Abstract
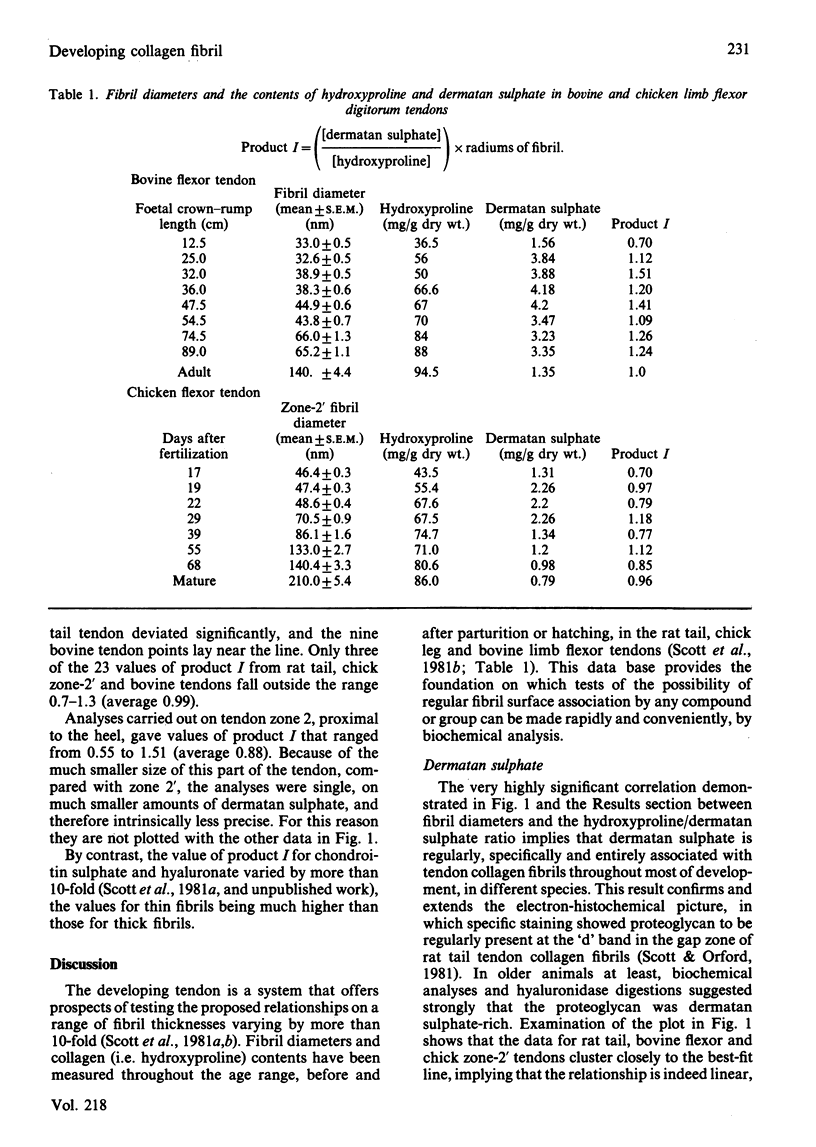
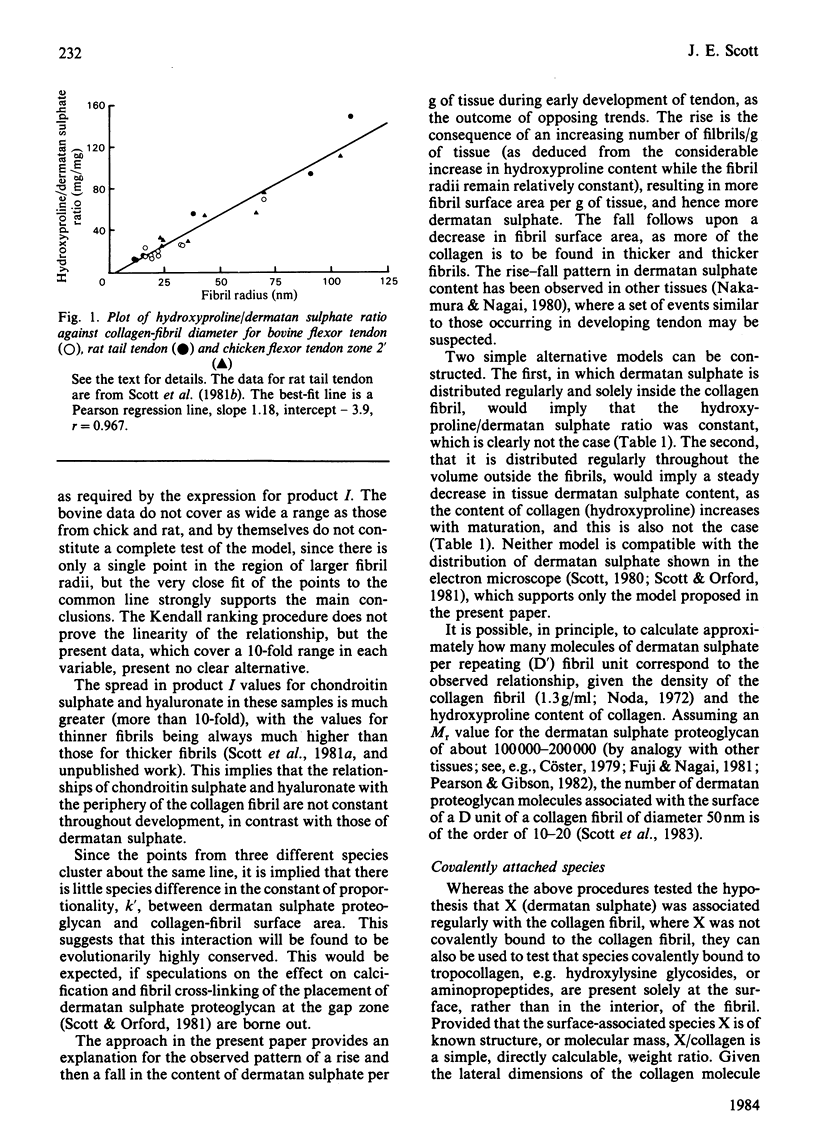
Dermatan sulphate, hydroxyproline and collagen fibril diameters were measured in flexor tendons from chick and calf limbs, from early in embryonic development to maturity. The collagen fibril is viewed as a long thin cylinder. A species X present at the periphery of the cylinder, regularly and specifically arrayed along the fibril, should then satisfy the relationship [X]/[collagen]r = k where [X] and [collagen] are tissue concentrations of X and collagen, and r is the fibril radius. Throughout the developmental period studied, dermatan sulphate (i.e.X) in chick, calf and rat tendons fits the relationship, implying that it is specifically, regularly and entirely associated with collagen fibrils, thus confirming and extended previous electron histochemistry [Scott & Orford (1981) Biochem. J. 197, 213-216]. This approach explains the pattern of change of dermatan sulphate content during development of the tendon. The findings imply that the dermatan sulphate proteoglycan-collagen interaction is evolutionarily highly conserved. The relationship [X]/[collagen]r = k is used to show that surface concentrations of covalently bound species, such as extension propeptides, can be easily assessed, given the data base described in paragraph 1 above.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujii N., Nagai Y. Isolation and characterization of a proteodermatan sulfate from calf skin. J Biochem. 1981 Nov;90(5):1249–1258. doi: 10.1093/oxfordjournals.jbchem.a133589. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Smith D., Wendelschafer-Crabb G., Mosher D. F., Foidart J. M. Fibronectin presence in native collagen fibrils of human fibroblasts: immunoperoxidase and immunoferritin localization. J Histochem Cytochem. 1980 Dec;28(12):1319–1333. doi: 10.1177/28.12.7014712. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A. Quasi-hexagonal molecular packing in collagen fibrils. Nature. 1979 Dec 20;282(5741):878–880. doi: 10.1038/282878a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nagai Y. Developmental changes in the synthesis of glycosaminoglycans and collagen in embryonic chick skin. J Biochem. 1980 Feb;87(2):629–637. doi: 10.1093/oxfordjournals.jbchem.a132787. [DOI] [PubMed] [Google Scholar]
- Noda H. Partial specific volume of collagen. J Biochem. 1972 Apr;71(4):699–703. [PubMed] [Google Scholar]
- Pearson C. H., Gibson G. J. Proteoglycans of bovine periodontal ligament and skin. Occurrence of different hybrid-sulphated galactosaminoglycans in distinct proteoglycans. Biochem J. 1982 Jan 1;201(1):27–37. doi: 10.1042/bj2010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Becker U., Nowack H., Timpl R. Antigenic structure of the aminoterminal region in type I procollagen. Characterization of sequential and conformational determinants. Immunochemistry. 1976 Dec;13(12):967–974. doi: 10.1016/0019-2791(76)90266-4. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Collagen--proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem J. 1980 Jun 1;187(3):887–891. doi: 10.1042/bj1870887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]



