 5 patients (42%) in the 7.5 mg/kg group). Of these 24 patients, 14 patients (31%) in the 5.0 mg/kg group resumed crizanlizumab treatment through commercial supply, while 5 patients (42%) in the 7.5 mg/kg group continued treatment through enrollment in a roll-over clinical study.
For both the dose groups (5.0 and 7.5 mg/kg), the median duration of crizanlizumab treatment was 206.1 weeks (IQR: 98.0, 242.1 weeks) and 169.6 weeks (IQR: 120.0, 216.6 weeks), respectively. In the 5.0 mg/kg group, 39 patients (87%) received treatment for at least 54 weeks, and 31 patients (69%) received treatment for at least 106 weeks. In the 7.5 mg/kg group, 10 patients (83%) received treatment for at least 54 weeks, and 9 patients (75%) received treatment for at least 106 weeks.
Change in PK and PD parameters
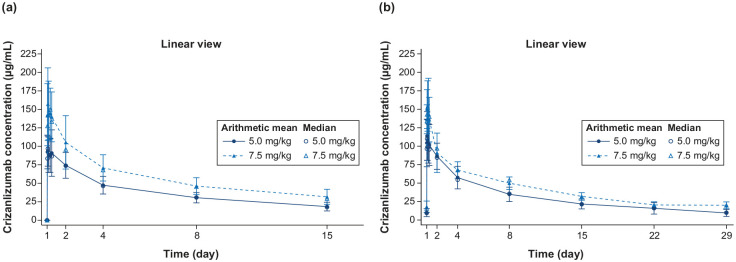
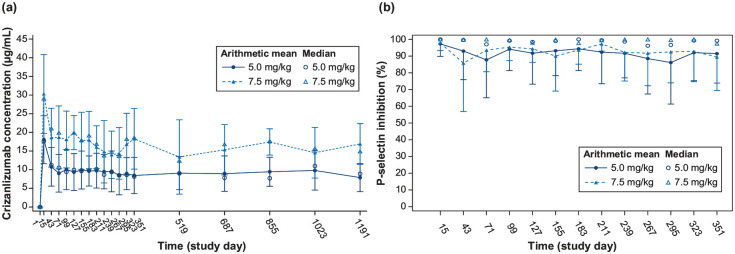
The serum concentrations of crizanlizumab increased to almost maximum levels (Cmax) within 30 min of infusion for the 5.0 and 7.5 mg/kg doses and remained steady for 6 h after infusion, both at starting dose and at steady state. The Cmax at weeks 1 and 15 were similar for each dose, indicating no significant accumulation over time. Moreover, both AUC and Cmax increased in a dose-proportional manner from 5.0 to 7.5 mg/kg (Figure 1(a) and (b)). The interpatient variability for both AUC and Cmax was relatively low (Table 2). Throughout the dosing interval at steady state, there was near-complete inhibition of P-selectin (ex vivo) for both 5.0 and 7.5 mg/kg doses. The inhibition ranged from 92% to 98% for the 5.0 mg/kg dose and 90% to 96% for the 7.5 mg/kg dose, as shown in Supplemental Figure S1. The pre-dose concentration of crizanlizumab increased almost proportionally from 5.0 to 7.5 mg/kg with a consistent relationship throughout the study (Figure 2(a)). The inhibition of P-selectin remained consistent and stable before each dose throughout the study (Figure 2(b)).
Figure 1.
Mean (SD) and median serum concentration-time profiles for the crizanlizumab 5.0 and 7.5 mg/kg dose groups at (a) starting dose (up to day 15 post-dose) and (b) steady state (up to day 29 post-dose).
The pharmacokinetic analysis set included all patients who received at least one planned treatment of 5.0 or 7.5 mg/kg and provided at least one corresponding evaluable PK concentration. SD, standard deviation.
Table 2.
PK and PD parameters for crizanlizumab at starting dose (week 1 day 1) and at steady state (week 15 day 1).
| PK parameters a | Crizanlizumab (5.0 mg/kg) | Crizanlizumab (7.5 mg/kg) |
|---|---|---|
| Number of individuals at each dose level | n = 45 | n = 11 |
| Starting dose (week 1, day 1) | ||
| AUCd15 (h × µg/mL) | n = 38 | n = 10 |
| Mean ± SD (CV%) | 13,100 ± 2810 (21.6) | 19,800 ± 4490 (22.7) |
| Cmax (µg/mL) | n = 42 | n = 10 |
| Mean ± SD (CV%) | 102 ± 29.8 (29.4) | 174 ± 49.9 (28.7) |
| Tmax (h) | n = 42 | n = 10 |
| Median (range) | 1.32 (0.42–26.1) | 1.06 (0.55–23.6) |
| Steady state (week 15, day 1) | ||
| AUCtau (h × µg/mL) | n = 35 | n = 9 |
| Mean ± SD (CV%) | 20,800 ± 5030 (24.2) | 27,800 ± 2780 (10) |
| Cmax (µg/mL) | n = 36 | n = 10 |
| Mean ± SD (CV%) | 123 ± 36.4 (29.5) | 168 ± 34.2 (20.4) |
| Tmax (h) | n = 36 | n = 10 |
| Median (range) | 1.70 (0.55–6.25) | 3.04 (0.58–23.4) |
| T1/2 (days) | n = 35 | n = 10 |
| Mean ± SD | 10.9 (3.2) | 13.5 (2.4) |
| PD parameters b | n = 43 | n = 10 |
| Starting dose (week 1, day 1) | ||
| PD-AUCd15 (h × %) | n = 36 | n = 9 |
| Mean ± SD (CV%) | 33,200 ± 1830 (5.5) | 33,200 ± 2680 (8.1) |
| Steady state (week 15, day 1) | ||
| PD-AUCd29 (h × %) | n = 33 | n = 9 |
| Mean ± SD (CV%) | 66,900 ± 5540 (8.3) | 64,600 ± 5450 (8.4) |
AUCd15, area under the curve from time 0 to the last measurable concentration sampling time at starting dose; AUCtau, area under the curve from time 0 to the last measurable concentration sampling time at steady state; Cmax, maximum observed serum drug concentration after dose administration; CV%, coefficient of variation; PD, pharmacodynamics; PD-AUCd15, area under the curve from time 0 to the last measurable PD sampling time at starting dose; PD-AUCd29, area under the curve from time 0 to the last measurable PD sampling time at steady state; Tmax, time to reach maximum observed serum drug concentration after dose administration; T½, elimination half-life associated with the terminal slope of a semilogarithmic scale.
The PD analysis set included all patients with ⩾1 evaluable PK profile.
The PD analysis set included all patients who provided at least one evaluable PD profile.
Figure 2.
Median and arithmetic mean (SD) pre-dose (trough). (a) Serum concentration-time profiles and (b) serum PD (P-selection inhibition)-time profiles for crizanlizumab.
The PD analysis set included all patients who received at least one planned treatment of 5.0 or 7.5 mg/kg and provided at least one corresponding evaluable PK concentration. PD, pharmacokinetic; SD, standard deviation.
Efficacy
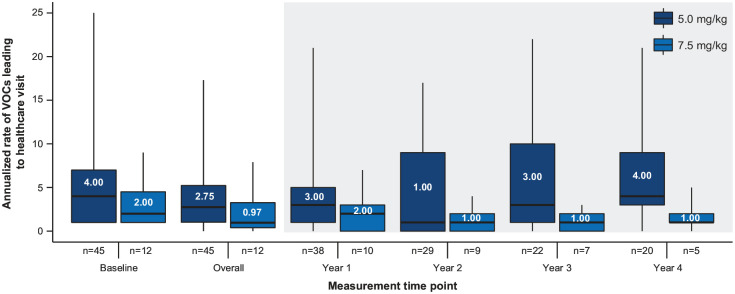
The median (IQR) absolute change in the annualized rate of VOCs from baseline was −0.79 (−3.04, 2.01) in the 5.0 mg/kg group and −0.98 (−1.11, −0.41) in the 7.5 mg/kg group. Figure 3 shows the baseline annualized rate of VOC, as well as overall annualized rate of VOC, during the whole study and by year. Note that, as only patients with complete data in a corresponding year were included, the sample size decreased as the study progressed. There were also several patients who did not experience a VOC during the entire treatment period: six patients (13%) in the 5.0 mg/kg group and two patients (17%) in the 7.5 mg/kg group.
Figure 3.
Annualized rate of VOCs leading to healthcare visit by each year in the crizanlizumab 5.0 and 7.5 mg/kg groups.
The whiskers are minimum and maximum, the boxes are interquartile ranges, and horizontal lines and displayed values in the boxes are medians. VOC, vaso-occlusive crisis.
Safety
In this study, all patients in both dose groups experienced at least one AE, regardless of relationship to study treatment. The most common AEs reported (⩾25%) in the 5.0 and 7.5 mg/kg dose groups, respectively, included pyrexia (31% and 25%), headache (27% and 17%), hypokalemia (26% and 17%), COVID-19 (18% and 25%), and vomiting (9% and 25%). Grade ⩾3 AEs occurred in 27 patients (60%) in the 5.0 mg/kg dose group and in 7 patients (58%) in the 7.5 mg/kg dose group. Hypokalemia reported in 7 patients ((16%); all in 5.0 mg/kg group) was the most common grade ⩾3 AE. None of these events were related to treatment and all had resolved (Table 3). At least 1 treatment-related AE was reported in 11 patients (24%) and 3 patients (25%) in the 5.0 and 7.5 mg/kg dose groups, respectively. Grade ⩾3 treatment-related AEs including IRR with sickle cell crisis was reported in one patient (2%) in the 5.0 mg/kg dose group and increased blood bilirubin was reported in one patient (8%) in the 7.5 mg/kg dose group, which led to treatment withdrawal. None of the patients reported grade 4 treatment-related AEs. Two patients (4%) in the 5.0 mg/kg dose group reported grade 1 or 2 electrocardiogram QT (QT interval is defined from the beginning of the QRS complex to the end of the T wave on EKG) (QT prolongation. Other treatment-related AEs of grade 1 or 2, such as headache, alopecia areata, contusion, deafness neurosensory, heavy menstrual bleeding, infusion-site pain, iron overload, lip edema, rash, and urinary retention, were reported by one patient each in the 5.0 mg/kg dose group. In the 7.5 mg/kg dose group, increased alanine aminotransferase, aspartate aminotransferase, blood bilirubin, diarrhea, pyrexia, and vomiting were reported by one patient each. Almost 50% of the patients reported ⩾1 serious AE (SAE): 22 patients (49%) in the 5.0 mg/kg group and 6 patients (50%) in the 7.5 mg/kg group. One SAE related to a sickle cell crisis was considered related to the study drug in the 5.0 mg/kg group, whereas no SAEs related to the drug were reported in the 7.5 mg/kg group.
Table 3.
AEs (reported ⩾10% in all grades and in all patients) regardless of relationship with drug and AESIs.
| Preferred term | Crizanlizumab 5.0 mg/kg (N = 45) | Crizanlizumab 7.5 mg/kg (N = 12) | All patients (N = 57) | |||
|---|---|---|---|---|---|---|
| All grades, n (%) | Grade ⩾3, n (%) | All grades, n (%) | Grade ⩾3, n (%) | All grades, n (%) | Grade ⩾3, n (%) | |
| Patients with ⩾1 AE | 45 (100) | 27 (60.0) | 12 (100) | 7 (58.3) | 57 (100) | 34 (59.6) |
| Pyrexia | 14 (31.1) | 0 | 3 (25.0) | 1 (8.3) | 17 (29.8) | 1 (1.8) |
| Headache | 12 (26.7) | 0 | 2 (16.7) | 0 | 14 (24.6) | 0 |
| Hypokalemia | 12 (26.7) | 7 (15.6) | 2 (16.7) | 0 | 14 (24.6) | 7 (12.3) |
| Upper respiratory tract infection | 11 (24.4) | 2 (4.4) | 2 (16.7) | 0 | 13 (22.8) | 2 (3.5) |
| Arthralgia | 9 (20.0) | 0 | 2 (16.7) | 0 | 11 (19.3) | 0 |
| COVID-19 | 8 (17.8) | 3 (6.7) | 3 (25.0) | 0 | 11 (19.3) | 3 (3.5) |
| Chest pain | 8 (17.8) | 0 | 1 (8.3) | 0 | 9 (15.8) | 0 |
| Nausea | 6 (13.3) | 0 | 2 (16.7) | 0 | 8 (14.0) | 0 |
| Back pain | 6 (13.3) | 0 | 1 (8.3) | 0 | 7 (12.3) | 0 |
| Road traffic accident | 5 (11.1) | 0 | 2 (16.7) | 0 | 7 (12.3) | 0 |
| Vomiting | 4 (8.9) | 0 | 3 (25.0) | 0 | 7 (12.3) | 0 |
| Diarrhea | 4 (8.9) | 0 | 2 (16.7) | 0 | 6 (10.5) | 0 |
| Peripheral edema | 6 (13.3) | 0 | 0 | 0 | 6 (10.5) | 0 |
| Pain in extremity | 6 (13.3) | 0 | 0 | 0 | 6 (10.5) | 0 |
| Pneumonia | 5 (11.1) | 4 (8.9) | 1 (8.3) | 0 | 6 (10.5) | 4 (7.0) |
| Pruritus | 5 (11.1) | 0 | 1 (8.3) | 0 | 6 (10.5) | 0 |
| Patients with ⩾1 AESI | ||||||
| Infections | 31 (68.9) | 13 (28.9) | 8 (66.7) | 1 (8.3) | 39 (68.4) | 14 (24.6) |
| IRRs (standard search) a | 18 (40.0) | 1 (2.2) | 5 (41.7) | 0 | 23 (40.4) | 1 (1.8) |
| Pain events | 11 (24.4) | 2 (4.4) | 1 (8.3) | 0 | 12 (21.1) | 2 (3.5) |
AE, adverse event; AESI, AEs of special interest; COVID-19, Coronavirus disease 2019; IRR, infusion-related reaction.
Nonspecific, potential signs and symptoms indicative of IRRs were identified through standard search.
During the treatment period, one patient in the 5.0 mg/kg group died due to multiple organ dysfunction, while another patient in the 7.5 mg/kg group died due to anoxic brain injury. Both deaths did not occur within 3 weeks of receiving the infusion and were not deemed related to the study drug. No patient developed anti-drug antibodies to crizanlizumab treatment.
AEs of special interest
In the 5.0 mg/kg group, infections were reported in 31 patients (69%), with most commonly (>10%) reported being upper respiratory tract infection (11 (24%)), COVID-19 (8 (18%)), pneumonia (5 (11%)), and urinary tract infection (5 (11%)). In the 7.5 mg/kg group, infections were reported in eight patients (67%) with upper respiratory tract infection reported in two patients (17%) and COVID-19 in three patients (25%). Grade ⩾3 AESIs related to infection were reported in 14 patients (31%) in the 5.0 mg/kg group and 1 patient (8%) in 7.5 mg/kg group. No AEs of infection were considered treatment-related or led to treatment discontinuation.
Signs and symptoms possibly indicative of IRRs that occurred within 24 h were observed in 18 patients (40%) in the 5.0 mg/kg group and in 5 patients (41.7%) in the 7.5 mg/kg group. The most common signs and symptoms in the patients who experienced possible IRRs were headache (6 (13.3%), 1 (8.3%)), and nausea (3 (6.7%), 0) in the 5.0 and 7.5 mg/kg groups, respectively. All events were grade 1 or 2, except 1 grade 3 event in 5.0 mg/kg group. In the 5.0 mg/kg group, two patients had IRRs identified as a potential “severe reaction” where one patient had grade 2 pain and one reported grade 3 IRR. Both IRRs resolved without interrupting the drug infusion, although additional treatment was required. Both these AESIs were considered treatment-related by the investigator. In the 7.5 mg/kg group, one patient had an IRR identified as a potential “severe reaction”; however, the event was a grade 3 anaphylactic reaction due to the antibiotic ceftriaxone that occurred 22 days after the last infusion of crizanlizumab and resolved without stopping ongoing infusions of crizanlizumab. This AESI was not considered treatment-related by the investigator.
Other pain events were observed in 11 patients (24%) in the 5.0 mg/kg group, with headache (6 (13%)) and arthralgia (2 (4%)) being the most common. One “pain event,” a headache of grade 1 was considered as treatment-related and resolved without drug interruption, was observed in the 7.5 mg/kg group. None of the patients reported grade ⩾3 bleeding events during the study period.
Discussion
The phase II SOLACE-adults study investigated the PK/PD, safety, and efficacy of crizanlizumab in patients with SCD for ~4 years in 5.0 mg/kg group and ~3 years in 7.5 mg/kg group.
The PK analysis showed that Cmax was reached after 30 min of infusion at both doses of crizanlizumab and remained stable up to 6 h post-dose both at the initial dose as well as at steady state. The total exposure increased in an approximately dose-proportional manner from 5.0 to 7.5 mg/kg consistently over the course of the study. This is consistent with the PK profile of crizanlizumab (SelG1) observed in healthy volunteers and in the SUSTAIN study.6,7 The mean elimination T1/2 for the 5.0 mg/kg dose group in SOLACE-adults and in healthy volunteers are comparable (10.9 days vs 10.6 days). 7 The mean Cmax of crizanlizumab at the starting dose and at steady state were similar, indicating minimal or no accumulation. A relatively low inter-patient variability was observed for both AUC and Cmax.
The PD analysis revealed near-complete and sustained ex vivo inhibition of P-selectin expression following administration of both doses of crizanlizumab throughout the infusion interval, and these results were consistent with those reported earlier.6,7
Similar to the SUSTAIN study, 6 a sustained reduction (from baseline) in annualized rate of VOCs leading to healthcare visit was observed for both doses of crizanlizumab after >1 year of treatment. These changes in VOCs were generally consistent with previous data, showing a median absolute reduction of 0.79 and 0.98 VOC events from baseline with crizanlizumab 5.0 and 7.5 mg/kg doses, respectively, over the course of the study. Importantly, the two dose groups were enrolled sequentially and cannot be compared as they were not matched for sample size or baseline characteristics. Thus, no conclusions should be drawn as to the relative efficacy.
Of interest, a subsequent phase III study (STAND; NCT03814746) occurred during the same time as this study in patients aged ⩾12 years. Preliminary results from this study did not show statistically significant differences in the annualized rate of VOCs between the patients receiving crizanlizumab 5.0 or 7.5 mg/kg and those receiving placebo. 9 Consequently, in early 2023, crizanlizumab was removed from the European Medicine’s Agency’s approved medication list for SCD.10,11 The reasons for not meeting the efficacy endpoints in the STAND study could be multi-factorial including geographical differences such as variations in the use of healthcare and the low VOC rates in all arms. These factors could be attributed to inclusion of patients with milder disease, acute exacerbations of chronic pain not associated with VOCs, or underreporting during the COVID-19 pandemic. However, the safety profile was consistent with that in the SUSTAIN study.
Importantly, there were no new/unexpected safety concerns in the SOLACE-adult study, and no apparent differences between the two dose groups in terms of frequency and severity of AEs were observed after ⩾3 years of treatment with crizanlizumab. Consistent with the known safety profile of crizanlizumab, the most common AEs reported were mild to moderate and generally well tolerated, with very few discontinuations related to AEs, and resolved without complications. These findings were consistent with the SUSTAIN study, indicating that crizanlizumab has a manageable safety profile, reinforcing its potential for long-term treatment at either dose. 6
Furthermore, none of the infections were considered treatment-related and did not lead to treatment discontinuation, which is again in agreement with the findings of the SUSTAIN study. IRRs were not reported in the SUSTAIN study. In this study, there were a few severe treatment-related IRRs presenting as pain events, all of which resolved with additional treatment. There have been some instances of IRRs in the post-marketing setting as well,7,12,13 thus highlighting the need for continued evaluation and monitoring of the drug safety profile in real-world settings in larger patient cohorts to assess the real-world incidence of IRRs with crizanlizumab relative to that observed in clinical trials.
Key limitations: The phase II SOLACE-adults study was an open-label study and was not designed to make any direct comparisons between the 5.0 and 7.5 mg/kg dosing groups. The efficacy, safety, and tolerability of crizanlizumab in patients with SCD were the secondary objectives of this study. The 7.5 mg/kg group is an exploratory group, primarily planned to characterize the PK and PD of the dose. Patients were sequentially enrolled into two dosing groups without randomization, leading to unequal sample sizes and exposure times across groups. Small sample sizes limited the potential for a statistical evaluation of the impact of subgroups on VOC frequency.
Conclusions
The SOLACE-adults study in patients with SCD who had ⩾12 months of history experiencing VOCs showed that the exposure of crizanlizumab was approximately dose-proportional from 5.0 to 7.5 mg/kg, with no significant accumulation over time. Both doses achieved a near-complete ex vivo inhibition of P-selectin that was sustained for 4 weeks of the infusion interval. The median annualized rates of VOCs leading to healthcare visit on treatment decreased from baseline by 0.79 and 0.98 VOC events in the 5.0 and 7.5 mg/kg groups, respectively. These efficacy findings are consistent with previous studies on crizanlizumab, which demonstrated a reduction in VOC events leading to healthcare visits. Crizanlizumab, with or without HU/HC, was well tolerated with no differences in overall AEs between the two doses. No new or unexpected safety signals related to crizanlizumab were identified in this population.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207241292508 for Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study by Julie Kanter, Sarah Mennito, Santosh M. Nair, Deepa Manwani, Abdullah Kutlar, Nirmish Shah, Deborah Keefe, Hariprasad Madhamshetty, Michele Nassin, Evgeniya Reshetnyak, Anisha E. Mendonza and Darla Liles in Therapeutic Advances in Hematology
Acknowledgments
Pranitha Manchanapalli, Pharm D, of Novartis Healthcare Pvt Ltd. provided medical writing and editorial assistance in accordance with Good Publication Practice guidelines.
Footnotes
ORCID iDs: Julie Kanter  https://orcid.org/0000-0001-7002-8891
https://orcid.org/0000-0001-7002-8891
Nirmish Shah  https://orcid.org/0000-0002-7506-0935
https://orcid.org/0000-0002-7506-0935
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Julie Kanter, Division of Hematology–Oncology, University of Alabama, 1720, 2nd Street, Birmingham, AL 35233, USA.
Sarah Mennito, Department of Internal Medicine/Pediatrics, Medical University of South Carolina, Charleston, SC, USA.
Santosh M. Nair, Department of Hematology and Medical Oncology, Mid Florida Hematology and Oncology Center, Orange City, FL, USA
Deepa Manwani, Pediatric Hematology, Oncology and Cellular Therapy, Albert Einstein College of Medicine, Bronx, NY, USA.
Abdullah Kutlar, Department of Medicine, Medical College of Georgia, Augusta, GA, USA.
Nirmish Shah, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Deborah Keefe, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Hariprasad Madhamshetty, Novartis Healthcare Private Limited, Hyderabad, India.
Michele Nassin, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Evgeniya Reshetnyak, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Anisha E. Mendonza, BioMedical Research, Novartis, Cambridge, MA, USA
Darla Liles, Division of Hematology–Oncology, East Carolina University, Greenville, NC, USA.
Declarations
Ethics approval and consent to participate: The study was approved by the institutional review board or independent ethics committee of each participating center and was carried out in accordance with the recommendations of the Declaration of Helsinki. All participants gave written informed consent.
Consent for publication: Not applicable.
Author contributions: Julie Kanter: Conceptualization; Investigation; Methodology; Writing – review & editing.
Sarah Mennito: Conceptualization; Investigation; Writing – review & editing.
Santosh M Nair: Conceptualization; Investigation; Writing – review & editing.
Deepa Manwani: Conceptualization; Investigation; Writing – review & editing.
Abdullah Kutlar: Conceptualization; Investigation; Writing – review & editing.
Nirmish Shah: Conceptualization; Investigation; Writing – review & editing.
Deborah Keefe: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Hariprasad Madhamshetty: Methodology; Supervision; Validation; Writing – review & editing.
Michele Nassin: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Writing – review & editing.
Evgeniya Reshetnyak: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Visualization; Writing – review & editing.
Anisha E. Mendonza: Formal analysis; Methodology; Validation; Visualization; Writing – review & editing.
Darla Liles: Conceptualization; Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Novartis Pharma AG funded this study and the writing of this article.
J.K. reports receipt of research funding from NHLBI, BEAM, Bluebird Bio, CDC, HRSA, Novartis, Novo Nordisk, and Takeda; consultancy for Austin Pharmaceuticals, Bausch, BEAM, Bluebird Bio, ECOR-1, Fulcrum, Novartis, and Watkins, Lourie, Roll & Chance; membership on an entity’s Board of Directors or advisory committees with Austin Pharmaceuticals, Bluebird Bio, Ciesi, Glycomimetics, and Novartis; and honoraria from Guidepoint Global; D.M. reports consultancy from Editas, GBT, Novartis, Novo Nordisk, and Pfizer; A.K. reports receipt of research funding from Akira Bio, Forma/Novo-Nordisk, GBT/Pfizer, and Novartis; consultancy for Novartis; membership on an entity’s Board of Directors or advisory committees with Bluebird Bio, GBT/Pfizer, and Novartis; and event adjudication committee (EAC) Chair for Vertex; N.S. reports receipt of research funding from GBT/Pfizer; consultancy for Agios Pharmaceuticals, Bluebird Bio, Forma, GBT/Pfizer, and Vertex; and speaker bureau for Alexion Pharmaceuticals and GBT/Pfizer; D.L. reports clinical trial activity as principal investigator or sub-investigator for Annexon Biosciences Inc., Baxalta, BeiGene Ltd., Bioverativ Inc., Celgene, Delta, Exact Sciences, Janssen Research and Development, Immunovant Inc., Incyte Corp., Novartis, Partner Therapeutics, Principia Biopharma Inc., Sanofi-Aventis LLC, Takeda, and Vifor Pharma; D.K., M.N., and E.R. are employees of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA; H.M. is an employee of Novartis Healthcare Private Limited, Hyderabad, India; and A.E.M. is an employee of BioMedical Research, Cambridge, MA, USA; S.M. and S.M.N. have nothing to disclose.
Availability of data and materials: Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
References
- 1. GBD 2021 SCD collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol 2023; 10: e585–e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah N, Bhor M, Xie L, et al. Sickle cell disease complications: prevalence and resource utilization. PLoS One 2019; 14(7): e0214355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udeze C, Evans KA, Yang Y, et al. Economic and clinical burden of managing sickle cell disease with recurrent vaso-occlusive crises in the United States. Adv Ther 2023; 40: 3543–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 2013; 122(24): 3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karki NR, Saunders K, Kutlar AA. critical evaluation of crizanlizumab for the treatment of sickle cell disease. Expert Rev Hematol 2022; 15(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 6. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. NEJM 2017; 376(5): 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Novartis. ADAKVEO® (crizanlizumab-tmca) injection. Prescribing information, https://www.novartis.com/us-en/sites/novartis_us/files/adakveo.pdf (Revised September 2022, accessed 4 March 2024).
- 8. Kanter J, Brown RC, Norris C, et al. Pharmacokinetics, pharmacodynamics, safety and efficacy of crizanlizumab in patients with sickle cell disease. Blood Adv 2023; 7(6): 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abboud MR, Cançado RD, De Montalembert M, et al. Efficacy, safety, and biomarker analysis of 5 mg and 7.5 mg doses of crizanlizumab in patients with sickle cell disease: primary analyses from the Phase III STAND study. Blood 2023; 142(Suppl. 1): 272. [Google Scholar]
- 10. European Medicines Agency. Revocation of authorisation for sickle cell disease medicine Adakveo, https://www.ema.europa.eu/en/news/revocation-authorisation-sickle-cell-disease-medicine-adakveo (2023, accessed 4 March 2024).
- 11. European Medicines Agency. Adakveo Summary of product characteristics. Novartis. https://www.ema.europa.eu/en/documents/product-information/adakveo-epar-product-information_en.pdf (2022, accessed 4 March 2024).
- 12. Kanter J, Shah A, Joshi V, et al. Rare cases of infusion-related reactions (IRRs) presenting as pain events during or after crizanlizumab infusion in patients (pts) with sickle cell disease (SCD): a systematic evaluation of post-marketing (PM) reports [abstract]. Blood 2021; 138(Suppl. 1): 3112. Abstract. [Google Scholar]
- 13. Kanter J, Hellemann G, Cohen A, et al. Early evaluation of the use of crizanlizumab in sickle cell disease: a National Alliance of Sickle Cell Centers Study [abstract]. Blood 2021; 138(Suppl 1): 3113. Abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207241292508 for Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study by Julie Kanter, Sarah Mennito, Santosh M. Nair, Deepa Manwani, Abdullah Kutlar, Nirmish Shah, Deborah Keefe, Hariprasad Madhamshetty, Michele Nassin, Evgeniya Reshetnyak, Anisha E. Mendonza and Darla Liles in Therapeutic Advances in Hematology