Abstract
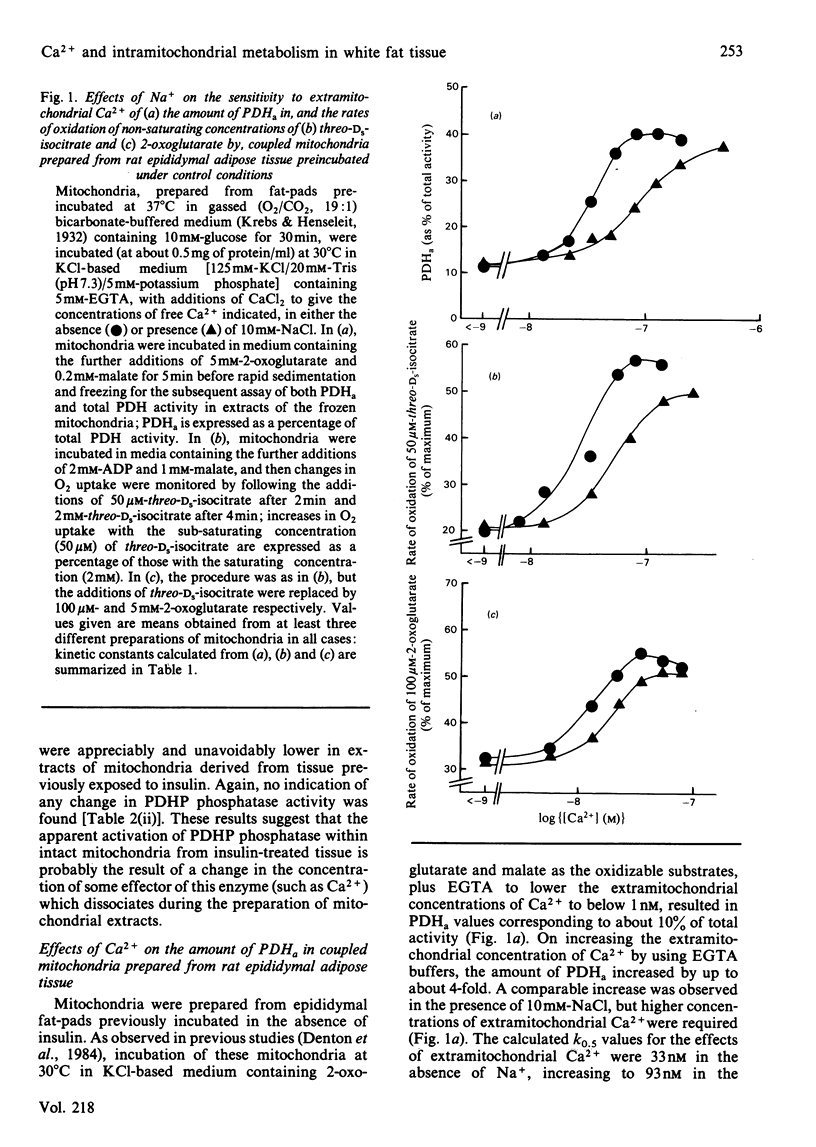
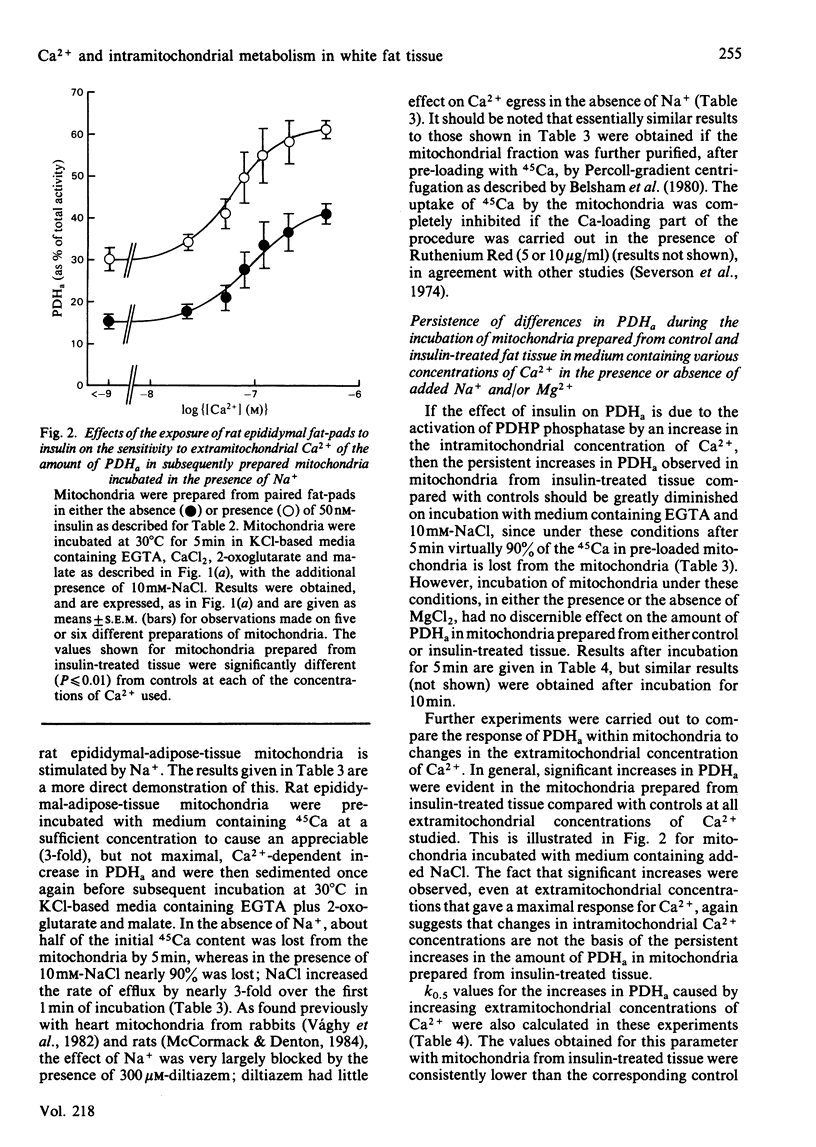
The sensitivity of rat epididymal-adipose-tissue pyruvate dehydrogenase phosphate phosphatase, NAD+-isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase to Ca2+ ions was studied both in mitochondrial extracts and within intact coupled mitochondria. It is concluded that all three enzymes may be activated by increases in the intramitochondrial concentration of Ca2+ and that the distribution of Ca2+ across the mitochondrial inner membrane is determined, as in rat heart mitochondria, by the relative activities of a uniporter (which transports Ca2+ into mitochondria and is inhibited by Mg2+ and Ruthenium Red) and an antiporter (which allows Ca2+ to leave mitochondria in exchange for Na+ and is inhibited by diltiazem). Previous studies with incubated fat-cell mitochondria have indicated that the increases in the amount of active non-phosphorylated pyruvate dehydrogenase in rat epididymal tissue exposed to insulin are the result of activation of pyruvate dehydrogenase phosphate phosphatase. In the present studies, no changes in the activity of the phosphatase were found in extracts of mitochondria, and thus it seemed likely that insulin altered the intramitochondrial concentration of some effector of the phosphatase. Incubation of rat epididymal adipose tissue with medium containing a high concentration of CaCl2 (5mM) was found to increase the active form of pyruvate dehydrogenase to much the same extent as insulin. However, the increases caused by high [Ca2+] in the medium were blocked by Ruthenium Red, whereas those caused by insulin were not. Moreover, whereas the increases resulting from both treatments persisted during the preparation of mitochondria and their subsequent incubation in the absence of Na+, only the increases caused by treatment of the tissue with insulin persisted when the mitochondria were incubated in the presence of Na+ under conditions where the mitochondria are largely depleted of Ca2+. It is concluded that insulin does not act by increasing the intramitochondrial concentration of Ca2+. This conclusion was supported by finding no increases in the activities of the other two Ca2+-responsive intramitochondrial enzymes (NAD+-isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase) in mitochondria prepared from insulin-treated tissue compared with controls.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belsham G. J., Denton R. M., Tanner M. J. Use of a novel rapid preparation of fat-cell plasma membranes employing Percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat-cells. Biochem J. 1980 Nov 15;192(2):457–467. doi: 10.1042/bj1920457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The calcium cycle of mitochondria. FEBS Lett. 1979 Aug 1;104(1):1–5. doi: 10.1016/0014-5793(79)81073-x. [DOI] [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- Crompton M., Künzi M., Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977 Oct 3;79(2):549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Moser R., Lüdi H., Carafoli E. The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues. Eur J Biochem. 1978 Jan 2;82(1):25–31. doi: 10.1111/j.1432-1033.1978.tb11993.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W. The role of phosphorylation in the regulation of fatty acid synthesis by insulin and other hormones. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):33–45. doi: 10.1098/rstb.1983.0036. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Coore H. G., Martin B. R., Randle P. J. Insulin activates pyruvate dehydrogenase in rat epididymal adipose tissue. Nat New Biol. 1971 May 26;231(21):115–116. doi: 10.1038/newbio231115a0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Hughes W. A. Pyruvate dehydrogenase and the hormonal regulation of fat synthesis in mammalian tissues. Int J Biochem. 1978;9(8):545–552. doi: 10.1016/0020-711x(78)90113-1. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Calcium ions, hormones and mitochondrial metabolism. Clin Sci (Lond) 1981 Aug;61(2):135–140. doi: 10.1042/cs0610135. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Marshall S. E. Persistence of the effect of insulin on pyruvate dehydrogenase activity in rat white and brown adipose tissue during the preparation and subsequent incubation of mitochondria. Biochem J. 1984 Jan 15;217(2):441–452. doi: 10.1042/bj2170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Effects of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem J. 1981 Sep 15;198(3):525–533. doi: 10.1042/bj1980525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Effect of micromolar concentrations of free Ca2+ ions on pyruvate dehydrogenase interconversion in intact rat heart mitochondria. Biochem J. 1981 Mar 15;194(3):721–732. doi: 10.1042/bj1940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Hughes W. A., Denton R. M. Incorporation of 32Pi into pyruvate dehydrogenase phosphate in mitochondria from control and insulin-treated adipose tissue. Nature. 1976 Dec 2;264(5585):471–473. doi: 10.1038/264471a0. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Effect of insulin on fatty acid ynthesis from pyruvate, lactage, or endogenous sources in adipose tissue: evidence for the hormonal regulation of pyruvate dehydrogenase. Endocrinology. 1970 Jun;86(6):1368–1375. doi: 10.1210/endo-86-6-1368. [DOI] [PubMed] [Google Scholar]
- Kiechle F. L., Jarett L., Kotagal N., Popp D. A. Partial purification from rat adipocyte plasma membranes of a chemical mediator which simulates the action of insulin on pyruvate dehydrogenase. J Biol Chem. 1981 Mar 25;256(6):2945–2951. [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. A comparative study of the regulation of Ca2+ of the activities of the 2-oxoglutarate dehydrogenase complex and NAD+-isocitrate dehydrogenase from a variety of sources. Biochem J. 1981 May 15;196(2):619–624. doi: 10.1042/bj1960619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984 Feb 15;218(1):235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980 Jul 15;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The activation of pyruvate dehydrogenase in the perfused rat heart by adrenaline and other inotropic agents. Biochem J. 1981 Feb 15;194(2):639–643. doi: 10.1042/bj1940639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Edgell N. J., Denton R. M. Studies on the interactions of Ca2+ and pyruvate in the regulation of rat heart pyruvate dehydrogenase activity. Effects of starvation and diabetes. Biochem J. 1982 Feb 15;202(2):419–427. doi: 10.1042/bj2020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980 Mar 10;111(2):261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Evidence that insulin activates an intrinsic plasma membrane protease in generating a secondary chemical mediator. J Biol Chem. 1980 Jul 25;255(14):6529–6531. [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Bridges B. J., Randle P. J. Exchangeable and total calcium pools in mitochondria of rat epididymal fat-pads and isolated fat-cells. Role in the regulation of pyruvate dehydrogenase activity. Biochem J. 1976 Jan 15;154(1):209–223. doi: 10.1042/bj1540209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vághy P. L., Johnson J. D., Matlib M. A., Wang T., Schwartz A. Selective inhibition of Na+-induced Ca2+ release from heart mitochondria by diltiazem and certain other Ca2+ antagonist drugs. J Biol Chem. 1982 Jun 10;257(11):6000–6002. [PubMed] [Google Scholar]
- Weiss L., Löffler G., Schirmann A., Wieland O. Control of pyruvate dehydrogenase interconversion in adipose tissue by insulin. FEBS Lett. 1971 Jun 24;15(3):229–231. doi: 10.1016/0014-5793(71)80318-6. [DOI] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]



