Abstract
Background:
The latest guidelines discourage the use of long-acting beta2-agonists/inhaled corticosteroids (LABA/ICS) for chronic obstructive pulmonary disease (COPD). However, there is a lack of evidence regarding the optimal subsequent treatment after discontinuing LABA/ICS.
Objectives:
To compare the effectiveness and safety of switching from LABA/ICS to triple therapy (LABA/long-acting muscarinic antagonists (LAMA)/ICS) or to dual bronchodilators (LABA/LAMA) in COPD patients.
Design:
This was a new-user, active-comparator, and propensity score-matched cohort study analyzing the Taiwanese nationwide healthcare insurance claims.
Methods:
We recruited COPD patients switching from LABA/ICS to triple therapy or to dual bronchodilators from 2015 to 2019. The primary effectiveness outcome was the annual rate of exacerbations, and safety outcomes included severe pneumonia and all-cause mortality. Stratification by prior exacerbations was conducted.
Results:
After matching, each group comprised 1892 patients, 55% of whom experienced no exacerbations in the prior year. Treatment with LABA/LAMA/ICS versus LABA/LAMA showed comparable annual rate of moderate-to-severe exacerbations (incidence rate ratio, 1.04; 95% confidence interval (CI), 0.91–1.19). However, switching to LABA/LAMA/ICS was associated with increased risks of severe pneumonia (hazard ratio (HR), 1.65; 95% CI, 1.30–2.09) and all-cause death (HR, 1.39; 95% CI, 1.09–1.78). In patients with⩾2 prior exacerbations, LABA/LAMA/ICS versus LABA/LAMA was related to a 21% reduced rate of exacerbations but with a twofold increased pneumonia risk and a 49% elevated risk of all-cause mortality.
Conclusion:
Switching from LABA/ICS to triple therapy versus dual bronchodilators in COPD patients was associated with similar rates of annual exacerbations but was related to elevated risks of severe pneumonia and all-cause mortality. Among frequent exacerbators, triple therapy was associated with lower rates of exacerbation but was accompanied by increased risks of pneumonia and mortality compared to LABA/LAMA. Careful consideration of the examined safety events is necessary when switching from LABA/ICS to triple therapy in COPD management.
Keywords: comparative effectiveness and safety, COPD, dual therapy, observational study, triple therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory lung disease that currently affects 200 million people 1 and was the third major cause of death in 2019 worldwide. 2 Exacerbation of COPD, characterized by an acute worsening of dyspnea, cough, sputum production, sputum purulence, and airway obstruction,3,4 can lead to significant morbidity and mortality.5,6
Inhaled long-acting medications, including inhaled corticosteroids (ICS), long-acting beta2-agonists (LABA), and long-acting muscarinic antagonists (LAMA), used alone or in combination, particularly LABA/ICS, LABA/LAMA, and LABA/LAMA/ICS, are the mainstay therapies for preventing COPD exacerbation. 4 LABA/ICS remains one of the most prescribed combination therapies; for instance, approximately half of COPD patients receive this dual therapy as the first maintenance therapy in the United States. 7 However, the 2024 update of the GOLD guidelines discourages LABA/ICS usage, indicating an urgent need to identify the optimal treatment option among patients receiving LABA/ICS. The 2024 GOLD guidelines recommend that patients with LABA/ICS who require treatment changes could either switch to LABA/LAMA or escalate to LABA/LAMA/ICS, 4 but no prior COPD studies have assessed the comparative effects of the two suggested pharmacotherapy alternatives in LABA/ICS-receiving patients.
Previous studies assessing the comparative effects between LABA/LAMA/ICS and LABA/LAMA have yielded inconsistent results. Three randomized controlled trials (RCTs)8–10 reported a reduced exacerbation rate associated with LABA/LAMA/ICS versus LABA/LAMA, although these studies included patients with a history of asthma9,10 and adopted rigorous selection criteria,8–10 which might have introduced confounding factors and limited the generalizability of the reported data. Conversely, real-world studies revealed that LABA/LAMA had a lower exacerbation rate than LABA/LAMA/ICS,11–13 whereas a cohort study 14 reported comparable exacerbation rates between the two inhaled combination therapies. These observational studies, however, might be subject to an imbalance of prior ICS-containing medication usage12,13 and prior exacerbations 11 between the two comparison groups. Additionally, despite frequent LABA/ICS use, none of the prior studies analyzed both switchers from LABA/ICS to LABA/LAMA and escalators from LABA/ICS to triple therapy. Despite inconsistent findings in the literature, we hypothesized that escalating to triple inhalation therapy is more effective than switching to dual bronchodilator therapy in preventing COPD exacerbations after discontinuing LABA/ICS. This hypothesis was formulated based on the reduced risk of COPD exacerbation observed in pivotal large RCTs comparing LABA/LAMA/ICS to LABA/LAMA, as well as on the previously identified limitations in prior observational studies, which would be addressed in this study.
The primary objectives of the current study were to assess the comparative effectiveness and safety outcomes of escalating to triple therapy versus switching to dual bronchodilator therapy in COPD patients receiving LABA/ICS.
Methods
Study design and data source
This retrospective observational study utilized a propensity score (PS)-matched and new-user study design, analyzing the Taiwanese National Health Insurance Research Database (NHIRD) between January 1, 2014, and December 31, 2020. The NHIRD comprises nationwide administrative healthcare records of >99% of Taiwanese inhabitants, including all medical diagnoses, procedures, and prescription refill records of outpatient, emergency, and inpatient visits. Multiple diagnosis codes in the NHIRD have been validated with high accuracy.15,16 To obtain death records, the NHIRD was linked with the National Death Registry records. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 17
Study population
We identified a population of COPD patients who received LABA/ICS fixed-dose combination therapy from January 1, 2015, to December 31, 2019. Patients with COPD were defined as those having ⩾two COPD-related outpatient visits or one COPD-related hospitalization based on the International Classification of Diseases, Ninth Revision diagnoses codes of 491–492 and 496 or International Classification of Diseases, Tenth Revision diagnoses codes of J41-44 in a given year.18,19 Initiators of LABA/ICS were defined as those without any LABA/ICS refill prescription records in the previous year. We followed the source population from the first prescription refill date of LABA/ICS until LABA/ICS therapy discontinuation, defined as >90 days between successive prescriptions being refilled, or until patients switched to LABA/LAMA or escalated to triple therapy, both of whom composed the study cohort. The cohort entry date was defined as the date of the first prescription of dual or triple combination medications during the follow-up of the source population. The study cohort was further required to be aged ⩾40 years. Patients were excluded if they had any diagnoses of lung cancer, asthma, or <1 year of National Health Insurance (NHI) coverage in the year preceding cohort entry, as detailed in Supplemental e-Table 1.
We defined new users of triple therapy or dual bronchodilators as those without any prescription refill records of both regimens in the prior year. Each new user of LABA/LAMA/ICS was matched with an initiator of LABA/LAMA in terms of the duration of LABA/ICS therapy (in quintiles) received prior to cohort entry, the baseline exacerbation risk defined by GOLD guidelines, and PSs using nearest neighbor matching without replacement and a caliper width equal to 0.2 standard deviations of the logit function of PSs.
Follow-up of the study cohort started from cohort entry until treatment discontinuation, treatment switching, death, the end of NHI enrollments, end of the study period, or up to a 1-year follow-up period, whichever came first. We defined treatment discontinuation using a 60-day grace period. To assess each time-to-first event outcome, we included the occurrence of the first event as an additional censoring criterion.
Outcome measurement
We assessed the annual rate of moderate-to-severe exacerbation of COPD as the main effectiveness outcome19,20 and evaluated the first hospitalization with a primary discharge diagnosis of pneumonia as the primary safety endpoint. 19 We defined severe exacerbation of COPD as a hospital admission or emergency visit with a primary COPD diagnosis accompanied by systemic corticosteroids and/or respiratory antibiotic prescription records.19,20 Moderate exacerbation referred to an outpatient visit for a primary COPD diagnosis with 3–14 days of systematic corticosteroids and/or respiratory antibiotics.19,21 Secondary outcomes included the annual rate and the first separate severe and moderate exacerbations, cardiovascular disease, cardiovascular-specific mortality, respiratory-specific mortality, and all-cause mortality. A 14-day gap was considered to indicate a single exacerbation episode. 20 Supplemental e-Table 1 presents the diagnosis codes and prescription drugs used to define the outcomes.
Covariate measurement
We examined patients’ demographic and clinical characteristics, including age, sex, entry year, and hospital level at the cohort entry date, as well as proxy indicators for COPD severity (e.g., number of severe and/or moderate COPD exacerbations), monthly income-based insurance, comorbidities, and co-medications evaluated at the baseline year (see details in Table 1).
Table 1.
Baseline characteristics of the initiators of LABA/LAMA/ICS and LABA/LAMA.
| Characteristics a | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| LABA/LAMA/ICS (N = 2270) | LABA/LAMA (N = 2467) | aSMD b | LABA/LAMA/ICS (N = 1892) | LABA/LAMA (N = 1892) | aSMD b | |
| No (%) or mean (SD) | No (%) or mean (SD) | |||||
| Age (years) | 73.8 (11.4) | 72.6 (11.2) | 0.105 | 73.3 (11.3) | 73.0 (11.3) | 0.023 |
| Sex, male | 1929 (85.0) | 2176 (88.2) | 0.095 | 1629 (86.1) | 1642 (86.8) | 0.020 |
| Entry year | ||||||
| 2015 | 358 (15.8) | 174 (7.1) | 0.277 | 197 (10.4) | 173 (9.1) | 0.043 |
| 2016 | 555 (24.5) | 492 (19.9) | 0.109 | 446 (23.6) | 435 (23.0) | 0.014 |
| 2017 | 494 (21.8) | 692 (28.1) | 0.146 | 463 (24.5) | 483 (25.5) | 0.024 |
| 2018 | 456 (20.1) | 571 (23.2) | 0.074 | 415 (21.9) | 410 (21.7) | 0.006 |
| 2019 | 407 (17.9) | 538 (21.8) | 0.097 | 371 (19.6) | 391 (20.7) | 0.026 |
| Matching criteria | ||||||
| High exacerbation risk by GOLD definition | 735 (32.4) | 651 (26.4) | 0.132 | 540 (28.5) | 540 (28.5) | <0.001 |
| Duration of LABA/ICS therapy (months) c | 7.8 (9.9) | 7.1 (9.1) | 0.072 | 8.0 (10.0) | 7.8 (9.7) | 0.020 |
| COPD severity indicators | ||||||
| Number of severe COPD exacerbation | ||||||
| 0 | 1791 (78.9) | 2062 (83.6) | 0.120 | 1549 (81.9) | 1560 (82.5) | 0.015 |
| 1 | 367 (16.2) | 343 (13.9) | 0.063 | 274 (14.5) | 273 (14.4) | 0.002 |
| ⩾2 | 112 (4.9) | 62 (2.5) | 0.128 | 69 (3.7) | 59 (3.1) | 0.029 |
| Number of moderate COPD exacerbation | ||||||
| 0 | 1398 (61.6) | 1697 (68.8) | 0.152 | 1224 (64.7) | 1244 (65.8) | 0.022 |
| 1 | 565 (24.9) | 523 (21.2) | 0.088 | 441 (23.3) | 433 (22.9) | 0.010 |
| ⩾2 | 307 (13.5) | 247 (10.0) | 0.109 | 227 (12.0) | 215 (11.4) | 0.020 |
| Location of initial COPD diagnosis | ||||||
| Outpatient | 1775 (78.2) | 1908 (77.3) | 0.021 | 1476 (78.0) | 1483 (78.4) | 0.009 |
| ER | 124 (5.5) | 131 (5.3) | 0.007 | 98 (5.2) | 100 (5.3) | 0.005 |
| Inpatient | 371 (16.3) | 428 (17.4) | 0.027 | 318 (16.8) | 309 (16.3) | 0.013 |
| LABA/ICS component | ||||||
| Salmeterol/Fluticasone | 1125 (49.6) | 1090 (44.2) | 0.108 | 908 (48.0) | 893 (47.2) | 0.016 |
| Formoterol/Beclomethasone | 500 (22.0) | 508 (20.6) | 0.035 | 407 (21.5) | 396 (20.9) | 0.014 |
| Formoterol/Budesonide | 408 (18.0) | 489 (19.8) | 0.047 | 347 (18.3) | 358 (18.9) | 0.015 |
| Others d | 237 (10.4) | 380 (15.4) | 0.148 | 230 (12.2) | 245 (13.0) | 0.024 |
| Prescription no. of COPD medications | ||||||
| Short-acting β2 agonists | ||||||
| Nebulized | 1065 (46.9) | 1040 (42.2) | 0.096 | 841 (44.5) | 828 (43.8) | 0.014 |
| Inhaled | ||||||
| 0 | 1138 (50.1) | 1473 (59.7) | 0.193 | 1013 (53.5) | 1043 (55.1) | 0.032 |
| 1–2 | 687 (30.3) | 667 (27.0) | 0.071 | 554 (29.3) | 549 (29.0) | 0.006 |
| ⩾3 | 445 (19.6) | 327 (13.3) | 0.172 | 325 (17.2) | 300 (15.9) | 0.036 |
| Short-acting muscarinic antagonists | ||||||
| Nebulized | 1049 (46.2) | 1010 (40.9) | 0.107 | 827 (43.7) | 813 (43.0) | 0.015 |
| Inhaled | ||||||
| 0 | 1869 (82.3) | 2110 (85.5) | 0.087 | 1594 (84.3) | 1585 (83.8) | 0.013 |
| 1–2 | 235 (10.4) | 241 (9.8) | 0.019 | 178 (9.4) | 198 (10.5) | 0.035 |
| ⩾3 | 166 (7.3) | 116 (4.7) | 0.110 | 120 (6.3) | 109 (5.8) | 0.024 |
| Methylxanthines e | ||||||
| 0 | 682 (30.0) | 770 (31.2) | 0.025 | 581 (30.7) | 586 (31.0) | 0.006 |
| 1–6 | 957 (42.2) | 1161 (47.1) | 0.099 | 824 (43.6) | 840 (44.4) | 0.017 |
| ⩾7 | 631 (27.8) | 536 (21.7) | 0.141 | 487 (25.7) | 466 (24.6) | 0.026 |
| Oral β2 agonists f | ||||||
| 0 | 1609 (70.9) | 1743 (70.7) | 0.005 | 1367 (72.3) | 1338 (70.7) | 0.034 |
| 1–6 | 558 (24.6) | 612 (24.8) | 0.005 | 445 (23.5) | 453 (23.9) | 0.010 |
| ⩾7 | 103 (4.5) | 112 (4.5) | <0.001 | 80 (4.2) | 101 (5.3) | 0.052 |
| Monthly income-based insurance premium (NTD) | ||||||
| First tertile | 756 (33.3) | 703 (28.5) | 0.104 | 580 (30.7) | 537 (30.3) | 0.008 |
| Second tertile | 764 (33.7) | 892 (36.2) | 0.053 | 660 (34.9) | 660 (34.9) | <0.001 |
| Third tertile | 750 (33.0) | 872 (35.4) | 0.049 | 652 (34.5) | 659 (34.8) | 0.008 |
| Hospital level | ||||||
| Academic medical centers | 748 (33.0) | 835 (33.9) | 0.019 | 632 (33.4) | 661 (34.9) | 0.032 |
| Metropolitan hospitals | 1113 (49.0) | 1071 (43.4) | 0.113 | 896 (47.4) | 865 (45.7) | 0.033 |
| Local community hospitals | 371 (16.3) | 489 (19.8) | 0.091 | 328 (17.3) | 328 (17.3) | <0.001 |
| Physician clinics | 38 (1.7) | 72 (2.9) | 0.083 | 36 (1.9) | 38 (2.0) | 0.008 |
| Comorbidities | ||||||
| CV diseases | ||||||
| Coronary artery disease | ||||||
| None | 1601 (70.5) | 1732 (70.2) | 0.007 | 1348 (71.3) | 1344 (71.0) | 0.005 |
| History | 630 (27.8) | 718 (29.1) | 0.030 | 523 (27.6) | 531 (28.1) | 0.009 |
| Hospitalization | 39 (1.7) | 17 (0.7) | 0.095 | 21 (1.1) | 17 (0.9) | 0.021 |
| Cardiac arrhythmia | ||||||
| None | 1891 (83.3) | 2067 (83.8) | 0.013 | 1585 (83.8) | 1581 (83.6) | 0.006 |
| History | 353 (15.6) | 375 (15.2) | 0.010 | 289 (15.3) | 292 (15.4) | 0.004 |
| Hospitalization | 26 (1.2) | 25 (1.0) | 0.013 | 18 (1.0) | 19 (1.0) | 0.005 |
| Heart failure | ||||||
| None | 1854 (81.7) | 2099 (85.1) | 0.092 | 1574 (83.2) | 1587 (83.9) | 0.019 |
| History | 351 (15.5) | 304 (12.3) | 0.091 | 266 (14.1) | 251 (13.3) | 0.023 |
| Hospitalization | 65 (2.9) | 64 (2.6) | 0.017 | 52 (2.8) | 54 (2.9) | 0.006 |
| Ischemic stroke | ||||||
| None | 2095 (92.3) | 2323 (94.2) | 0.075 | 1762 (93.1) | 1765 (93.3) | 0.006 |
| History | 141 (6.2) | 114 (4.6) | 0.070 | 105 (5.6) | 103 (5.4) | 0.005 |
| Hospitalization | 34 (1.5) | 30 (1.2) | 0.024 | 25 (1.3) | 24 (1.3) | 0.005 |
| Other strokes | 303 (13.4) | 312 (12.7) | 0.021 | 242 (12.8) | 250 (13.2) | 0.013 |
| Dyslipidemia | 579 (25.5) | 675 (27.4) | 0.042 | 496 (26.2) | 500 (26.4) | 0.005 |
| Hypertension | 1332 (58.7) | 1449 (58.7) | 0.001 | 1101 (58.2) | 1103 (58.3) | 0.002 |
| Peripheral vascular disease | 89 (3.9) | 60 (2.4) | 0.085 | 58 (3.1) | 47 (2.5) | 0.035 |
| Rheumatic heart disease | 50 (2.2) | 52 (2.1) | 0.007 | 43 (2.3) | 39 (2.1) | 0.015 |
| Pulmonary diseases | ||||||
| Pneumonia | ||||||
| None | 1554 (68.5) | 1755 (71.1) | 0.058 | 1326 (70.1) | 1337 (70.7) | 0.013 |
| History | 259 (11.4) | 281 (11.4) | 0.001 | 215 (11.4) | 206 (10.9) | 0.015 |
| Hospitalization | 457 (20.1) | 431 (17.5) | 0.068 | 351 (18.6) | 349 (18.5) | 0.003 |
| Acute bronchitis | 769 (33.9) | 914 (37.1) | 0.066 | 654 (34.6) | 678 (35.8) | 0.027 |
| Influenza | 176 (7.8) | 215 (8.7) | 0.035 | 152 (8.0) | 145 (7.7) | 0.014 |
| Mental diseases | ||||||
| Schizophrenia | 10 (0.4) | 14 (0.6) | 0.018 | 8 (0.4) | 10 (0.5) | 0.015 |
| Depression | 138 (6.1) | 138 (5.6) | 0.021 | 111 (5.9) | 104 (5.5) | 0.016 |
| Dementia | 181 (8.0) | 144 (5.8) | 0.084 | 132 (7.0) | 124 (6.6) | 0.017 |
| Diabetes mellitus | 709 (31.2) | 731 (29.6) | 0.035 | 583 (30.8) | 582 (30.8) | 0.001 |
| Parkinson’s disease | 83 (3.7) | 65 (2.6) | 0.059 | 64 (3.4) | 57 (3.0) | 0.021 |
| Chronic renal disease | 385 (17.0) | 386 (15.7) | 0.036 | 311 (16.4) | 299 (15.8) | 0.017 |
| Chronic liver disease | 208 (9.2) | 228 (9.2) | 0.003 | 181 (9.6) | 186 (9.8) | 0.009 |
| GERD | 385 (15.8) | 469 (19.0) | 0.086 | 309 (16.3) | 312 (16.5) | 0.004 |
| Cancer | 284 (12.5) | 298 (12.1) | 0.013 | 228 (12.1) | 232 (12.3) | 0.007 |
| Sepsis | 170 (7.5) | 170 (6.9) | 0.023 | 133 (7.0) | 132 (7.0) | 0.002 |
| Smoking cessation | 128 (5.6) | 153 (6.2) | 0.024 | 113 (6.0) | 107 (5.7) | 0.014 |
| Co-medications | ||||||
| CV medications | ||||||
| Antiplatelets | 1037 (45.7) | 1107 (44.9) | 0.016 | 851 (45.0) | 848 (44.8) | 0.003 |
| Calcium channel blockers | 1193 (52.6) | 1248 (50.6) | 0.039 | 969 (51.2) | 969 (51.2) | <0.001 |
| Diuretics | 1310 (57.7) | 1356 (55.0) | 0.055 | 1065 (56.3) | 1059 (56.0) | 0.006 |
| Angiotensin-converting enzyme inhibitors | 179 (7.9) | 246 (10.0) | 0.073 | 164 (8.7) | 146 (7.7) | 0.035 |
| Angiotensin receptor blockers | 996 (43.9) | 1019 (41.3) | 0.052 | 810 (42.8) | 813 (43.0) | 0.003 |
| CV-selective β-blockers | 504 (22.2) | 568 (23.0) | 0.020 | 432 (22.8) | 430 (22.7) | 0.003 |
| Non-CV-selective β-blockers | 337 (14.9) | 394 (16.0) | 0.031 | 285 (15.1) | 287 (15.2) | 0.003 |
| Digoxin | 127 (5.6) | 131 (5.3) | 0.013 | 103 (5.4) | 98 (5.2) | 0.012 |
| Antiarrhythmic agents | 201 (8.9) | 202 (8.2) | 0.024 | 164 (8.7) | 162 (8.6) | 0.004 |
| Nitrates | 395 (17.4) | 384 (15.6) | 0.050 | 305 (16.1) | 304 (16.1) | 0.001 |
| Anticoagulants | 319 (14.1) | 330 (13.4) | 0.020 | 256 (13.5) | 257 (13.6) | 0.002 |
| Lipid-lowering agents | ||||||
| Statins | 607 (26.7) | 694 (28.1) | 0.031 | 521 (27.5) | 524 (27.7) | 0.004 |
| Others | 91 (4.0) | 123 (5.0) | 0.047 | 79 (4.2) | 82 (4.3) | 0.008 |
| Gastric acid suppressants | ||||||
| PPIs | 481 (21.2) | 573 (23.2) | 0.049 | 397 (21.0) | 405 (21.4) | 0.010 |
| H2-blockers | 1138 (50.1) | 1297 (52.6) | 0.049 | 956 (50.5) | 968 (51.2) | 0.013 |
| Psychotropic drugs | ||||||
| Benzodiazepines and Z-drugs | 1270 (56.0) | 1449 (58.7) | 0.056 | 1070 (56.6) | 1085 (57.4) | 0.016 |
| Antipsychotics | 428 (18.9) | 435 (17.6) | 0.032 | 342 (18.1) | 343 (18.1) | 0.001 |
| Antidepressants | 376 (16.6) | 399 (16.2) | 0.011 | 311 (16.4) | 304 (16.1) | 0.010 |
| Anti-inflammatory agents | ||||||
| NSAIDs | 1649 (72.6) | 1876 (76.0) | 0.078 | 1395 (73.7) | 1392 (73.6) | 0.004 |
| Aspirin (⩾325 mg) | 911 (40.1) | 979 (39.7) | 0.009 | 755 (39.9) | 744 (39.3) | 0.012 |
| Systemic corticosteroids | 1687 (74.3) | 1705 (69.1) | 0.116 | 1368 (72.3) | 1359 (71.8) | 0.011 |
| Respiratory antibiotics | 1809 (79.7) | 2009 (81.4) | 0.044 | 1515 (80.1) | 1521 (80.4) | 0.008 |
| Opioids | 967 (42.6) | 1029 (41.7) | 0.018 | 788 (41.7) | 792 (41.9) | 0.004 |
| Vaccines (influenza and pneumonia) | 875 (38.6) | 985 (39.9) | 0.028 | 745 (39.4) | 759 (40.1) | 0.015 |
All comorbidities, co-medications, COPD severity indicators, and monthly income were measured in the year preceding the cohort entry date; age, sex, entry year, and hospital level were measured at the cohort entry date.
Absolute standardized mean difference >0.1 represented meaningful differences between two groups.
Duration of LABA/ICS prescriptions between initial use of LABA/ICS and cohort entry date was measured in months.
Other LABA/ICS component included vilanterol/fluticasone and formoterol/fluticasone.
Use of methylxanthines was required to be accompanied with a COPD diagnosis and was measured in the year preceding the cohort entry date and at the cohort entry date.
Use of oral β2 agonists was required to be accompanied with a COPD diagnosis.
aSMD, absolute standardized mean difference; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; GERD, gastroesophageal reflux disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, inhaled long-acting β2 agonists; LAMA, inhaled long-acting muscarinic antagonists; No, number; NSAIDs, nonsteroidal anti-inflammatory drugs; NTD, New Taiwan dollar; PPIs, proton pump inhibitors; SD, standard deviation.
Additional analyses
We conducted multiple sensitivity analyses (detailed in Supplemental Table 2), such as performing intention-to-treat analysis, restricting patients with a medication possession ratio ⩾80%, 22 performing PS-based treatment inverse weighting, 23 considering all-cause death as a competing risk for time-to-first outcomes, 24 and employing a high-dimensional PS-matching approach 25 (detailed in Supplemental e-Methods), as well as performed a negative outcome analysis to examine the risk of gastroesophageal reflux diseases. Additionally, we performed subgroup analyses according to prior COPD exacerbations (defined as 0, 1, or ⩾2 time(s) in the baseline year), history of pneumonia, and prior cardiovascular disease. We re-estimated the PS for each subgroup analysis. Finally, we assessed whether there was a potential effect of ICS withdrawal on the effectiveness outcome in the LABA/LAMA group.
Statistical analyses
A sample size of 589 patients per group followed-up for 1 year was required at a 5% significance level and 80% power to detect a 20% difference in the exacerbation rate. 26 The exacerbation rate was assumed to be 0.6 per person-year in the LABA/LAMA group based on previous studies with annual rates ranging from 0.59 to 1.42.8–10,14 The absolute standardized mean difference was adopted to assess the characteristics between the LABA/LAMA/ICS group and LABA/LAMA group, with a magnitude >0.1 representing imbalances between groups. 27 A generalized linear regression model with a log link function and negative binomial distribution was used to calculate the incidence rate ratio (IRR) of COPD exacerbations. We employed the Kaplan–Meier approach and Cox proportional hazard regressions to estimate the cumulative incidence rate and hazard ratio (HR) with 95% CI of each time-to-first outcome, respectively. The proportional hazard assumption for conducting Cox regression analysis was tested through Schoenfeld residuals, with all assumptions being met. A P value less than 0.05 was set as the statistical significance threshold. We used STATA 16.0 (StataCorp LLC, College Station, TX, USA) to perform competing risk analysis and construct Kaplan–Meier survival curves as well as forest plots of sensitivity and subgroup analyses and employed SAS 9.4 (SAS Institute, Cary, NC, USA) for all data cleaning and the remaining statistical analyses.
Results
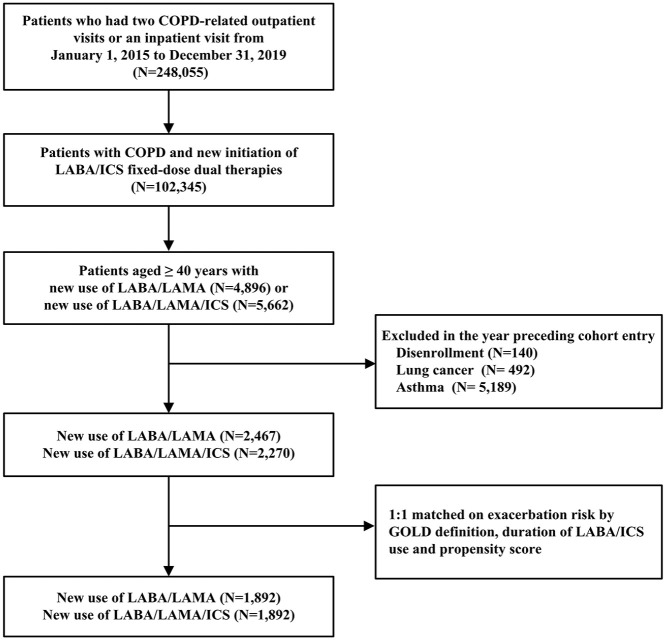
The base cohort comprised 102,345 patients receiving LABA/ICS fixed-dose dual therapy. After applying the exclusion criteria and the 1:1 matching scheme, the LABA/LAMA/ICS and LABA/LAMA groups comprised 1892 eligible patients in the study cohort (Figure 1). The mean follow-up duration was 7.8 months in the LABA/LAMA/ICS group and 7.5 months in the LABA/LAMA group, with similar censoring reasons across the two groups (Supplemental e-Table 3). Individual components of the analyzed triple therapies and LABA/LAMA are presented in Supplemental e-Table 4.
Figure 1.
Flowchart showing the selection of the base and study cohorts.
COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, inhaled long-acting β2 agonists; LAMA, inhaled long-acting muscarinic antagonists.
Before matching, most examined factors were balanced, except for the use of short-acting bronchodilators, salmeterol/fluticasone, and systemic corticosteroids. After matching, all characteristics were well-balanced between the two groups, with mean ages of 73.3 years in the LABA/LAMA/ICS group and 73.0 years in the LABA/LAMA group, and 86.1% men in the former group compared with 86.8% men in the latter group (Table 1).
According to our primary analyses, the annual rate of severe or moderate exacerbations per 100 person-years was 105 in the triple therapy group and 101.1 in the dual bronchodilator group; the former group had a greater incidence of severe pneumonia leading to hospitalization than did the latter group (16 per 100 person-year vs 9.8 per 100 person-year; Table 2). Comparative analyses revealed no increase in the annual rate of moderate-to-severe exacerbations between LABA/LAMA/ICS and LABA/LAMA (IRR, 1.04; 95% CI, 0.91–1.19). Likewise, we observed no associations when assessing the rate of moderate and severe exacerbations individually or when examining the time-to-first moderate or severe exacerbations. However, patients who escalated to triple therapy had a 1.65-fold (95% CI, 1.30–2.09) greater pneumonia risk than those who switched to LABA/LAMA therapy. Additionally, the HRs were 1.39 (95% CI, 1.09–1.78) and 1.45 (95% CI, 1.09–1.92) for all-cause death and respiratory-specific death, respectively. The cumulative incidence rates of the outcomes are presented in Supplemental e-Figures 1 to 2.
Table 2.
Comparative effectiveness and safety outcomes of LABA/LAMA/ICS versus LABA/LAMA.
| Outcomes | LABA/LAMA/ICS (N = 1892) | LABA/LAMA (N = 1892) | |||||
|---|---|---|---|---|---|---|---|
| No. with events | Person-years | Incidence rate (100 person-year) | No. with events | Person-years | Incidence rate (100 person-year) | IRR (95% CI) | |
| Annual rate of moderate-to-severe exacerbation | 1157 | 1207.72 | 105.03 | 1013 | 1169.76 | 101.10 | 1.04 (0.91–1.19) |
| Severe exacerbation | 372 | 1207.72 | 36.19 | 330 | 1169.76 | 34.13 | 1.06 (0.85–1.32) |
| Moderate exacerbation | 785 | 1207.72 | 67.78 | 683 | 1169.76 | 65.47 | 1.04 (0.88–1.21) |
| Time to first events | No. with events | Person-years | Incidence rate (100 person-year) | No. with events | Person-years | Incidence rate (100 person-year) | HR (95% CI) |
| Time to first moderate-to-severe exacerbation | 486 | 986.55 | 49.26 | 443 | 983.43 | 45.05 | 1.09 (0.96–1.24) |
| Severe exacerbation | 215 | 1126.84 | 19.08 | 184 | 1103.74 | 16.67 | 1.15 (0.94–1.40) |
| Moderate exacerbation | 346 | 1038.31 | 33.32 | 332 | 1026.23 | 32.35 | 1.03 (0.89–1.20) |
| First hospitalization for pneumonia | 184 | 1147.54 | 16.03 | 111 | 1136.25 | 9.77 | 1.65 (1.30–2.09) a |
| First hospitalization for CVD | 73 | 1182.06 | 6.18 | 57 | 1150.63 | 4.95 | 1.25 (0.89–1.77) |
| All-cause mortality | 156 | 1207.72 | 12.92 | 109 | 1169.76 | 9.32 | 1.39 (1.09–1.78) a |
| Cardiac-specific mortality | 60 | 1207.72 | 4.97 | 40 | 1169.76 | 3.42 | 1.46 (0.98–2.18) |
| Respiratory-specific mortality | 119 | 1207.72 | 9.85 | 80 | 1169.76 | 6.84 | 1.45 (1.09–1.92) a |
p < 0.05.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HR, hazard ratio; ICS, inhaled corticosteroids; IRR, incidence rate ratio; LABA, inhaled long-acting β2 agonists; LAMA, inhaled long-acting muscarinic antagonists.
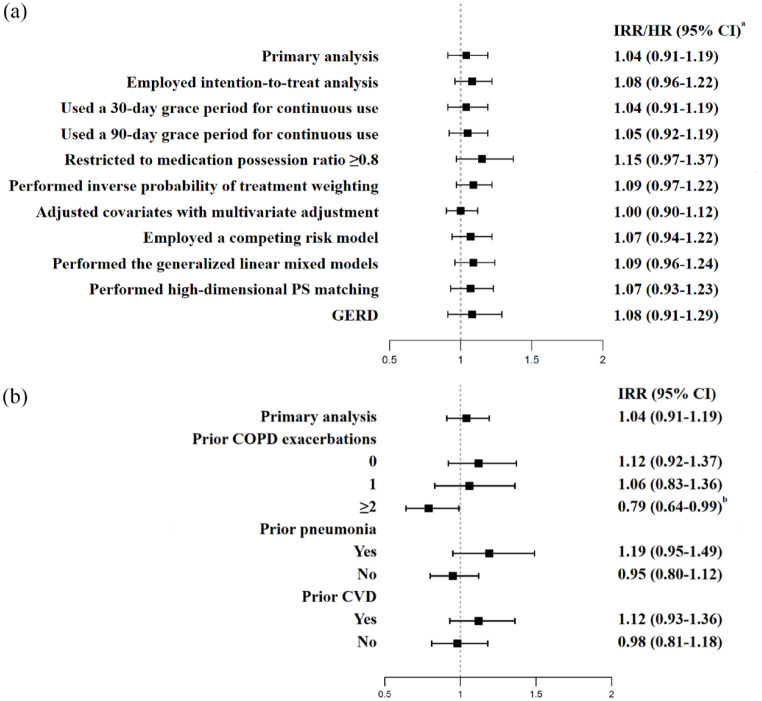
All sensitivity analyses yielded consistent results regarding the primary effectiveness and safety findings (Figure 2 and Supplementary e-Figure 3). Examination of the negative control outcome yielded a null association, which was expected.
Figure 2.
Forest plots showing the (a) sensitivity analyses and (b) subgroup analyses of the annual rate of exacerbation.
aHRs with 95% CIs were estimated to study the outcomes of competing risk models, GERD, and high-dimensional PS matching.
bp < 0.05.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GERD, gastroesophageal reflux disease; HR, hazard ratio; IRR, incidence rate ratio; PS, propensity score.
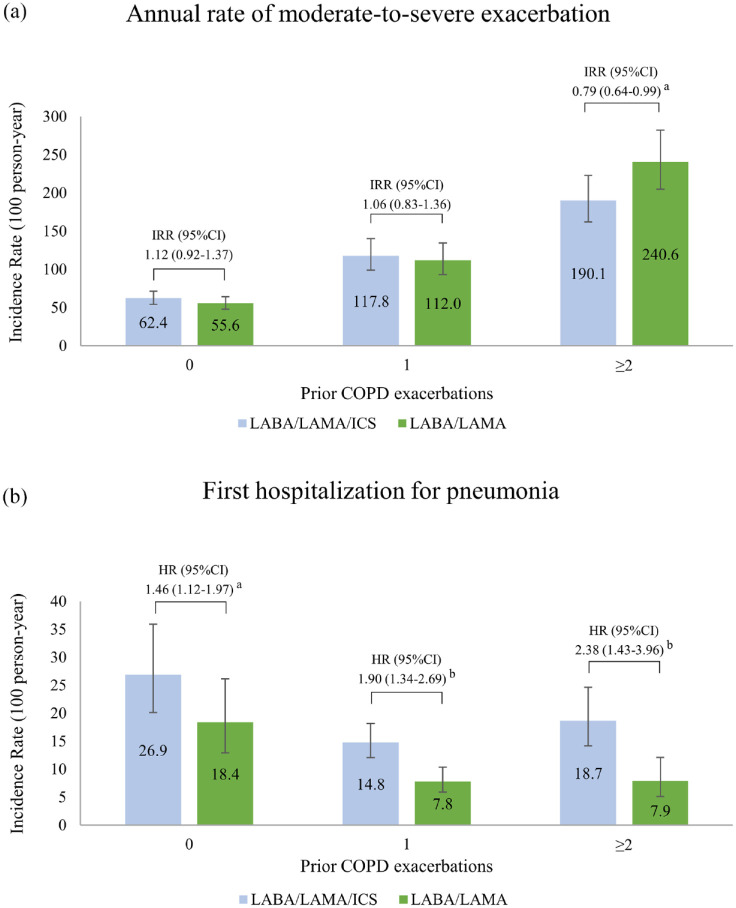
Stratifications by prior pneumonia and prior cardiovascular events did not produce apparent changes in the main effectiveness results (Figure 2). Nevertheless, among patients who experienced ⩾2 exacerbations in the prior year, there was a 21% reduction in the rate of exacerbations associated with the use of LABA/LAMA/ICS compared with LABA/LAMA (IRR, 0.79; 95% CI, 0.64–0.99); however, in this subgroup of patients, LABA/LAMA/ICS therapy was related to a 2.38-fold increase in pneumonia risk relative to the use of LABA/LAMA (IRR, 2.38; 95% CI, 1.43–3.96; Supplemental e-Figure 4). Figure 3 presents the annual rates of exacerbation and first hospitalization for pneumonia in the two groups stratified by 0, 1, and ⩾2 prior exacerbations. There was no apparent ICS withdrawal effect in the dual bronchodilator group (Supplemental e-Figure 5).
Figure 3.
Bar charts illustrating (a) the annual rate of COPD moderate-to-severe exacerbations and (b) incidence rate of first hospitalization for pneumonia stratified by prior exacerbation(s).
ap < .05.
bp < .001.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; ICS, inhaled corticosteroids; IRR, incidence rate ratio; LABA, inhaled long-acting β2 agonists; LAMA, inhaled long-acting muscarinic antagonists.
Discussion
In this observational study with a nationwide COPD population receiving LABA/ICS, most of whom had no prior exacerbations at baseline, escalation to triple therapy versus switching to dual bronchodilator therapy was related to a comparable annual rate of moderate-to-severe COPD exacerbations but with significantly increased risks of pneumonia, respiratory-specific death, and all-cause death. The primary effectiveness and safety findings were robust across multiple sensitivity analyses. However, among patients with ⩾2 prior exacerbations, the use of LABA/LAMA/ICS versus LABA/LAMA was beneficial for preventing COPD exacerbations but was accompanied by a more than twofold increased risk of severe pneumonia.
Our reported comparative effectiveness data are congruent with those of a well-designed cohort study 14 reporting a comparable effect in preventing exacerbations (HR, 0.97; 95% CI, 0.87–1.08) between LABA/LAMA/ICS and LABA/LAMA therapies in COPD management. The consistent findings could be attributable to similar patient characteristics between the two studies, such as no prior asthma diagnosis and no prior exacerbations in most patients. Nevertheless, previous RCTs8–10 reported that LABA/LAMA/ICS versus LABA/LAMA caused significant 15% to 25% reductions in the annual rate of exacerbations. However, these studies included patients with frequent exacerbations8–10 or prior asthma diagnoses,9,10 most of whom had previously received ICS-containing regimens before trial enrollment.8,9 These characteristics of the enrolled patients raised concerns about the potential effect of ICS withdrawal in the LABA/LAMA arm.28,29 Conversely, several real-world studies11–13 reported LABA/LAMA/ICS versus LABA/LAMA to have an increased risk of exacerbations, although the triple therapy group had more prior exacerbations 11 and more ICS-containing regimen usage than did the dual bronchodilator group.12,13 Collectively, previous studies have reported conflicting comparative effectiveness data, possibly owing to the inclusion of patients with different baseline risks for exacerbation and confounding by prior COPD medications or a history of asthma. These confounding effects were addressed in the current study.
Our safety data were in line with the results from the Informing the Pathway of COPD Treatment (IMPACT) trial, the Efficacy and Safety of Triple Therapy in Obstructive Lung Disease (ETHOS) trial, and several observational studies.8,9,13,14,30 We observed a 1.65-fold increased risk of severe pneumonia with triple therapy compared with dual bronchodilator therapy, which was consistent with previous observations8,9,13,14,30 reporting a 1.29- to 1.53-fold increased risk of severe pneumonia under the same comparison. Additionally, our observed greater risk of all-cause death associated with LABA/LAMA/ICS than with LABA/LAMA was probably primarily driven by increased mortality related to respiratory-specific causes, including pneumonia. Specifically, we further examined all-cause death events and found that >70% of all observed death events resulted from respiratory-related causes; in particular, 55% of all death events were pneumonia-related in the triple therapy group, as opposed to 39% in the dual bronchodilator group. These data were consistent with previous findings reporting that LABA/LAMA/ICS versus LABA/LAMA for the management of COPD was associated with a 1.17- to 1.53-fold increased risk of death from any cause13,30 and with a 1.48-fold increased risk of pneumonia-related hospitalization leading to death, 13 respectively. In contrast, there were 42% and 46% decreases in the risk of mortality from any cause when comparing LABA/LAMA/ICS with LABA/LAMA in the IMPACT 9 and ETHOS 8 trials, respectively, although the patients in these trials had a greater COPD severity and greater exacerbation risk than did those in our study.
The ICS withdrawal effect in our study was minimal. We focused on a source COPD population of LABA/ICS initiators because they were a subgroup of COPD patients for whom the choice between LABA/LAMA/ICS and LABA/LAMA needed to be considered the most if major symptoms or exacerbation occurred. Nevertheless, the LABA/LAMA switchers in our study may have experienced an ICS withdrawal effect, although we did not observe such an effect according to the cumulative incidence rates of exacerbations by the duration of both therapies. Several reasons may underlie the observed phenomenon. In the real-world setting, our included study cohort may not benefit from or respond well to ICS-containing therapies, as we excluded patients with a prior history of asthma, and most of them did not have prior exacerbations. In the SUNSET 31 trial, LABA/LAMA/ICS de-escalation to LABA/LAMA among non-frequently-exacerbating patients did not increase the rate of COPD exacerbations. Accordingly, probably because most of our study population had non-frequent exacerbations, switching from LABA/ICS to LAMA/LAMA did not result in an apparent effect of ICS withdrawal.
There are substantial disagreements between the real-world use of triple therapy and LABA/LAMA and the GOLD guideline recommendations. Most triple therapy and LABA/LAMA users did not have prior exacerbations, indicating that these patients were at a lower future exacerbation risk, for whom the use of these inhaled therapies is not suggested. The potentially inappropriate use of inhaled therapies is not uncommon in real-world settings across the world.32–34 Accordingly, there is an urgent need to align the real-world use of inhaled combination therapies with the GOLD guidelines, particularly for LABA/LAMA/ICS treatment, given the associated potential harms of severe pneumonia and all-cause mortality. In most patients with a lower future exacerbation risk, we found that the use of triple therapy versus LABA/LAMA was not beneficial for reducing exacerbations but was related to increased risks of pneumonia and all-cause mortality. Consequently, considering these characteristics of COPD patients and the associated benefits and risks of triple therapy, we recommend the use of LABA/LAMA over LABA/LAMA/ICS when a change in LABA/ICS treatment is needed for COPD management. However, in patients in group E (having ⩾2 prior exacerbations) who were more likely to receive ICS-containing therapy according to the current treatment guidelines, we observed a decreased rate of COPD exacerbation with the use of LABA/LAMA/ICS versus LABA/LAMA. Likewise, a cohort study reported a 17% decreased exacerbation risk for the same comparison in this group of patients, 30 among whom, however, our study further revealed a profoundly increased pneumonia risk with LABA/LAMA/ICS relative to LABA/LAMA use. Accordingly, despite the beneficial effect of LABA/LAMA/ICS on patients who have ⩾2 prior exacerbations, healthcare professionals need to be vigilant about the pneumonia risk in COPD patients with this phenotype.
This study had several strengths. The unique attributes of the present study were the inclusion of a nationwide population of COPD patients receiving LABA/ICS and the adoption of parsimonious exclusion criteria, both of which have led to the high generalizability of our reported data. Additionally, to our knowledge, the present study was the first to take prior use of LABA/ICS into consideration when comparing the effectiveness and safety of LABA/LAMA/ICS with LABA/LAMA in real-world situations. Furthermore, confounding by indication bias is anticipated to be minimal owing to the adoption of several approaches, such as implementing a new-user design, an active-comparator analysis, and a PS-matching approach.
Several limitations of this study merit discussion. First, due to the nature of observational studies, the present cohort study was subject to inherent limitations existing in an observational study design, such as confounding by indication bias and selection bias; however, we achieved well-balanced baseline characteristics and disease severity indicators between groups and collected data from a nationwide COPD population. Second, although the differences in the reasons for censoring between the two groups were less than 5%, the presence of informative censoring could not be ruled out. However, we alternatively conducted an intention-to-treat analysis and observed similar findings. Third, our findings might represent short-term effects owing to the short duration of the inhalation therapies. The observed short-term use of triple and dual bronchodilator therapy, however, reflects the real-world use of inhaled combination therapies. Fourth, owing to a lack of important clinical data such as blood eosinophil levels and lung function data in the analyzed databases, unmeasured confounders could have posed a threat to the internal validity of the reported data. Nevertheless, we reached consistent findings as the main results when employing the high-dimensional PS approach and observed the expected findings with negative outcome analysis. Fifth, medication adherence may act as another confounding factor because most patients receiving LABA/LAMA/ICS used multiple inhalers as opposed to the single inhalers used by the majority of LABA/LAMA-receiving patients. Considering this potential confounding factor, we restricted patients to those with a medication possession ratio ⩾80% and observed consistent findings.
Conclusion
In this real-world study of a nationwide population of COPD patients receiving LABA/ICS, most of whom had no prior exacerbation history, escalation to LABA/LAMA/ICS versus switching to LABA/LAMA was associated with a similar annual rate of moderate-to-severe exacerbations but was related to elevated risks of pneumonia, all-cause mortality, and respiratory-specific mortality. Among patients with ⩾2 prior exacerbations, triple therapy was associated with a lower rate of exacerbation but was accompanied by an increased pneumonia risk compared with dual bronchodilator therapy. Given that the 2024 GOLD guidelines discourage the use of LABA/ICS, our study provides empirical evidence to allow physicians to choose between LABA/LAMA/ICS and LABA/LAMA.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666241292242 for Comparative effectiveness and safety of escalating to triple therapy versus switching to dual bronchodilators after discontinuing LABA/ICS in patients with COPD: a retrospective cohort study by Li-Wei Wu, Tzu-Chieh Lin, Tzu-Han Lin, Ying-Jay Liou, Chen-Liang Tsai, Kuang-Yao Yang and Meng-Ting Wang in Therapeutic Advances in Respiratory Disease
Acknowledgments
We express our appreciation to the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW), Taiwan, for providing access to analyze the database. The interpretations and conclusions contained herein do not represent those of HWDC, MOHW, or the Ministry of Science and Technology, Taiwan. The authors also express our appreciation to Ms. Bi-Juian Wu, affiliated with the Department of Pharmacy, National Yang Ming Chiao Tung University, Taipei, Taiwan, for administrative assistance and technical assistance.
The authors used ChatGPT 3.5 only for editing the manuscript to enhance readability and refine English expression. After using the tool, the authors reviewed the content for accuracy and made additional edits as necessary. The authors take full responsibility for all content in this publication
Appendix
Abbreviations
CI confidence Interval
COPD chronic obstructive pulmonary disease
CVD cardiovascular disease
GERD gastroesophageal reflux disease
GOLD Global Initiative for Chronic Obstructive Lung Disease
HR hazard ratio
ICS inhaled corticosteroids
IPTW inverse probability of treatment weighting
IRR incident rate ratio
LABA inhaled long-acting β2 agonists
LAMA inhaled long-acting muscarinic antagonists
NHI National Health Insurance
NHIRD National Health Insurance Research Database
PS propensity score
RCTs randomized controlled trials
Footnotes
ORCID iDs: Kuang-Yao Yang  https://orcid.org/0000-0002-2803-980X
https://orcid.org/0000-0002-2803-980X
Meng-Ting Wang  https://orcid.org/0000-0003-2153-6632
https://orcid.org/0000-0003-2153-6632
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Li-Wei Wu, School of Pharmacy, National Defense Medical Center, Taipei, Taiwan, Republic of China.
Tzu-Chieh Lin, Department of Pharmacy, National Yang Ming Chiao Tung University, Taipei, Taiwan, Republic of China.
Tzu-Han Lin, Department of Pharmacy, National Yang Ming Chiao Tung University, Taipei, Taiwan, Republic of China.
Ying-Jay Liou, Department of Psychiatry, Taipei Veterans General Hospital, Taipei, Taiwan, Republic of China; Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan, Republic of China.
Chen-Liang Tsai, Division of Pulmonary and Critical Care, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan, Republic of China.
Kuang-Yao Yang, Department of Chest Medicine, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Road, Beitou District, Taipei City, Taiwan 11217, Republic of China; Institute of Emergency and Critical Care Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Meng-Ting Wang, Department of Pharmacy, National Yang Ming Chiao Tung University, No. 155, Sec. 2, Linong Street, Taipei 112, Taiwan 8267250, Republic of China.
Declarations
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of National Yang Ming Chiao Tung University (YM110129E), the Taipei Veterans General Hospital (2021-01-021CC), and the Tri-Service General Hospital, National Defense Medical Center (C202105007). Dates of approval for the three Institutional Review Boards are September 3, 2021, January 25, 2021, and February 17, 2021, respectively. The waiver of written informed consent was granted owing to analysis of the de-identified claims data.
Consent for publication: Not applicable.
Author contributions: Li-Wei Wu: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Tzu-Chieh Lin: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Tzu-Han Lin: Conceptualization; Methodology; Writing – review & editing.
Ying-Jay Liou: Conceptualization; Methodology; Writing – review & editing.
Chen-Liang Tsai: Conceptualization; Methodology; Writing – review & editing.
Kuang-Yao Yang: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Meng-Ting Wang: Conceptualization; Formal analysis; Funding acquisition; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Ministry of Science and Technology (MOST), Taiwan (MOST 110-2320-B-A49A-534). Part of the content of this manuscript was submitted to the MOST as a final report to the funding agency.
The authors declare that there is no conflict of interest.
Availability of data and materials: The authors are not allowed to share the analyzed data of this study because public access to the Taiwan NHIRD is forbidden according to the current regulations of the Ministry of Health and Welfare, Taiwan (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html). To request access to data, please contact the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html).
References
- 1. Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ 2022; 378(e069679): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396(10258): 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aaron SD, Donaldson G, Whitmore GA, et al. Time course and pattern of COPD exacerbation onset. Thorax 2012; 67(3): 238–243. [DOI] [PubMed] [Google Scholar]
- 4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2024 report. Global initiative for chronic obstructive lung disease website, https://goldcopd.org/2024-gold-report/ (2024, accessed 15 May 2024).
- 5. Lareau S, Moseson E, Slatore CG. Exacerbation of COPD. Am J Respir Crit Care Med 2018; 198(11): 21–22. [DOI] [PubMed] [Google Scholar]
- 6. Fortis S, Wan ES, Kunisaki K, et al. Increased mortality associated with frequent exacerbations in COPD patients with mild-to-moderate lung function impairment, and smokers with normal spirometry. Respir Med X 2021; 3: 100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloom CI, Montonen J, Jöns O, et al. First maintenance therapy for chronic obstructive pulmonary disease: retrospective analyses of US and UK healthcare databases. Pulm Ther 2022; 8(1): 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. NEJM 2020; 383(1): 35–48. [DOI] [PubMed] [Google Scholar]
- 9. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. NEJM 2018; 378(18): 1671–1680. [DOI] [PubMed] [Google Scholar]
- 10. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018; 391(10125): 1076–1084. [DOI] [PubMed] [Google Scholar]
- 11. Vogelmeier CF, Worth H, Buhl R, et al. Impact of switching from triple therapy to dual bronchodilation in COPD: the DACCORD “real world” study. Respir Res 2022; 23(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buhl R, Criée C-P, Kardos P, et al. Dual bronchodilation vs triple therapy in the “real-life” COPD DACCORD study. Int J Chron Obstruct Pulmon Dis 2018; 13: 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suissa S, Dell’Aniello S, Ernst P. Triple inhaler versus dual bronchodilator therapy in COPD: real-world effectiveness on mortality. COPD 2022; 19(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: cohort study in real-world clinical practice. Chest 2020; 157(4): 846–855. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh CY, Su CC, Shao SC, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol 2019; 11: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186(6): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 18. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med 2018; 178(2): 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang MT LJ, Huang YL, et al. Comparative effectiveness and safety of different types of inhaled long-acting β2-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β2-agonist plus inhaled corticosteroid fixed-dose combinations in COPD: a propensity score-inverse probability of treatment weighting cohort study. Chest 2021; 160(4): 1255–1270. [DOI] [PubMed] [Google Scholar]
- 20. Samp JC, Joo M, Schumock GT, et al. Comparative effectiveness of long-acting beta2-agonist combined with a long-acting muscarinic antagonist or inhaled corticosteroid in chronic obstructive pulmonary disease. Pharmacotherapy 2017; 37(4): 447–455. [DOI] [PubMed] [Google Scholar]
- 21. Perrone V, Sangiorgi D, Buda S, et al. Comparative analysis of budesonide/formoterol and fluticasone/salmeterol combinations in COPD patients: findings from a real-world analysis in an Italian setting. Int J Chron Obstruct Pulmon Dis 2016; 11: 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006; 40(7–8): 1280–1288. [DOI] [PubMed] [Google Scholar]
- 23. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34(28): 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36(27): 4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneeweiss S, Rassen JA, Glynn RJ, et al. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009; 20(4): 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pandis N, Machin D. Sample calculations for comparing rates. Am J Orthod Dentofacial Orthop 2012; 142(4): 565–567. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009; 38(6): 1228–1234. [Google Scholar]
- 28. Suissa S. Perplexing mortality data from triple therapy trials in COPD. Lancet Respir Med 2021; 9(7): 684–685. [DOI] [PubMed] [Google Scholar]
- 29. Suissa S, Ariel A. Triple therapy trials in COPD: a precision medicine opportunity. Eur Respir J 2018; 52(6): 1801848. [DOI] [PubMed] [Google Scholar]
- 30. Suissa S, Dell’Aniello S, Ernst P. Single-inhaler triple versus dual bronchodilator therapy in COPD: real-world comparative effectiveness and safety. Int J Chron Obstruct Pulmon Dis 2022; 17: 1975–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med 2018; 198(3): 329–339. [DOI] [PubMed] [Google Scholar]
- 32. Miyazaki M, Nakamura H, Takahashi S, et al. The reasons for triple therapy in stable COPD patients in Japanese clinical practice. Int J Chron Obstruct Pulmon Dis 2015; 10: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014; 9: 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis 2017; 12: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666241292242 for Comparative effectiveness and safety of escalating to triple therapy versus switching to dual bronchodilators after discontinuing LABA/ICS in patients with COPD: a retrospective cohort study by Li-Wei Wu, Tzu-Chieh Lin, Tzu-Han Lin, Ying-Jay Liou, Chen-Liang Tsai, Kuang-Yao Yang and Meng-Ting Wang in Therapeutic Advances in Respiratory Disease