Abstract
Objectives
The frozen elephant trunk (FET) technique is effective for treating extended aortic arch aneurysms. This study compares hand-made and factory-made devices in this context.
Methods
A retrospective case-control study was conducted on 68 patients who underwent FET for distal aortic arch aneurysm at our institution. We used two different types of devices: hand-made stent graft in group Z (17 cases, 25.0%) and a commercialized stent graft in group J (51 cases, 75.0%). The study compared demographic characteristics and the outcomes between the two groups.
Results
In-hospital mortality was equivalent in both groups (5.9%). Spinal cord injury rates were 5.9% in group Z and 3.9% in group J. Group Z had a higher rate of aortic events (55.9% vs 96.9%, p < 0.001) and more frequent stent migration. The number of cases with aneurysm diameter shrinkage was lower in group Z. The landing zone angle at insertion influenced aneurysm changes, being 17.6 degrees in shrink cases and 26.7 degrees in unchanged or enlarged cases (p = 0.045).
Conclusions
FROZENIX device notably reduced incidences of postoperative complications compared to hand-made prostheses. Factors such as insertion angle and stent size, rather than changes in the landing zone angle, appeared to influence aneurysm shrinkage. The use of FROZENIX in TARFET procedures has shown benefits in reducing complications and improving long-term prognosis, taking into account the landing zone angle.
Keywords: Total arch replacement, frozen elephant trunk technique, aortic arch aneurysm
Introduction
Distal aortic arch aneurysms present significant surgical challenges, often requiring complex repair strategies and limited access via median sternotomy. 1 The frozen elephant trunk (FET) technique, introduced by Kato et al. in 1996 2 and further developed by Karck et al., 3 represents a pivotal advancement in treating these aneurysms. It simplifies the placement of stent grafts in the distal aortic arch and enables treatment of extensive aortic diseases in a single operation. While the FET, particularly the Total Arch Replacement using FET (TARFET), has shown promise in treating aortic dissection, its effectiveness in distal aortic arch aneurysms, especially in terms of long-term outcomes, remains under-explored. Japan has reported increasing use of TARFET in distal aortic arch diseases, demonstrating notable success. 3 Prior to commercial device availability, our institution was instrumental in employing hand-made stent grafts for TARFET. The focus on Japan stems from its innovative cardiovascular techniques and high volume of TARFET procedures. This study aims to evaluate TARFET outcomes in distal aortic arch aneurysms, comparing the efficacy of commercial stent grafts with hand-made prostheses, focusing on both early and long-term results.
Patients and methods
Patients and study design
The present study was conducted as a retrospective observational single-center case control investigation. From January 2009 to June 2023, Anjo Kosei Hospital (Aichi, Japan) performed 288 total arch replacements (TARs), including 150 (52.1%) for true aneurysms and 138 (47.9%) for dissections and other cases. This study included 70 consecutive TARFET procedures for true extended aortic arch aneurysms. Two cases involving two-stage thoracic endovascular aortic repair (TEVAR) were excluded. The study encompassed patients with degenerative aortic aneurysms extending from the aortic arch to the proximal descending aorta, including two ruptured aneurysms. We defined a distal aortic arch aneurysm as an aneurysm situated at the aortic arch and extending from the root of the left subclavian artery (SCA) to the descending thoracic aorta. The conventional TAR procedure was considered the primary choice for degenerative distal arch aneurysm repair. If the distance from the left SCA bifurcation to the proximal end of the aneurysm was adequate and the aortic morphology suitable for TEVAR, endovascular treatment was selected. For patients under 70 years, aggressive open surgery was considered the primary treatment, barring any contraindications. FET procedure criteria included age over 80 years, challenging distal anastomosis with aneurysms, and a favorable landing zone for FET in the descending aorta. A two-stage operation consisting of TEVAR following TAR with FET was performed for two patients. If the FET distal landing to cover the entire distal aortic arch aneurysm was below the Th9 level, a two-staged surgery followed by either descending aorta replacement or secondary TEVAR was preferred. After excluding two cases, 68 cases were enrolled in this retrospective study. Patients with hand-made stent grafts (Gianturco Z stent, Cook, USA) were included in group Z (17 patients, 25.0%), and those with commercialized stent grafts, J Graft FROZENIX (formerly J graft Open Stent Graft, Japan Lifeline Co., Tokyo, Japan), were included in group J (51 patients, 75.0%). We retrospectively compared the surgical outcomes of these two groups to establish the effectiveness and safety of the commercialized stent graft.
Stent grafts
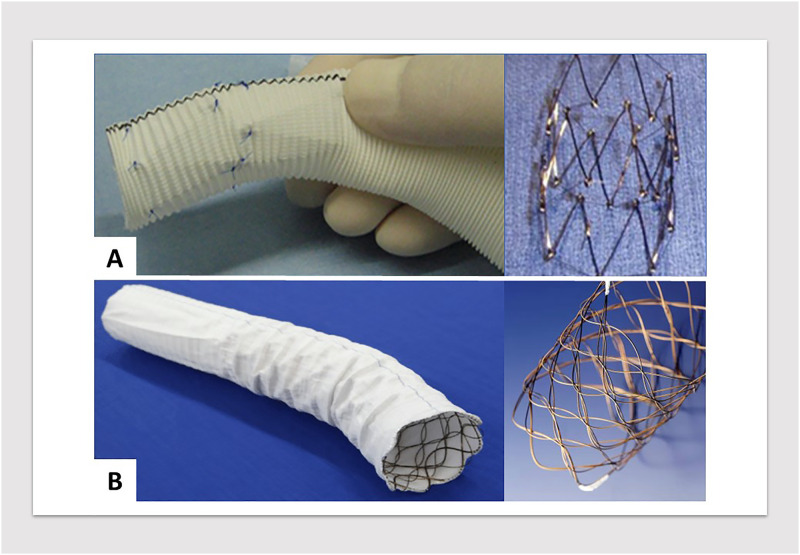
Two different types of devices were used in this study. When TARFET was initiated in 2009, there were no approved devices for FET in Japan. Consequently, in the early years, hand-made stent grafts were fabricated. These consisted of a Gianturco Z stent, either 30 or 40 mm in diameter and 50 mm in length, inserted into the distal end of a 22–30 mm diameter Dacron graft. The stent was then sutured inside the graft. The Japan-made commercialized FET prosthesis, J Graft FROZENIX (Japan Lifeline, Tokyo), was first launched in 2014.3,4 Since its introduction, the TARFET using a 4-branched graft for TAR and the FROZENIX prosthesis for the FET has replaced hand-made stent grafts as the primary treatment for distal aortic arch aneurysms at our institute (Figure 1).
Figure 1.
(A) Hand-made stent graft featuring a Gianturco stainless-steel endoskeleton, composed of Z-shaped stent bodies. (B) Commercialized stent graft with a flexible Nitinol wire-woven structure, enhancing trackability to the aortic arch.
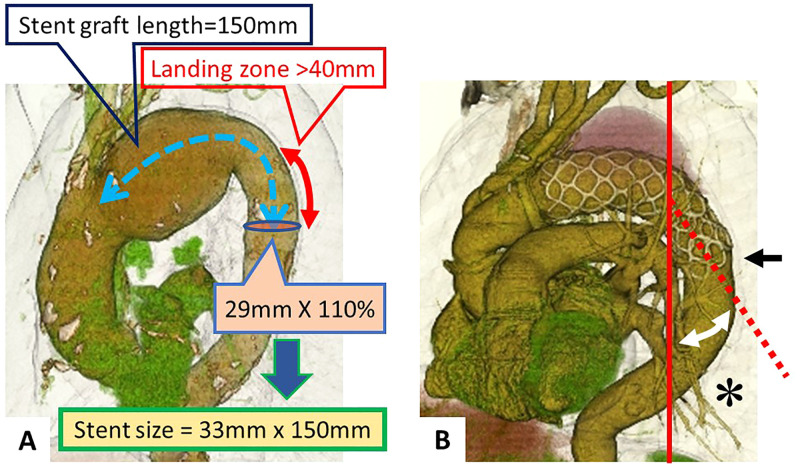
Regarding the sizing of stent grafts, the optimal size was determined based on preoperative CT scans. Measurements were taken between the planned distal anastomosis of the tetrafurcated tube graft for TAR and the target distal end of the FET prosthesis. The selected stent graft was designed to cover the straight descending aorta above the thoracic vertebra (Th) 8 level, ensuring a landing zone of at least 40 mm and a diameter between 110% and 120% of the descending aorta (Figure 2(A)).
Figure 2.
(A) Illustration of the stent graft sizing process. The length of the stent graft is determined from the subclavian artery bifurcation to 4 cm past the end of the aneurysm. The diameter of the stent graft is chosen based on 110% of the aortic diameter at the landing zone. (B) Measurement of the stent distal end level at the vertebral body (indicated by the black arrow). The landing zone angle (marked with an asterisk) is the angle between the distal end of the stent and the vertical axis.
FROZENIX consists of a Dacron polyester fabric vascular prosthesis with nitinol stents affixed on the inner aspect. Its delivery system comprises a malleable rod, which can be advanced into the descending aorta. FROZENIX is available in lengths of 60, 90, 120, and 150 mm for the stented portions and diameters ranging from 21 to 39 mm.
Operative technique
All TARFET procedures were performed through median sternotomy. Cerebrospinal fluid drainage was not employed in any of the cases. The primary method for arterial cannulation was direct cannulation of the ascending aorta, with the left side of the SCA serving as an alternative. Femoral artery cannulation was not additionally employed. Once cardiopulmonary bypass was established, patients were cooled until their rectal or bladder temperatures reached 26 °C. Under circulatory arrest conditions, selective cerebral perfusion was initiated. Myocardial protection was ensured through the infusion of cold blood cardioplegia into the coronary sinus. The aortic arch was typically incised between the left common carotid artery and the left SCA (Zone 2). In some cases, incision was made distal to the left SCA (Zone 3). The stent graft was inserted into the descending aorta and deployed, ensuring it was filled with blood to prevent air embolism. For the FROZENIX cases, the non-stented part was minimized to less than 5 mm, allowing the formation of the distal stump without continuous suturing, facilitated by the self-expanding force of the stent. In contrast, for Z-stent grafts, the non-stented part was longer, necessitating continuous suturing for distal stump formation. The modification time of the stent in the Z group was about 30 min (Image). Subsequently, another 4-branched graft was anastomosed to the stump in an open distal anastomosis fashion, reinforced with an outer felt strip coated with Hydrofit hemostatic sealant (Sanyo Chemical Industries, Kyoto, Japan). In cases employing FROZENIX, the ease of distal anastomosis was enhanced by direct anastomosis between the graft and the stent graft without needing to construct a distal stump, thereby simplifying the surgical technique. After establishing antegrade systemic perfusion from the 4-branched graft, proximal anastomosis was performed. Subsequently, the left subclavian, left carotid, and brachiocephalic arteries were anastomosed to each branch of the graft.
Measurements of CT images
Preoperative evaluation included measuring the maximum aneurysm diameter, aneurysm morphology (classified as saccular or fusiform), distance from the SCA to the distal end of the aneurysm, and the diameter of the distal descending aorta (landing zone). Patients who underwent TARFET were systematically followed up at our hospital's outpatient clinic. Follow-up CT evaluations were scheduled at discharge, six months post-procedure, and annually thereafter. The purpose of these CT scans was to assess aneurysm remodeling by monitoring changes in the aneurysm diameter, stent graft position, and the angle of the stent graft over time (Figure 2(B)).
Aneurysm diameter changes
Changes in aneurysm diameter were classified as follows: a decrease of 10% or more from the initial postoperative measurement was defined as the “shrink group.” Conversely, cases with either an increase of 10% or more or a change of less than 10% were classified as the “no change group.”
Distal landing zone Th level
The positioning of the distal side of the stent graft was quantified in relation to vertebral levels. To enhance the precision of stent graft placement, the height of the vertebral body was divided into ten equidistant segments. The change in the stent's position was meticulously assessed by comparing segmental locations across different time points, allowing for an accurate evaluation of migration or stability.
Landing zone angle
The angle of the stent graft landing zone relative to the body's longitudinal axis was carefully measured. Both the initial values at the time of insertion and the postoperative changes in angle were evaluated. These measurements provided insights into the stent's positional integrity and potential risks for endoleak or migration.
Endpoints and follow-up
The primary endpoint of the study was defined as in-hospital mortality and the necessity for subsequent aortic procedures, such as TEVAR or additional surgeries. Secondary endpoints included surgical parameters and the incidence of major complications. Neurological dysfunctions, such as stroke or spinal cord injury (SCI), were identified as persistent brain dysfunctions at discharge, with new postoperative lesions evident on CT scans or magnetic resonance imaging. SCI was further characterized as paraplegia or paraparesis.
Definitions
Coronary disease was defined as a history of coronary intervention or the presence of concomitant coronary artery stenosis. Chronic obstructive pulmonary disease (COPD) was defined as having a forced expiratory volume in one second (FEV1) less than 70% of the predicted value or requiring the use of a bronchial inhaler. Renal failure was defined as a serum creatinine level greater than 1.3 g/dL or an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2.
Ethics and data collection
This study was conducted in accordance with the latest version of the Declaration of Helsinki. The study design was approved by the Institutional Review Board of Anjo Kosei Hospital (approval no. R23-081). The approval date was 11 December 2023. Owing to the retrospective nature of the research, the Ethics Committee waived the requirement for written patient informed consent, as the study did not alter the course of treatment and utilized a database designed to protect patients’ identities. The reporting of this study conforms to the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 5
Statistical analysis
Patient data were collected retrospectively. Continuous variables are presented as mean and standard deviation (SD) if normally distributed, and as median with interquartile range for non-normal distributions. The χ2 test was used for univariate analysis to identify significant differences. Changes within subjects were compared using the paired t-test. A P-value of less than 0.05 was considered statistically significant. Outcomes were estimated using Kaplan–Meier analysis. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
Results
Patients characteristics
Among the 68 patients who underwent TARFET surgery, two different types of devices were used. Group Z comprised 17 patients (25.0%) who received hand-made stent grafts with Gianturco Z stents, and Group J consisted of 51 patients (75.0%) who were treated with the commercialized stent graft (J Graft FROZENIX). The preoperative characteristics of both groups are outlined in Table 1. The average age and gender distribution were similar across groups. The prevalence of comorbidities such as diabetes mellitus, chronic kidney disease, and COPD showed no significant differences between the groups. The mean aneurysm diameter was 57.3 ± 8.8 mm (range 42–70 mm) in Group Z and 57.9 ± 9.7 mm (range 35–89 mm) in Group J, with the majority of aneurysms being of the saccular type. The length of the aneurysm was 70 mm or greater in both groups. None of these comparisons showed statistically significant differences.
Table 1.
Patient preoperative demographics.
| Variable | Group Z (n = 17) | Group J (n = 51) | p-value |
|---|---|---|---|
| Age (range) | 75.2 ± 3.94 (69–84) | 75.3 ± 5.34 (61–85) | 0.912 |
| Male(%)/Female(%) | 16(94%)/1 (6%) | 46(90%)/5 (10%) | 0.885 |
| Diabetes mellitus | 3 (17.6%) | 12 (23.5%) | 0.880 |
| Chronic kidney disease | 6 (35.3%) | 14 (27.5%) | 0.828 |
| COPD | 5 (29.4%) | 23 (45.1%) | 0.523 |
| Coronary artery disease | 4 (23.5%) | 13 (25.5%) | 0.987 |
| Aortic operation | 4 (23.5%) | 9 (17.6%) | 0.867 |
| Cerebral vascular disease | 2 (11.8%) | 10 (19.6%) | 0.764 |
| Peripheral artery disease | 3 (17.6%) | 7 (13.7%) | 0.925 |
| Poor LVEF (<50%) | 2 (11.8%) | 3 (5.9%) | 0.723 |
| Aneurysm diameter (mm) (range) | 57.3 ± 8.8 (42–70) | 57.9 ± 9.7 (35–89) | 0.859 |
| Aneurysm type, saccular/fusiform | 16 (94%)/1 (6%) | 43 (86%)/8 (14%) | 0.586 |
| Length of aneurysm (mm) (from SCA to the end of aneurysm) | 72.5 ± 22.0 | 72.8 ± 20.7 | 0.883 |
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction.
Operative data and results
Surgical characteristics between the two groups are summarized in Table 2. Operative time was comparable across groups. The stent graft diameter and length showed no significant differences. However, the stent length varied significantly, with Group Z having a uniform length of 50.0 mm and Group J averaging 118.8 ± 18.9 mm (p < 0.001). This difference is attributable to the structural design, as Z stents only have a 50 mm length, while FROZENIX varies from 60 to 150 mm. The ratio of stent diameter to aorta diameter was similar between the groups (1.12 ± 0.12 for Group Z and 1.11 ± 0.06 for Group J, p = 0.937).
Table 2.
Surgical characteristics.
| Variable | Group Z (n = 17) | Group J (n = 51) | p-value |
|---|---|---|---|
| Stent graft diameter (mm) | 31.5 ± 2.96 | 31.3 ± 2.58 | 0.754 |
| Stent graft length (mm) | 127.5 ± 19.6 | 133.7 ± 21.3 | 0.295 |
| Stent length (mm) | 50.0 ± 0 | 118.8 ± 18.9 | <0.001 |
| Stent diameter/Aorta diameter | 1.12 ± 0.12 | 1.11 ± 0.06 | 0.937 |
| Operative time (min) | 351 ± 65.9 | 368 ± 109 | 0.541 |
| Concomitant operation | |||
| CABG | 0 | 2 | |
| AVR | 0 | 2 | |
| ASD closure + TAP | 0 | 1 | |
CABG: coronary artery bypass grafting; AVR: aortic valve replacement; ASD: atrial septal defect; TAP: tricuspid annuloplasty.
The postoperative outcomes are detailed in Table 3. Prolonged intubation occurred in 11.8% of Group Z and 3.9% of Group J, with no significant difference in incidence. The in-hospital mortality rate was identical for both groups at 5.9%. The incidence of early morbidity, such as stroke, was 17.6% in Group Z and 11.8% in Group J, and the rate of SCI was 5.9% in Group Z and 3.9% in Group J, with no significant differences observed between the groups (p = 0.825 and p = 0.943, respectively). Neither group experienced cases of paraplegia, while paraparesis occurred in 5.9% of Group Z and 3.9% of Group J, again without a significant difference (p = 0.825).
Table 3.
Postoperative results.
| Variable | Group Z (n = 17) | Group J (n = 51) | p-value |
|---|---|---|---|
| Prolonged intubation | 2 (11.8%) | 2 (3.9%) | 0.492 |
| Renal failure/new dialysis | 0 | 0 | |
| Early mortality | |||
| In-hospital mortality | 1 (5.9%) | 3 (5.9%) | 1.0 |
| Early morbidity | |||
| Stroke | 3 (17.6%) | 6 (11.8%) | 0.825 |
| Spinal cord injury | 1 (5.9%) | 2 (3.9%) | 0.943 |
| Paraplegia | 0 | 0 | |
| Paraparesis | 1 (5.9%) | 2 (3.9%) | 0.825 |
Postoperative CT findings
Significant differences between both groups were observed in several aspects of the postoperative CT findings, as detailed in Table 4. The average proximal non-stent length in Group Z was 77.5 ± 19.6 mm, significantly longer than the 15.5 ± 14.7 mm in Group J (p < 0.0001). The distal landing length was shorter in Group Z, averaging 42.6 ± 7.1 mm, compared to 51.3 ± 16.9 mm in Group J, a difference that was statistically significant (p = 0.0467). The mean level of the distal landing zone at the thoracic vertebrae (Th) was Th7.1 ± 0.84 for Group Z and Th7.5 ± 0.73 for Group J, with no significant difference observed (p = 0.0659). Stent migration length, measured by vertebral body range, was significantly greater in Group Z (0.96 ± 0.99, range 0–3.1) compared to Group J (0.45 ± 0.41, range 0–1.6) (p = 0.0472). The incidence of stent migration over one vertebral body was higher in Group Z (37.5%) compared to Group J (12.8%), though this difference was not statistically significant (p = 0.1250). A significant difference was noted in the landing zone angle from the axis, with Group Z showing a larger variance of 28.4 ± 22.4° (range 0–81) compared to 17.6 ± 15.2° (range 0–62) in Group J (p = 0.0342). However, the change in landing zone angle (Δ) showed no significant difference, with 16.1 ± 13.0° (range 0–43) in Group Z and 11.2 ± 11.6° (range 0–34) in Group J (p = 0.1605). Type Ib endoleak occurred in 43.8% of Group Z, significantly higher compared to 2.3% in Group J (p = 0.0002). TEVAR was considered as an additional intervention for patients with Type Ib endoleak. Aneurysm shrinkage was observed in 43.8% of Group Z and 63.6% of Group J, showing no significant difference (p = 0.3849).
Table 4.
Postoperative CT findings 1.
| Variable | Group Z (n = 16) | Group J (n = 44) | p-value |
|---|---|---|---|
| Proximal non-stent length (mm) | 77.5 ± 19.6 | 15.5 ± 14.7 | <0.0001 |
| Distal landing length (mm) | 42.6 ± 7.1 | 51.3 ± 16.9 | 0.0467 |
| Distal landing zone Th level (mean) | Th7.1 ± 0.84 | Th7.5 ± 0.73 | 0.0659 |
| Th5 | 1 (5.9%) | 1 (2.0%) | |
| Th6 | 5 (2.9%) | 5 (10.2%) | |
| Th7 | 7 (41.2%) | 27 (55.1%) | |
| Th8 | 3 (17.6%) | 16 (32.7%) | |
| Stent migration length (vertebral body) (range) | 0.96 ± 0.99 (0–3.1) | 0.45 ± 0.41 (0–1.6) | 0.0472 |
| Stent migration length >1 vertebral body (n) | 6 (37.5%) | 6 (12.8%) | 0.1250 |
| landing zone angle from axis (range) | 28.4 ± 22.4° (0–81) | 17.6 ± 15.2° (0–62) | 0.0342 |
| Landing zone angle change Δ (range) | 16.1 ± 13.0° (0–43) | 11.2 ± 11.6° (0–34) | 0.1605 |
| Endoleak (type Ib) | 7/16 (43.8%) | 1/44 (2.3%) | 0.0002 |
| Aneurysm shrinkage | 7/16(43.8%) | 28 /44(63.6%) | 0.3849 |
CT: computed tomography; Th: thoracic vertebrae.
Postoperative CT findings were compared between the shrink group (n = 35) and the no change group (n = 25) (Table 5). The level of the landing zone and the landing length revealed no significant difference. Stent migration length, measured in terms of vertebral bodies, was slightly less in the shrink group at 0.45 (range 0–1) compared to 0.69 (range 0–3) in the no change group, but this difference approached significance (p = 0.0678). The incidence of stent migration over one vertebral body was not significantly different between the groups, with 8.6% in the shrink group and 28.0% in the no change group (p = 0.1378), indicating a trend toward more cases in the no change group. The landing zone angles from the axis were significantly lower in the shrink group at 17.6° (range 0–66) compared to a larger angle of 26.7° (range 2–81) in the no change group (p = 0.0445). However, the change in the landing zone angle (Δ) did not differ significantly, with the shrink group showing a change of 12.8° (range 0–49) compared to 10.9° (range 2–43) in the no change group (p = 0.7040).
Table 5.
Postoperative CT findings 2.
| Variable | Shrink group (n = 35) | No change group (n = 25) | p |
|---|---|---|---|
| Distal landing zone Th level (Th)(range) | Th 7.5 (6–8) | Th 7.4 (5–8) | 0.5909 |
| Landing length (mm) | 46.0 | 47.7 | 0.8173 |
| Stent migration length (vertebra) | 0.45 | 0.69 | 0.0678 |
| Stent migration length >1 vertebra (n) | 3 (8.6%) | 7 (28.0%) | 0.1378 |
| Landing zone angle from axis | 17.6° | 26.7° | 0.0445 |
| Landing zone angle change Δ | 12.8° | 10.9° | 0.7040 |
Follow-up outcomes
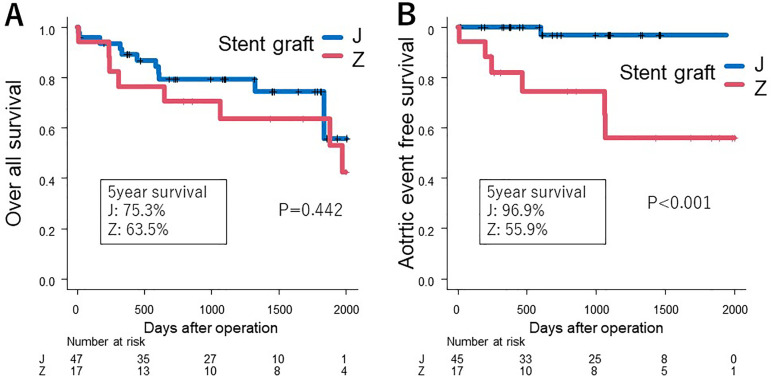
The mean follow-up period was 40.9 months. The Kaplan-Meier survival curves were used to illustrate the postoperative outcomes of patients receiving stent grafts in the two groups (Figure 3(A)). In Group Z, five patients (18.5%) underwent additional TEVAR, and two patients experienced rupture before TEVAR. The overall survival rate at the five-year period showed that Group J had a survival rate of 75.3%, while Group Z had a survival rate of 63.5%; however, this difference was not statistically significant (p = 0.442). In contrast, the aortic event-free survival rate differed markedly between the groups, with Group J demonstrating a significantly higher event-free survival compared to Group Z (p < 0.001) (Figure 3(B)). This suggests a potential difference in the long-term efficacy or complications associated with the stent grafts used in the two groups.
Figure 3.
Comparison of outcomes between the groups. (A) The overall survival rates, showing no significant difference between the two groups. (B) Postoperative aortic event rates, indicating a significant difference between the groups.
Discussion
TAR is the gold standard technique for addressing degenerative aortic arch aneurysms. Despite its technical complexity, particularly in terms of aneurysm morphology, the FET technique, first reported in Japan in 1996, 2 has gained international acclaim. FET simplifies the distal anastomosis, the most intricate part of TAR, allowing for comprehensive treatment of arch lesions extending into the descending aorta within a single procedure. Initial experiences with hand-made stent grafts in Japan highlighted a significant frequency of SCI. This was attributed to extensive coverage of segmental arteries crucial for spinal cord perfusion or embolization of thrombus, atheroma, or air within these arteries.6–9 Several European studies have demonstrated that the FET method10–12 has a relatively low mortality rate. Parallel to the development of factory-produced devices, a growing body of literature on TARFET has emerged, discussing its suitability for a range of aortic pathologies. FET for extensive thoracic aortic diseases has received a class IIa recommendation from the Vascular Domain of the European Association for Cardio-Thoracic Surgery. 13 The first Japan-made commercialized FET device, J Graft Open Stent Graft (now FROZENIX), became clinically available in Japan in 2014. 3 This prosthesis, with its unique design featuring oval-shaped nitinol wire secured inside the vascular prosthesis, minimizes the risk of intimal injury and provides high flexibility to conform to the aortic curvature. 4 The delivery system's malleable rod allows for easy deployment, and sheath markers aid in precise placement of the stent's distal end.4,14 FROZENIX's design enables the minimization of the non-stented portion, an advantage over single-piece devices such as Thoraflex (Vascutek, Inchinnan, Scotland, UK) or E-vita (Jotec Inc., Hechingen, Germany) used elsewhere. Our study's mid-term outcomes for TARFET with FROZENIX in treating distal aortic arch aneurysms were satisfactory, with a 5-year survival rate of 75.3%, comparable to previous reports.15–19 Notably, most prior TARFET studies focused on aortic dissection, with only a few addressing aortic arch aneurysms.20,21 Leone et al.'s TARFET sub-analysis indicated that patients with degenerative aortic aneurysms were typically older and had more preoperative conditions, including heart and lung diseases, and exhibited a higher death rate compared to aortic dissection cases (19.3% vs 12.9%). 18 The course of aneurysm diameter and endoleak are issues in TARFET for true aneurysms, and there are no reports comparing hand-made devices with factory-made devices. Our study compares the short- and mid-term outcomes of hand-made stent grafts (Group Z) with factory-produced devices (Group J) in patients with degenerative aortic arch aneurysms. While mortality and neurological complication rates were lower in Group J, the small sample size limits the significance of these findings. The study also noted that improved devices reduced neurological morbidity, but it remained high. According to a position paper by the EACTS Vascular Domain, aggregated morbidity and mortality rates were described as in-hospital mortality between 1.8% and 17.2%, stroke between 2.5% and 20%, and SCI between 0% and 21%. 13 A meta-analysis reported in-hospital mortality at 8.8% (0%–40.9%), stroke at 7.6% (0%–24%), and paraplegia at 4.7% (0%–21.6%). 8 In a clinical trial of this FET device among nine major centers, the rates of in-hospital mortality, stroke, and paraplegia were 5.1%, 10.0%, and 1.7%, respectively. 3 Compared with these results, our outcomes for TEAFET using FROZENIX with an in-hospital mortality rate of 5.9%, stroke rate of 11.8%, and paraplegia rate of 0% were comparable or more favorable. Furthermore, a recent Japanese multicenter study using FROZENIX reported in-hospital mortality at 1.9%, stroke at 5.6%, and paraplegia at 1.6%, 22 superior to other series. Recent results at our institution have shown improvement over earlier series. Katayama et al. 9 identified significant independent risk factors for SCI, including the distal position of the stent-graft below the Th9 and low mean blood pressure below 70 mmHg. Therefore, we aim to deploy the distal end of the FET prosthesis above the Th8 level and maintain stable perioperative hemodynamics to avoid critical postoperative complications, including SCI.
Postoperative CT evaluation is crucial for monitoring remaining aneurysms. Our study closely examined postoperative CT images, providing objective criteria for assessing aneurysm repair success and stent graft performance. There were more postoperative aortic events and fewer cases of aneurysm size reduction in Group Z. Postoperative type Ib endoleak into the remaining aneurysm remains a concern for TARFET. A retrospective study 22 using FROZENIX for thoracic aortic aneurysm showed only one case (0.15%) of type Ib endoleak. In our series, endoleak Ib was significantly reduced with FROZENIX (from 43.8% to 2.3%, p = 0.0002), while the rate of aneurysm shrinkage improved but not significantly (from 43.8% to 63.6%, p = 0.3849). Compared with Z stents, FROZENIX is expected to enhance trackability to the aortic arch of the thoracic aorta due to its unique Nitinol wire-woven structure. The improvement in the device did not significantly reduce the shrinkage rate, prompting a detailed analysis of postoperative CT images to determine the cause. The landing zone angle (the angle of the distal portion of the stent graft) was found to influence the change in aneurysm diameter. However, the challenge remains in selecting a short FET to prevent type IB endoleak from an insufficient distal landing zone. To minimize these complications, we choose the length of the stent-graft to be inserted by maintaining more than a 4 cm distal landing zone in the descending aorta. If the patient's anatomy does not meet these criteria, we opt for conventional TAR or a two-stage procedure (TAR with FET followed by conventional TEVAR). Tokunaga et al. reported that the cumulative incidence of secondary intervention after TARFET for distal aortic arch aneurysms was 16.6% at 1 year and 25.5% at 5 years. 20 Indeed, there is a tendency to choose a more proximal implantation of the distal end of the FET prosthesis to avoid SCI. Cases in which complete exclusion and remodeling could not be achieved due to the aneurysm's morphology were excluded from this study, as they were scheduled for secondary aortic intervention.
Regarding neurological complications, the incidence of stroke was higher in TARFET, likely due to the aneurysm-specific shaggy aorta and redo surgeries. However, the incidence of paraplegia could be reduced by avoiding deep FET device insertion below the T8 level for spinal cord safety. Another caution should be taken to promote aneurysmal shrinkage. This study confirms the importance of the stent landing zone angle and the height of the stent ends. We believe that overcoming the problems of the hand-made Z stent graft, the advantages of FROZENIX may contribute to better outcomes in TARFET for distal aortic arch aneurysms. Intensive clinical assessment with close imaging evaluation, while waiting for secondary intervention, is mandatory to avoid any interval aortic events. In addition, preoperative evaluation of aneurysm morphology with CT and designing the appropriate diameter and length of the FET prosthesis are essential to obtain the maximum advantage of TARFET. These complications, derived from the severely atherosclerotic aorta in patients with degenerative aortic aneurysms, are still challenging to manage and remain a future issue to be addressed.
Study limitations
This study has several limitations. First, it was a single-center, retrospective study, and the number of patients was small. Second, the observational periods for the two groups differed, as FROZENIX has been used since 2014. Third, the ratios of the two groups were disparate because the procedure selection was nonrandomized and depended on the era of each device. Finally, the follow-up period was relatively short, especially for the FROZENIX group. Despite these limitations, few studies have focused on distal aortic arch aneurysm repair with TARFET. Our experience supports the use of TARFET with FROZENIX as a significant therapeutic option for distal aortic arch aneurysms. Further investigations with a larger cohort and longer follow-up are warranted.
Conclusions
This study revealed no significant difference in all-cause mortality between the groups. However, the hand-made Z stent group experienced a higher frequency of aortic events, including stent migration, endoleak, graft kink, and aneurysm enlargement, necessitating additional interventions, suggesting that the TARFET with FROZENIX is more effective treatment option for distal aortic arch aneurysms. FROZENIX notably reduced the postoperative aortic events. Although there were cases where aneurysms did not shrink, this outcome appears to be influenced by factors such as the insertion stent angle, rather than changes in the landing zone angle. Importantly, the use of FROZENIX in TARFET procedures, as part of the evolution of the Japan-made FET device, has shown to be beneficial in reducing early complications and potentially improving long-term prognosis, considering the landing zone angle. To further ascertain the precise impact of FROZENIX, long-term outcomes, including follow-up CT image analyses, are crucial.
Footnotes
Author Contributions: Shinji Kanemitsu designed the research and supervised the study. Shunsuke Sakamoto conducted the surgeries and analyzed the data. Renta Ishikawa contributed to data collection and interpretation. Toru Mizumoto assisted in manuscript preparation and provided critical revisions. All authors reviewed and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shinji Kanemitsu https://orcid.org/0000-0002-1823-5962
References
- 1.Minatoya K, Ogino H, Matsuda H, et al. Surgical management of distal arch aneurysm: another approach with improved results. Ann Thorac Surg 2006; 81: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 2.Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996; 94: 188–193. [PubMed] [Google Scholar]
- 3.Uchida N, Katayama A, Higashiue S, et al. A new device as an open stent graft for extended aortic repair: a multicentre early experience in Japan. Eur J Cardiothorac Surg 2016; 49: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 4.Okita Y. Frozen elephant trunk with Frozenix prosthesis. Ann Cardiothorac Surg 2020; 9: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 6.Usui A, Fujimoto K, Ishiguchi T, et al. Cerebrospinal dysfunction after endovascular stent-grafting via a median sternotomy: the frozen elephant trunk procedure. Ann Thorac Surg 2002; 74: S1821–S1824. [DOI] [PubMed] [Google Scholar]
- 7.Flores J, Kunihara T, Shiiya N, et al. Extensive deployment of the stented elephant trunk is associated with an increased risk of spinal cord injury. J Thorac Cardiovasc Surg 2006; 131: 336–342. [DOI] [PubMed] [Google Scholar]
- 8.Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: a meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg 2019; 160: 20–33. [DOI] [PubMed] [Google Scholar]
- 9.Katayama K, Uchida N, Katayama A, et al. Multiple factors predict the risk of spinal cord injury after the frozen elephant trunk technique for extended thoracic aortic disease. Eur J Cardiothorac Surg 2015; 47: 616–620. [DOI] [PubMed] [Google Scholar]
- 10.Di Bartolomeo R, Murana G, Di Marco L, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. Eur J Cardiothorac Surg 2017; 51: i20–i28. [DOI] [PubMed] [Google Scholar]
- 11.Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg 2013; 44: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: the Hannover experience. J Thorac Cardiovasc Surg 2015; 149: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 13.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the vascular domain of EACTS. Eur J Cardiothorac Surg 2015; 47: 759–769. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka K. Editorial comment regarding “total aortic arch replacement using the frozen elephant trunk technique with J graft open stent graft for distal aortic arch aneurysm”. Gen Thorac Cardiovasc Surg 2018; 66: 501–503. [DOI] [PubMed] [Google Scholar]
- 15.Sueda T, Takahashi S, Katayama K, et al. The long-term outcomes of partial arch repair using the frozen elephant trunk technique for distal arch aortic aneurysm. Surg Today 2018; 48: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitake A, Tochii M, Tokunaga C, et al. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg 2020; 58: 707–713. [DOI] [PubMed] [Google Scholar]
- 17.Di Bartolomeo R, Murana G, Di Marco L, et al. Is the frozen elephant trunk frozen? Gen Thorac Cardiovasc Surg 2019; 67: 111–117. [DOI] [PubMed] [Google Scholar]
- 18.Leone A, Beckmann E, Aandreas M, et al. Total aortic arch replacement with frozen elephant trunk technique: results from two European institutes. J Thorac Cardiovasc Surg 2020; 159: 1201–1211. [DOI] [PubMed] [Google Scholar]
- 19.Uchida N. Open stent grafting for complex diseases of the thoracic aorta: clinical utility. Gen Thorac Cardiovasc Surg 2013; 61: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokunaga C, Kumagai Y, Chubachi F, et al. Total arch replacement using frozen elephant trunk technique with Frozenix for distal aortic arch aneurysms. Interact Cardiovasc Thorac Surg 2022; 35: ivac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koizumi S, Ishigami M, Tsubota Het al. et al. Short- and mid-term outcomes of the frozen elephant-trunk procedure for degenerative aortic arch aneurysm. Surg Today 2022; 52: 324–329. [DOI] [PubMed] [Google Scholar]
- 22.Ogino H, Okita Y, Uchida N, et al. J-open cardiac aortic arch disease replacement surgical therapy study investigators. Comparative study of Japanese frozen elephant trunk device for open aortic arch repairs. J Thorac Cardiovasc Surg 2022; 164: 1681–1692.e2. [DOI] [PubMed] [Google Scholar]