Abstract
Objective
Atrial cardiomyopathy is closely associated with atrial fibrillation (AF), and some patients exhibit no dysfunction at rest but demonstrate evident changes in left atrial (LA) function and LA volume during exercise. This study aimed to identify distinguishing signs during exercise stress echocardiography (ESE) among patients in sinus rhythm (SR), with and without history of paroxysmal/persistent AF (PAF).
Methods
A prospective cohort of 1055 patients in SR was enrolled across 12 centers. The main study cohort was divided into two groups: the modeling group (n = 513) and the verification group (n = 542). All patients underwent ESE, which included B-lines, LA volume index (LAVi), and LA strain of the reservoir phase (LASr).
Results
Age, resting and stress LAVi and LASr, and B-lines were identified as a combination of detectors for PAF in both groups. In the entire cohort, aside from resting and stress LAVi and LASr, additional parameters differentiating PAF and non-PAF patients were the presence of systemic hypertension, exercise E/e’ > 7, worse right ventricle (RV) contraction during exercise (∆ tricuspid annular plane systolic excursion < 5 mm), a lower left ventricular contractile reserve (< 1.6), and a reduced chronotropic reserve (heart rate reserve < 1.64). The composite score, summing all 9 items, yielded a score of > 4 as the best sensitivity (79%) and specificity (65%).
Conclusion
ESE can complement rest echocardiography in the identification of previous PAF in patients with SR through the evaluation of LA functional reservoir and volume reserve, LV chronotropic, diastolic, and systolic reserve, and RV contractile reserve.
Graphical Abstract
A scoring system predicting the probability of PAF. The score was computed using the cutoff values as in the illustration. The score >4 demonstrated a sensitivity of 79% and a specificity of 65% of PAF.
Keywords: Atrial fibrillation, B-lines, Left atrium, Reservoir strain, Exercise stress echo
Background
Atrial fibrillation (AF) is the most prevalent atrial tachyarrhythmia, affecting an estimated 2% to 4% of adults worldwide, excluding those with asymptomatic forms of the disease [1]. AF is a well-recognized and treatable risk factor for stroke, but it often remains asymptomatic or subclinical, leading to underdiagnosis. It is noteworthy that approximately 25% of cryptogenic strokes are attributed to asymptomatic AF, highlighting the substantial thromboembolic risk associated with this condition [2].
The pathogenesis of AF is believed to be closely linked to structural and functional changes in the atria, which fall under the newly proposed concept of "atrial cardiomyopathy" [3, 4]. Excessive tension in the wall of the left atrium (LA) is thought to be responsible for mechanical and structural remodeling, resulting in the replacement of muscle fibers with connective tissue. This increased tension and reduced muscle fibers ultimately lead to impaired LA functional reserve.
Two-dimensional (2D) strain imaging of the LA is an innovative echocardiographic parameter that holds the potential for early AF detection. In particular, the LA strain of the reservoir phase (LASr) is informative and highly reproducible, therefore suitable for a multicenter study [5, 6]. Our hypothesis is that patients with paroxysmal/persistent AF may not exhibit LA dysfunction at rest, yet they may display evidence of LA functional changes and LA volume alterations during exercise. Patients with permanent AF tend to have more pronounced LA impairment, but given their arrhythmia, they typically receive appropriate treatment for stroke prevention without additional diagnostic methods. These stress atrial markers can be useful in the early identification of atrial cardiomyopathy and incipient rhythm disturbances, showing persistent functional abnormalities in patients with previous AF history despite current SR.
The aim of this study was to identify distinguishing signs during exercise stress echo (ESE) between patients in sinus rhythm (SR) with and without history of paroxysmal/persistent AF (PAF).
Methods
A prospective cohort of 1146 consecutive patients was initially considered from 12 cardiology institutions in 11 countries. These patients were referred for clinically-driven ESE as part of the Stress Echo 2020-2030 study network [7, 8]. Recruitment occurred between November 2020 and February 2024. The patients were referred for ESE due to conditions such as heart failure and/or chronic coronary syndromes (CCS).
Inclusion criteria encompassed patients aged over 18 years who underwent an analysis of LA function during ESE, including the assessment of LA volume index (LAVI) and LASr. Exclusion criteria comprised the presence of AF during the test, including permanent AF, as well as severe valvular or congenital heart disease or LA views of inadequate image quality.
The main study cohort was subdivided into two groups: the modeling group and the verification group. All consecutive patients in whom LASr assessment was feasible at rest from November 2020 until July 2022 were included in the modeling group. Subsequently, all consecutive patients with feasible LASr assessment from August 2022 until February 2024 were included in the verification group. Data from patients in the modeling group were utilized to develop a formula for detecting PAF. The verification group was established to assess the formula's significance for independent "all-comer" patients. The study protocol was reviewed and approved by the institutional ethics committees, as a part of the more comprehensive stress echo 2020 study (Clinical trials.Gov Identifier NCT 030.49995) and stress echo 2030 study (Clinical trials.Gov Identifier NCT 050.81115) [9].
Echocardiography
Transthoracic echocardiography (TTE) was performed using commercially available ultrasound machines equipped with multifrequency phased-array sector scan probes and second harmonic technology. All patients underwent comprehensive TTE at rest. All measurements were taken by certified cardiologists according to the current recommendations [10].
Stress echocardiography
All patients underwent comprehensive ESE with the ABCDE + protocol [11]. There were two types of exercise tests: semi-supine bike and post-treadmill. Loops for wall motion, contractile reserve, and LASr analysis were obtained immediately at peak stress and post-stress, as soon as possible, up to 1.5 min. The B-lines, diastolic function parameters were obtained till 3 min after exercise. Regional wall motion abnormalities (RWMA), B-lines, and when possible, coronary flow velocity reserve in the mid-distal left anterior descending artery were assessed. Wall motion score index (WMSI) was calculated in each patient at baseline and peak stress, in a four-point score ranging from 1 (normal) to 4 (dyskinetic) in a 17-segment model of the LV [12]. B-lines were evaluated with a simplified 4-site scan in the third intercostal space, between mid-axillary to anterior axillary and anterior axillary to mid-clavicular lines, each space scored from 0 (normal horizontal A-lines) to 10 (white lung), with a cumulative score per patient from 0 (normal) to 40 (severely abnormal). The stress-rest change in B-lines was ∆ B-lines, with higher values indicating more pulmonary congestion during stress. Left ventricle (LV) contractile reserve was assessed as the stress/rest ratio of force, calculated as systolic blood pressure/end-systolic volume. Coronary flow velocity reserve was assessed during the standard SE examination using intermittent imaging of wall motion and left anterior descending artery. HRR was calculated as the peak/rest heart rate from a 12-lead electrocardiogram (ECG). The procedure of acquisition was standardized between centers through a web-based learning module before starting data collection. All readers (one for each center) underwent quality control as previously described [13, 14].
Additionally, we conducted assessments of several cardiac parameters at rest and peak or immediately post-ESE. These included LV ejection fraction (EF), LV end-diastolic volume, E/e’, pulmonary artery systolic pressure estimation, and the so-called step L for LA: LAVi and LASr. Continuous ECG monitoring was used throughout the test, and blood pressure measurements were taken at each stage. The criteria for interrupting the test included the following conditions: severe chest pain, diagnostic ST-segment abnormalities, excessive blood pressure increase (systolic blood pressure ≥ 240 mmHg, diastolic blood pressure ≥ 120 mmHg), achievement of > 85% of target HR, muscular exhaustion, and significant arrhythmias [15].
LA assessment
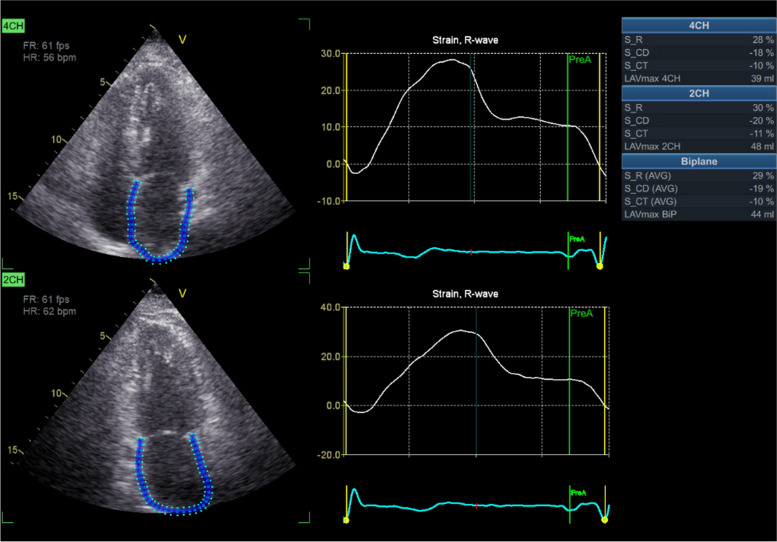
LAVI was measured from apical 4- and 2-chamber views using the modified method of disks and indexed for the body surface area. LASr was measured by speckle-tracking echocardiography using frame rates from 40 to 80/s. The speckle tracking technique is a postprocessing algorithm that quantifies LA deformation by tracking the motion of speckles within the whole myocardium through the cardiac cycle. The LASr was calculated from either an apical 4-chamber view (average from 6 LA segments) or combined 4- and 2-chamber views (average value from 12 LA segments) according to recommendations [16]. In each patient, the same approach (single apical or biplane views) was used at rest and peak ESE. LASr was calculated at LV-end diastole, with the QRS beginning as the zero-reference time point used as a surrogate of end-diastole. The first positive peak corresponds to the LA reservoir phase, and values are expressed as percentage points, algebraically positive (Fig. 1). Both LAVi and LASr were measured using offline echocardiography software from the rest and peak/near peak loops. All measurements were conducted by the sonographers without prior knowledge of the objective of this study.
Fig. 1.
Example of LAVI and LASr measurement
Statistical analysis. In the presentation of data, we used the following formats as appropriate: mean ± standard deviation (SD) for normally distributed continuous variables; number (percentage) for categorical variables; median (interquartile range) for skewed continuous variables. To assess the distribution of the data, we conducted a Kolmogorov-Smirnov test. Group differences were analyzed depending on the type of data: Student's t-test for normally distributed continuous variables, ANOVA for normally distributed continuous variables with multiple groups, Kruskal-Wallis test for skewed continuous variables with multiple groups; Chi-square or Fisher exact tests for categorical variables. In the modeling group, logistic regression was employed to create a detecting formula. The "enter" method was used, with variables included if they had a significance level (p-value) of < 0.1. Receiver operating characteristic (ROC) analysis was conducted to determine the cut-off values of prognostic discriminators in the modeling group and to assess their accuracy in predicting outcomes in the verification group. A probability value < 0.05 was considered statistically significant in all analyses. The Statistica package version 10.0 (Stat Soft Inc., Tulsa, Oklahoma, USA) and MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium) were used for statistical analysis.
Results
Rest LAVI and LASr parameters were obtained in all patients, by selection. The study population consisted of 935 patients without a history of PAF, 120 with paroxysmal/persistent AF, and 91 with permanent AF. A total of 91 patients were excluded from the study due to the presence of AF during the test, including those with permanent AF. The bike tests were performed on 678 patients (64%), the treadmill tests were conducted in 377 patients (36%). Extrasystolic beats were recorded in 423 patients (40%), of whom 73 (7%) had bigeminy, and 47 patients (4.5%) experienced supraventricular tachycardia during exercise.
There were 44 patients (4%) with moderate mitral regurgitation, 368 patients (38%) with mild to trivial mitral regurgitation, and the majority of the remaining patients had no mitral regurgitation.
The modeling group comprised 513 patients, while the verification group consisted of 542 patients. The baseline clinical characteristics, echocardiography, and ESE data are summarized in Table 1.
Table 1.
Clinical, echocardiographic and stress echo findings in the study population
| Modeling group N = 513 |
Verification group N = 542 |
p | |
|---|---|---|---|
| Age | 64.3 ± 11.1 | 62.3 ± 12.6 | 0.007 |
| Male/female | 315/198 | 291/251 | 0.014 |
| Prior PAF | 50 (9.7%) | 70 (12.9%) | 0.128 |
| Body mass index, kg/m2 | 28.4 (25.0-30.7) | 27.3 (24.6-30.1) | 0.003 |
| Body surface area, m2 | 1.94 ± 0.22 | 1.88 ± 0.21 | 0.001 |
| Hypertension | 75% | 75% | 0.939 |
| Diabetes | 19% | 16% | 0.252 |
| Smoking | 10% | 10% | 0.973 |
| Obesity | 33% | 26% | 0.014 |
| Prior MI | 21% | 13% | 0.0008 |
| Prior PCI | 26% | 18% | 0.006 |
| Dyspnea NYHA Class | 2 (1-2) | 1 (1-2) | 0.0006 |
| HR rest, beats/min | 70 (62-78) | 70 (62-78) | 0.906 |
| SBP rest, mmHg | 130 (118-145) | 128 (120-138) | 0.009 |
| DBP rest, mmHg | 80 (70-90) | 80 (70-86) | 0.026 |
| IMM, g/m2 | 90.1 (76.4-107.6) | 89.6 (74.2-109.8) | 0.599 |
| RWT | 0.42 (0.37-0.48) | 0.43 (0.37-0.49) | 0.098 |
| E rest, cm/s | 70 (58-84) | 75 (63-89) | 0.0001 |
| e’ rest, cm/s | 8.9 (7.4-10.5) | 9.5 (7.6-11.5) | 0.002 |
| E/e’ rest | 8.3 (6.5-10.4) | 8.3 (6.5-10.2) | 0.670 |
| LVEDV rest, ml | 94 (75-123) | 85 (58-107) | 0.0001 |
| LVESV rest, ml | 34 (26-50) | 30 (24-40) | 0.0001 |
| LVEF at rest, % | 61.6 (56.4-66.7) | 63.7 (58.1-67.9) | 0.002 |
| WMSI rest | 1.00 (1.00-1.06) | 1.00 (1.00-1.00) | 0.266 |
| GLS LV rest, |%| | 17.2 (14.0-20.0) | 17.0 (14.5-19.2) | 0.410 |
| TAPSE rest, mm | 23 (21-27) | 22 (20-25) | 0.0001 |
| LASr rest, |%| | 27.5 ± 9.2 | 27.6 ± 9.3 | 0.894 |
| LAVi_rest, ml/m2 | 28 (22-35) | 28 (22-36) | 0.890 |
| LAD flow rest (n = 469), cm/s | 25 (21-31) | 25 (21-31) | 0.791 |
| B-lines at rest, number | 0 (0-1) | 0 (0-2) | 0.025 |
| HR stress, beats/min | 130 (116-142) | 130 (115-143) | 0.610 |
| SBP stress, mmHg | 173 (155-195) | 176 (158-194) | 0.418 |
| DBP stress, mmHg | 85 (72-96) | 90 (80-99) | 0.0001 |
| LVEDV stress, ml | 85 (65-109) | 80 (65-100) | 0.026 |
| LVESV stress, ml | 26 (18-39) | 25 (19-34) | 0.247 |
| E stress, cm/s | 100 (82 -118) | 105 (90 -120) | 0.0001 |
| e’ stress, cm/s | 11.5 (9.5-13.2) | 11.5 (9.5-14.0) | 0.060 |
| E/e’stress | 8.7 (6.9-10.7) | 8.8 (7.3-11.0) | 0.164 |
| TAPSE stress, mm | 28 (24-31) | 25 (22-29) | 0.0001 |
| LASr stress, % | 29.1 ± 10.7 | 29.9 ± 10.4 | 0.251 |
| LAVi stress, ml/m2 | 28.3 (21.1-38.0) | 27.7 (22.4-36.0) | 0.990 |
| GLS LV stress, |%| | 17.0 (14.0-20.0) | 17.4 (12.6-20.6) | 0.936 |
| LVEF stress, % | 68.0 (60.2-74.5) | 68.4 (61.1-73.8) | 0.964 |
| WMSI stress, unit | 1.0 (1.00-1.25) | 1.0 (1.00-1.19) | 0.132 |
| LAD flow stress (n = 428), cm/s | 50.0 (39.8-62.3) | 54.0 (42.0-63.0) | 0.287 |
| B-lines stress, number | 1 (0-3) | 2 (0-4) | 0.027 |
| Ischemia | 36% | 41% | 0.123 |
| dWMSI | 0 (0-0.17) | 0 (0-0.18) | 0.578 |
| LV contractile reserve | 1.78 (1.38-2.23) | 1.69 (1.33-2.12) | 0.063 |
| CFVR | 2.02 (1.60-2.38) | 2.08 (1.71-2.36) | 0.181 |
| HRR | 1.82 (1.59-2.11) | 1.81 (1.57-2.07) | 0.502 |
| ∆ B-lines | 0 (0-2) | 0 (0-2) | 0.285 |
Abbreviations: CFVR coronary flow velocity reserve, DBP diastolic blood pressure, GLS global longitudinal strain, HR heart rate, HRR heart rate reserve, IMM index of myocardial mass is this LVMI, left ventricular mass index, LAD left anterior descending artery, LAS left atrial strain, LAVi left atrial volume index, LV left ventricle, LVEF left ventricle ejection fraction, LVEDV left ventricle end-diastolic volume, LVESV left ventricle end systolic volume, LVMI left ventricular mass index, MI myocardial infarction, NS non-significant, PCI percutaneous coronary intervention, RWT relative wall thickness, SBP systolic blood pressure, TAPSE tricuspid annular plane systolic excursion, WMSI wall motion score index
Within the modeling group, patients were further divided into subgroups based on the presence or absence of PAF. The key differences between these subgroups are presented in Table 2. Patients with PAF showed more prevalent systemic hypertension compared to patients in SR without a history of AF. In patients with PAF, TTE showed a larger LAVI (at rest and during exercise), reduced LASr (limited/absence of functional reserve during exercise), higher E wave velocity, and lower HRR, indicative of a reduced cardiac sympathetic reserve and cardiac autonomic imbalance.
Table 2.
Differences in clinic, echocardiography, and ESE parameters in patients with and without PAF among the modeling group
| Patients without PAF N = 463 |
Patients with PAF N = 50 |
p | |
|---|---|---|---|
| Age, years | 64.0 ± 11.4 | 66.0 ± 7.5 | 0.008 |
| Body mass index, kg/m2 | 27.9 (25.2-31.0) | 28.3 (25.4-32.4) | 0.445 |
| Body surface area, m2 | 1.93 ± 0.21 | 2.0 ± 0.25 | 0.04 |
| Hypertension | 73% | 88% | 0.033 |
| Diabetes | 19% | 13% | 0.331 |
| Smoking | 10% | 12% | 0.571 |
| Obesity | 33% | 36% | 0.769 |
| Prior MI | 22% | 18% | 0.667 |
| Prior PCI | 26% | 20% | 0.400 |
| Dyspnea NYHA Class | 2 (1-2) | 2 (1-2) | 0.601 |
| HR rest, beats/min | 70 (62-78) | 71 (63-77) | 0.874 |
| SBP rest, mmHg | 130 (118-145) | 129 (120-140) | 0.719 |
| DBP rest, mmHg | 80 (70-90) | 80 (68-87) | 0.726 |
| IMM, g/m2 | 83.3 (75.9-107.2) | 95.2 (84.9-109.9) | 0.114 |
| RWT | 0.42 (0.37-0.48) | 0.41 (0.37-0.46) | 0.591 |
| E rest, cm/s | 70 (58-84) | 70 (58-99) | 0.448 |
| e’ rest, cm/s | 9.0 (7.4-10.5) | 8.7 (7.5-9.9) | 0.645 |
| E/e’ rest | 8.3 (6.6-10.3) | 7.7 (6.4-13.7) | 0.499 |
| GLS LV rest, |%| | 17.0 ± 3.9 | 17.0 ± 3.6 | 0.948 |
| TAPSE rest, mm | 23 (21-27) | 23 (19-27) | 0.513 |
| LASr rest, |%| | 27.9 ± 8.9 | 23.5 ± 10.8 | 0.001 |
| LAVi_rest, ml/m2 | 27.2 (21.3-34.7) | 35.0 (31-44.7) | 0.0001 |
| LAD flow rest, cm/s | 25 (21-31) | 28 (22-31) | 0.483 |
| LVEDV rest, ml | 93 (75-122) | 98 (80-135) | 0.187 |
| LVESV rest, ml | 34 (26-49) | 36 (27-58) | 0.342 |
| LVEF at rest, % | 61.6 (56.4-66.5) | 62.1 (57.4-67.6) | 0.723 |
| WMSI rest | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 0.277 |
| B-lines at rest, number 4-region scan | 0 (0-1) | 1 (0-1) | 0.210 |
| HR stress, beats/min | 130 (117-144) | 125 (108-133) | 0.003 |
| SBP stress, mmHg | 174 (155-195) | 169(150 -190) | 0.227 |
| DBP stress, mmHg | 84 (72-96) | 87 (68-96) | 0.842 |
| LVEDV stress, ml | 85 (65-108) | 92 (65-124) | 0.112 |
| LVESV stress, ml | 26 (18-39) | 27 (18-44) | 0.517 |
| E stress, cm/s | 100 (90-120) | 112 (90-120) | 0.02 |
| e’ stress, cm/s | 11.5 (9.5-13.1) | 11.4 (9.2-13.5) | 0.775 |
| E/e’stress | 8.6 (6.8-10.7) | 9.8 (8.1-11.1) | 0.125 |
| TAPSE stress, mm | 28 (25-31) | 26 (23-31) | 0.261 |
| LASr stress, |%| | 29.6 ± 10.6 | 23.3 ± 10.0 | 0.002 |
| LAVi stress, ml/m2 | 27.6 (20.5-36.1) | 35.1 (30.0-46.4) | 0.0001 |
| GLS LV stress, |%|, (n = 222) | 16.8 ± 4.6 | 18.6 ± 4.2 | 0.078 |
| LVEF stress, % | 67.7 (60.3-74.2) | 69.8 (59.9-74.9) | 0.835 |
| WMSI stress, unit | 1.00 (1.00-1.25) | 1.00 (1.00-1.19) | 0.326 |
| LAD flow stress, cm/s | 51 (40-63) | 48 (35-54) | 0.224 |
| B-lines stress (number) 4-region scan | 1 (0-3) | 1 (0-3) | 0.401 |
| Ischemia | 37% | 27% | 0.235 |
| LV contractile reserve | 1.79 (1.38-2.23) | 1.79 (1.38-2.19) | 0.665 |
| CFVR (n = 153) | 2.04 (1.60-2.42) | 1.64 (1.42-1.94) | 0.101 |
| HRR | 1.83 (1.61-2.12) | 1.67 (1.50-1.93) | 0.023 |
| ∆ B-lines | 0 (0-2) | 0 (0-1) | 0.054 |
Abbreviations: CFVR coronary flow velocity reserve, DBP diastolic blood pressure, GLS global longitudinal strain, HR heart rate, HRR heart rate reserve, IMM index of myocardial mass is this LVMI, left ventricular mass index, LAD left anterior descending artery, LAS left atrial strain, LAVi left atrial volume index, LV left ventricle, LVEF left ventricle ejection fraction, LVEDV left ventricle end-diastolic volume, LVESV left ventricle end systolic volume, LVMI left ventricular mass index, MI myocardial infarction, NS non-significant, PCI percutaneous coronary intervention, RWT relative wall thickness, SBP systolic blood pressure, TAPSE tricuspid annular plane systolic excursion, WMSI wall motion score index
The differences in LA sizes and function during exercise for the entire study cohort are presented in Table 3.
Table 3.
Differences in left atrium parameters in patients with and without PAF history
| Variables | SR, no PAF history N = 935 |
SR, PAF history N = 120 |
P- values |
|---|---|---|---|
| Rest LAVI | 27.1 (21.7-34.7) | 34.3 (26.0-43.0) | < 0.0001 |
| Rest LASr | 28.0 ± 9.0 | 23.9 ± 10.5 | < 0.001 |
| Stress LAVI | 27.0 (21.0-35.9) | 33.9 (28.0-43.9) | < 0.0001 |
| Stress LASr | 30.3 ± 10.3 | 24.0 ± 10.2 | < 0.001 |
Abbreviations: LAVi left atrial volume index, LASr left atrial reservoir strain
LAVi during exercise did not significantly change in either group (p = 0.43 for one group and p = 0.85 for the other). LASr during exercise significantly increased in patients without a history of PAF (p < 0.000001), whereas it did not change significantly in patients with a history of PAF (p = 0.06).
There was no significant correlation between LASr at rest and B-lines at rest (r = -0.06, p = 0.06). However, there were mild but significant correlations between LASr at rest and exercise B-lines (r = -0.15, p < 0.0001), and between exercise LASr and exercise B-lines (r = -0.18, p < 0.0001).
Logistic Regression analysis
Logistic regression analysis resulted in the creation of a predictive model for PAF that exhibited a high level of statistical significance (p-value < 0.0001). The coefficients and constant for this formula are provided in Table 4.
Table 4.
Coefficients and Standard Errors in logistic regression analysis
| Variable | Coefficient | Std. Error |
|---|---|---|
| Age | 0.017290 | 0.02278 |
| Rest LAVi | 0.0066306 | 0.02054 |
| Rest LASr | -0.072410 | 0.03444 |
| Stress LAVi | 0.047206 | 0.02543 |
| Stress LASr | -0.0043906 | 0.02631 |
| ∆ B-lines during exercise | -0.16605 | 0.09263 |
| Constant | -3.3053 |
The formula generated from logistic regression analysis achieved a classification accuracy of 91.6%, correctly identifying cases in the modeling group. The discriminator was > 0.0893. The ROC analysis showed an area under the curve (AUC) of 0.78, with a highly significant p-value of < 0.0001, as illustrated in Fig. 2.
Fig. 2.
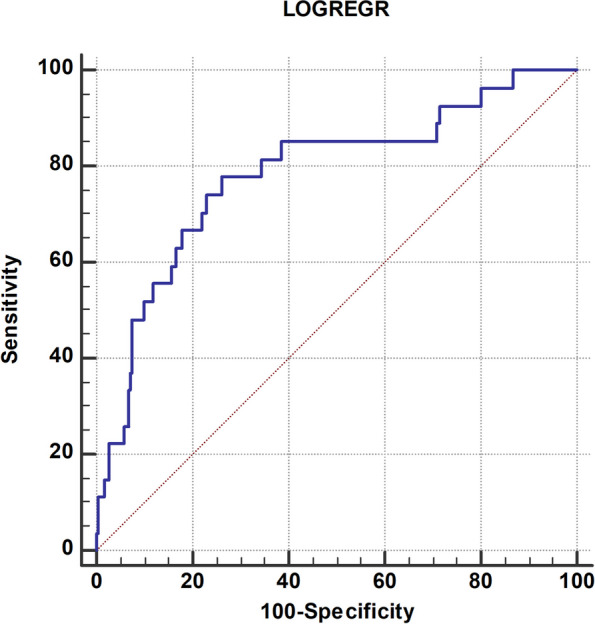
Logistic regression analysis formula predictive value by ROC-analysis
In the verification group, this predictive formula also demonstrated significant discriminative capabilities for PAF, with a sensitivity of 49% and specificity of 79%. The AUC in the verification group was 0.67, indicating a meaningful discriminatory power (p-value < 0.0001).
ROC-analysis for PAF prediction
For easier clinical application, a simplified scoring system was created to predict the probability of PAF. This scoring system is constructed using the variables that better differentiate the two groups (those with and without PAF) in the overall patient population, combining the modeling and verification groups (as shown in Table 5). The score was computed using the cutoff values. This system enables clinicians to evaluate the likelihood of PAF in patients based on these defined criteria.
Table 5.
Cut-off values of the parameters for calculating the score
| Variables | ROC-analysis cut-off value | SCORE |
|---|---|---|
| History of hypertension | yes | 1 |
| Rest LAVI | > 31 | 1 |
| Rest LASr | < 23 | 1 |
| Stress LAVI | > 29 | 1 |
| Stress LASr | < 22 | 1 |
| E/e’ stress (n = 609) | > 7 | 1 |
| ∆TAPSE (n = 820) | < 5 | 1 |
| HRR | < 1.64 | 1 |
| LVCR | < 1.6 | 1 |
| SCORE | > 4 |
Abbreviations: LAVi left atrial volume index, LASr left atrial reservoir strain, ∆-TAPSE difference between stress and rest tricuspid annular plane systolic excursion, HRR heart rate reserve, LVCR left ventricle contractile reserve
The ROC analysis of the scoring model using the entire cohort showed an AUC of 0.77, indicating a high ability to distinguish between patients with and without PAF (p-value < 0.0001). This scoring system demonstrated a sensitivity of 79% and a specificity of 65%. A higher score indicates a greater likelihood of PAF, as evidenced by a Chi-squared test for trend (p < 0.0001). Notably, the use of this score proved significantly more accurate compared to relying solely on the known increase in LAVi at rest in the main cohort (p < 0.003), as depicted in Fig. 3. Among the parameters, E/e’ and stress-rest variation of tricuspid annular plane systolic excursion (∆TAPSE) were less frequently obtained during SE, resulting in a patient group of 474. The model utilizing 7 parameters, excluding E/e’ and ∆TAPSE, also yielded high accuracy in detecting PAF. If the score exceeded 3, the AUC of the ROC curve was 0.73 (p-value < 0.0001), with a sensitivity of 66% and a specificity of 69%.
Fig. 3.
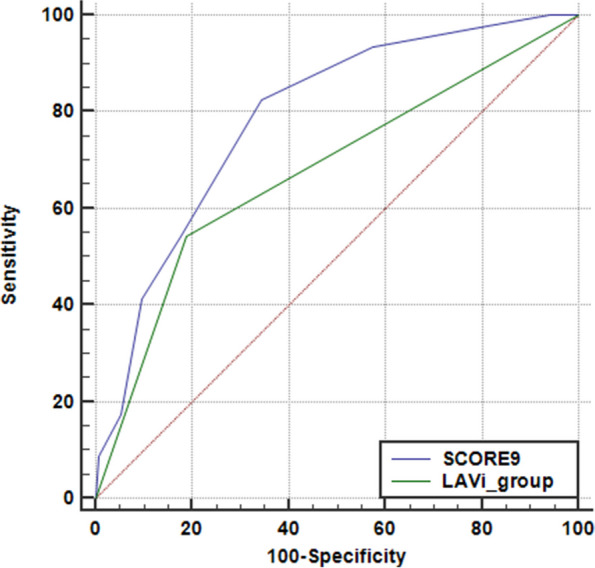
Comparison of predictive values of LAVi at rest (in blue) and a composite score (in green) inclusive of age, rest LAVI, and stress LASr
Two hundred thirty-eight patients comprised two matched groups. The average age was 67.9 ± 9.6 years in one group and 67.9 ± 9.7 years in the other (p = 0.996). There were no significant differences in the distribution of sex, diabetes mellitus, hypertension, or ischemia between the groups. Only LAVi and LASR at rest and during stress showed statistically significant differences among all the echocardiography and stress echocardiography parameters (Table 6). Additionally, Scores 9 and 7 differed significantly between the groups (p < 0.000005 for both comparisons).
Table 6.
Differences in left atrium parameters in patients with and without PAF history in the matched groups
| Variables | SR, no PAF history N = 119 |
SR, PAF history N = 119 |
P- values |
|---|---|---|---|
| Rest LAVI | 27.9 (22.2-36.0) | 34.3 (26.2-43.3) | < 0.001 |
| Rest LASr | 27.9 ± 9.8 | 23.8 ± 10.5 | < 0.01 |
| Stress LAVI | 30.0 (23.8-39.7) | 34.0 (28.0-43.9) | < 0.006 |
| Stress LASr | 29.0 ± 9.8 | 23.8 ± 10.2 | < 0.001 |
Abbreviations: LAVi left atrial volume index, LASr left atrial reservoir strain
Discussion
We evaluated a cohort consisting of 1055 patients in SR during ESE aiming to find distinguishing signs of those having PAF. This evaluation covered the assessment of LA morphology (using LAVI) and function (using LASr), as well as B-lines and HRR. The results suggest that this comprehensive method is practical and informative.
Not surprisingly, a history of PAF was associated with advanced age and a high prevalence of systemic hypertension. Resting TTE revealed higher LAVI and lower LASr values in patients with a history of PAF. Additionally, ESE demonstrated a diminished chronotropic, contractile response with more pronounced diastolic dysfunction of the LV, reduced right ventricular contractile reserve, and a lower atrial functional reserve in these patients compared to individuals without a history of PAF.
In the modeling group, a logistic regression formula was developed and subsequently validated in the independent verification group. Logistic regression, though useful, cannot be directly applied during tests due to its complex formula. Instead, Score9/Score7 offer a simpler, more immediate alternative during multiparameter stress echocardiography. The simplified scores was settled for the whole cohort. The primary hypothesis driving the study was that changes in the size and function of the remodeled LA would manifest at rest but sometimes only during ESE. The study confirmed this hypothesis by revealing significant differences in LAVi and LASr at rest and during ESE between groups with and without PAF, even when other clinical and echocardiographic characteristics were similar. Score 9 demonstrated high sensitivity with moderate specificity. We believe that emphasizing sensitivity is crucial for identifying patients with PAF who may require further examination, such as multi-day EKG monitoring.
The findings demonstrated that LAVi and LASr during ESE have additive value in identifying atrial dysfunction in PAF. Moreover, the inclusion of rest and stress parameters collectively provides a more informative approach to detecting atrial myopathy. Indeed, the simple score, which incorporates known parameters of LA size at rest along with age and contractile parameters of the LA during exercise, exhibited greater predictive power compared to relying solely on resting LAVI.
The main clinical implication is to offer a potential means to identify patients with PAF within the SR population, including those with asymptomatic/subclinical undiagnosed forms. Such identification could be instrumental in devising preventive strategies for strokes, especially considering that nearly 65% of patients with cryptogenic stroke are found to have atrial myopathy, with 25% of these cases attributed to asymptomatic AF, which carries a substantial risk of thromboembolism [17, 18]. Potentially, the study findings could provide valuable information on changes in atrial function, which may be useful for further examination and close follow-up of patients who do not experience noticeable arrhythmias.
Numerous previous studies have consistently demonstrated a connection between LA enlargement, decreased LASr at rest, and the presence of AF or stroke [3, 4, 18–20]. In patients with SR, these studies have also revealed an association between progressive LA remodeling, as assessed through serial TTE, and the progression of AF over extended follow-up periods. Patients who eventually developed persistent AF were found to have higher LAVi, and lower LASr compared to those in the PAF group [19].
Recent pilot and multicenter studies have further expanded our understanding by demonstrating that patients with AF exhibit LA dilation along with reduced LASr at rest and during stress. Importantly, LASr fails to increase during ESE, and patients with PAF display higher values of E/e’ during stress (Table 4), resembling those seen in individuals with heart failure, CCS, and symptomatic AF [21–23]. These studies have indicated that LA dysfunction progresses from SR to PAF and eventually to permanent AF, and it is associated with more significant LV systolic dysfunction, increased LV filling pressure, and pulmonary congestion. In contrast, the normal healthy cohort showed a significant increase in deformation without changes in atrial stiffness during maximum ESE [24].
While previous studies on the function of the LA during exercise, using LASr, have been conducted in relatively small groups of 177 and 252 patients [5, 22], the current study was designed to comprehensively assess signs of LA dysfunction in a large cohort of patients, excluding those with AF during the test. The current study was planned to compose signs of LA dysfunction in a large group, excluding patients with AF during the test, and further verification in the independent multicenter group in patients with heart failure and/or CCS.
In our study, we also noticed an increased E/e’ value during ESE in the PAF group, consistent with higher LV filling pressures during ESE, a well-known consequence and cause of LA dysfunction when LA dilation exceeds the range of LA Starling curve. This phenomenon is explained by atrial dysfunction, which can lead to exercise intolerance, as has been observed in previous studies [25, 26]. Additionally, the PAF group exhibited a reduced ability to achieve a higher heart rate during exercise, resulting in a smaller HRR, which is consistent with a reduced cardiac sympathetic reserve. Cardiac autonomic dysfunction is known to be a key player in the vulnerability to atrial arrhythmias, especially in the presence of LA dilation and reduced LA functional reserve.
Recognizing that some differences in echocardiographic parameters might be explained by age, the groups were matched by age. The main finding regarding differences in LA sizes and function at rest and during stress tests between patients with and without PAF remained consistent. However, due to the relatively small sample sizes, some echocardiographic and stress echocardiography parameters might not have reached statistical significance.
Further studies are needed to explore the value of stress and rest indicators of LA dysfunction in groups without a known history of PAF. This could prove invaluable for the active diagnosis of possible asymptomatic AF, which is an essential step in identifying and managing this condition, especially considering its association with stroke risk [27, 28]. In addition, ongoing outcome studies will clarify whether patients with high vulnerability scores for AF but in SR at the time of study will develop episodes of PAF in the follow-up.
Study limitations
We did not divide patients with persistent and paroxysmal AF. The multicenter nature of the study allowed a multi-vendor assessment of LA function, but LASr may show some inter-vendor variability, although the adopted cutoff values for abnormality have been validated across different vendors and the inter-vendor variability does not apply to stress-rest variation, evaluated in the same patient with the same vendor.
Among LA strain parameters, only LASr was measured to simplify the method. We did not separately analyze the conduit and contractile phases, as LASr is more reproducible, easier to measure, and provides a better representation of the overall function of the LA. We focused on LASr, but right atrial strain can be even more important for detecting AF.
We only considered ESE, but similar patterns of normal responses of LAVI (with a slight increase or decrease during stress) and LASr (with an increase during stress) were observed during dobutamine and vasodilator stress echo [5, 29].
The study is among the largest with ESE in AF. A larger data set analyzed with artificial-intelligence using ECG [30] may be needed to optimize the prognostic potential of a combined anatomic and functional approach based on ESE.
Conclusion
ESE evaluation can be a valuable adjunct to rest LAVI and rest LASr for identifying patients in SR with history of PAF. Six different measures applied during ESE in patients with SR may help to identify those with prior PAF: LA contractile reserve with LASr, LA volume reserve with LAVI, LV contractile reserve with force, chronotropic reserve with HRR, right ventricular contractile reserve with ∆-TAPSE, and LV diastolic reserve with E/e’. This multifaceted approach identifies new spectrum of abnormal physiological responses to exercise detectable during ESE which can support understanding of symptoms and clinical management of individuals in SR, e.g. proactive monitoring programs to identify recurrences of AF and subsequent increase of cardiovascular risk.
Acknowledgments
Appendix: Stress echo 2030 study group
Jorge Lowenstein1, Rosina Arbucci1, Diego M. Lowenstein Haber1, Sofia Marconi1, Pablo M Merlo1, Miguel Amor2, Hugo Mosto2, Michael Salamé2, Patricia Carral2, Germán Souto2, Ariel Saad3, Caroline M. Van De Heyning4, Miodrag Ostojic5, Bojan Stanetic5, Tamara Kovačević Preradović5, Clarissa Borguezan-Daros6, Ana Cristina Camarozano7, Iana Simova8, Yi Wang9, Zhang Hongmei9, Ding Geqi9, Zhang Qingfeng10, Yue Heng Wang10, Albert Varga11, Gergely Agoston11, Attila Palinkas12, Robert Sepp12, Eszter D. Palinkas12, Sergio Kobal13, Quirino Ciampi14, Bruno Villari14, Lauro Cortigiani15, Antonello D’Andrea16, Nicola Gaibazzi17, Domenico Tuttolomondo17, Elisa Merli18, Doralisa Morrone19, Fabio Mori20, Maria Grazia D’Alfonso20, Iacopo Olivotto20, Annamaria Del Franco20, Rodolfo Citro21, Rosangela Cocchia22, Eduardo Bossone22, Fausto Rigo23, Francesca Bursi24, Federica Re25, Paolo Colonna26, Ilaria Dentamaro26, Marco Fabio Costantino27, Fiorenzo Manganelli28, Jelena Celutkiene29, Hugo Rodriguez-Zanella30, Karina Wierzbowska-Drabik31, Jaroslaw D. Kasprzak32, Maciej Haberka33, Alla Boshchenko34, Olga Zhuravleva34, Natalia Sviazova34, Tamara Ryabova34, Ayten Safarova35, Tatiana Timofeeva35, Angela Zagatina36, Elena Kalinina36, Irina Begidova36, Aleksandra Nikolic37, Milica Dekleva38, Ana Djordievic-Dikic39, Nikola Boskovic39, Vojislav Giga39, Milorad Tesic39, Srdjan Dedic39, Eugenio Picano39, Jesus Peteiro Vazquez40, Nithima Chaowalit Ratanasit41, Patricia A Pellikka42, Adelaide M. Arruda-Olson42, Ratnasari Padang42, Garvan C. Kane42, Hector R. Villarraga42, Ylenia Bartolacelli43, Scipione Carerj44, Mauro Pepi45, Giovanni Benfari46, Andrea Barbieri47
1 Cardiodiagnosticos, Investigaciones Medicas, Buenos Aires, Argentina
2 Hospital Echocardiography Laboratory, Ramos Mejia Hospital, Buenos Aires, Argentina
3 División de Cardiología, Hospital de Clínicas José de San Martín, Buenos Aires, Argentina:
4 Department of Cardiology, Antwerp University Hospital, 2650 Edegem, Belgium:
5 Clinic of Cardiovascular Diseases, University of Banja Luka University Clinical Centre of the Republic of Srpska
6 Cardiology Division, Hospital San José, Criciuma, Brasil
7 Hospital de Clinicas UFPR, Medicine Department, Federal University of Paranà, Curitiba, Brasil
8 Heart and Brain Center of Excellence, University Hospital, Pleven, Bulgaria
9 Department of Cardiovascular Ultrasound and Non-invasive Cardiology, Sichuan Provincial People's Hospital, China
10 Key laboratory of ultrasound in cardiac electrophysiology and biomechanics, The Affiliated Sichuan Provincial People's Hospital of Electronic Science and Technology University of China, Chengdu, China
11 Institute of Family Medicine, University of Szeged, Hungary
12 Second Department of Internal medicine and Cardiology Center, University Hospital, Szeged, Hungary, and Elisabeth Hospital, Internal Medicine Department, Hódmezővásárhely, Hungary
13 Echocardiography Unit, Soroka University Medical Center, Israele
14 Cardiology Division, Fatebenefratelli Hospital, Benevento, Italy
15 Cardiology Department, San Luca Hospital, Lucca, Italy
16 PO Umberto I°, Nocera Inferiore, ASL Salerno, Salerno, Italy
17 Cardiology Department, Parma University Hospital, Italy
18 Department of Cardiology, Ospedale per gli Infermi, Faenza, Ravenna, Italy
19 Cardiothoracic Department, University of Pisa, Italy
20 SOD Diagnostica Cardiovascolare, DAI Cardio-Toraco-Vascolare, e Cardiomyopathy unit, Azienda Ospedaliera-Universitaria Careggi, Italy
21 Cardiology Department and Echocardiography Lab, University Hospital "San Giovanni di Dio e Ruggi d'Aragona," Salerno, Italy
22 Azienda Ospedaliera Rilevanza Nazionale A. Cardarelli Hospital, Naples, Italy:
23 Villa Salus Foundation, IRCCS San Camillo Hospital, Venice, Italy
24 ASST Santi Paolo e Carlo. Presidio Ospedale San Paolo, Milano, Italy
25 Ospedale San Camillo, Cardiology Division, Rome, Italy
26 Cardiology Hospital, Policlinico University Hospital of Bari, Italy:
27 Cardiology Division, San Carlo Hospital, Potenza, Italy
28 Ospedale Moscati Avellino, Cardiology Division, Province of Avellino, Italy
29 Centre of Cardiology and Angiology, Clinic of Cardiac and Vascular Diseases, Faculty of Medicine, Institute of Clinical Medicine, Vilnius University, State Research Institute Center for Innovative Medicine, LT-03101 Vilnius, Lithuania
30 Instituto Nacional de Cardiologia Ignacio Chavez, Mexico City, Mexico
31 Department of Internal Disease and Clinical Pharmacology, Lodz, Poland
32 Bieganski Hospital, Medical University, Lodz, Poland
33 University of Silesia, Cardiology Department, Katowice, Poland
34 Cardiology Research Institute, Tomsk National Research Medical Centre of the Russian Academy of Sciences, Tomsk, Russian Federation
35 Department of Internal Medicine with a course in Cardiology and functional Diagnostics at the Medical Institute of the Peoples' Friendship University of Russia, Moscow, Russian Federation
36 Cardiology Department, Research Cardiology Center “Medika”, Saint Petersburg, Russian Federation
37 Department of Noninvasive Cardiology, Institute for Cardiovascular Diseases Dedinje, School of Medicine, Belgrade, Serbia
38 Clinical Cardiology Department, Clinical Hospital Zvezdara, Medical School, University of Belgrade, Serbia
39 Cardiology Clinic, University Center Serbia, Medical School, University of Belgrade, Serbia
40 CHUAC- Complexo Hospitalario Universitario A Coruna- University of A Coruna, La Coruna, Spain
41 Division of Cardiology, Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
42 Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota, USA
43 Paediatric Cardiology and Adult Congenital Heart Disease Unit, S. Orsola-Malpighi Hospital, Bologna, Italy
44 Divisione di cardiologia, Policlinico Universitario, Università di Messina, Messina, Italy
45 Centro Cardiologico Monzino, IRCCS, Milan, Italy
46 University of Verona, Verona, Italy
47 Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, Policlinico di Modena, University of Modena and Reggio Emilia, Italy
Abbreviations
- AF
Atrial fibrillation
- CCS
Chronic coronary syndromes
- ECG
Electrocardiogram
- EF
Ejection fraction
- ESE
Exercise stress echocardiography
- HR
Hazard ratio
- HRR
Heart rate reserve
- LA
Left atrial
- LASr
Left atrial strain of the reservoir phase
- LAVi
Left atrial volume index
- LV
Left ventricle
- PAF
Paroxysmal-persistent atrial fibrillation
- RWMA
Regional wall motion abnormality
- SR
Sinus rhythm
- TAPSE
Tricuspid annular plane systolic excursion
- TTE
Transthoracic echocardiography
- 2DE
Two-dimensional echocardiography
- WMSI
Wall motion score index
Authors’ contributions
A.Z. originated idea, collected patients, prepared images and loops, and drafted the manuscript, Q.C. is the principal investigator of SE2030, collected patients, and drafted the manuscript, made the data quality control and contributed to data analysis, J.V.P. collected patients, E. K. collected patients, and drafted the manuscript, I. B. collected patients, prepared images and loops, and drafted the manuscript, R. P. collected patients, A. B. collected patients, E. M. collected patients, M. L. collected patients, H. R.-Z., S. K. collected patients, G.A. collected patients, A. V. collected patients, K. W.-D. collected patients, J. D. K. collected patients, R.A. collected patients, O. Z. collected patients, J. C. collected patients, J. L. collected patients, N. C. R. collected patients, P.A.P. served as the study co-chair, critically reviewed the protocol, helped to orient the data analysis, and critically revised the manuscript; E.P. served as the study chairman, designed the protocol, helped to orient the data analysis, and critically reviewed the manuscript; P. C., M.P. and S.C. are the Presidents and President-elect of the SIECVI, the scientific society that endorsed, organized, and funded the study. All authors contributed to the study design, undertook the quality control up to certification, critically revised the manuscript for an intellectually important contribution, and approved the submitted version.
Funding
Travel, publication, and infrastructural funding from Società Italiana di Ecocardiografia e Cardiovascular Imaging (SIECVI).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
The study was managed and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients before inclusion. The study protocol was reviewed and approved by the institutional ethics committees, as a part of the more comprehensive stress echo 2020 study (148-Comitato Etico Lazio-1, July 16, 2016; Clinical trials.Gov Identifier NCT 030.49995) and stress echo 2030 study (291/294/295 Comitato Etico Lazio-1, March 8, 2021; Clinical trials.Gov Identifier NCT 050.81115).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Angela Zagatina, Email: zag_angel@yahoo.com.
on behalf of the Stress Echo 2030 study group:
Angela Zagatina, Quirino Ciampi, Elena Kalinina, Irina Begidova, Ratnasari Padang, Alla Boshchenko, Elisa Merli, Hugo Rodriguez-Zanella, Sergio Kobal, Gergely Agoston, Albert Varga, Karina Wierzbowska-Drabik, Rosina Arbucci, Olga Zhuravleva, Jorge Lowenstein, Nithima Chaowalit Ratanasit, Paolo Colonna, Scipione Carerj, Mauro Pepi, Eugenio Picano, Diego M. Lowenstein Haber, Sofia Marconi, Pablo M. Merlo, Miguel Amor, Hugo Mosto, Michael Salamé, Patricia Carral, Germán Souto, Ariel Saad, Caroline M. Van De Heyning, Miodrag Ostojic, Bojan Stanetic, Tamara Kovačević Preradović, Clarissa Borguezan-Daros, Ana Cristina Camarozano, Iana Simova, Yi Wang, Zhang Hongmei, Ding Geqi, Zhang Qingfeng, Yue Heng Wang, Attila Palinkas, Robert Sepp, Eszter D. Palinkas, Bruno Villari, Lauro Cortigiani, Antonello D’Andrea, Nicola Gaibazzi, Domenico Tuttolomondo, Doralisa Morrone, Fabio Mori, Maria Grazia D’Alfonso, Iacopo Olivotto, Annamaria Del Franco, Rodolfo Citro, Rosangela Cocchia, Eduardo Bossone, Fausto Rigo, Francesca Bursi, Federica Re, Ilaria Dentamaro, Marco Fabio Costantino, Fiorenzo Manganelli, Jelena Celutkiene, Jaroslaw D. Kasprzak, Maciej Haberka, Natalia Sviazova, Tamara Ryabova, Ayten Safarova, Tatiana Timofeeva, Aleksandra Nikolic, Milica Dekleva, Ana Djordievic-Dikic, Nikola Boskovic, Vojislav Giga, Milorad Tesic, Srdjan Dedic, Jesus Peteiro Vazquez, Patricia A. Pellikka, Adelaide M. Arruda-Olson, Garvan C. Kane, Hector R. Villarraga, Ylenia Bartolacelli, Giovanni Benfari, and Andrea Barbieri
References
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, ESC Scientific Document Group, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, ASSERT Investigators, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 3.Olsen FJ, Møgelvang R, Jensen GB, Jensen JS, Biering-Sørensen T. Relationship between left atrial functional measures and incident atrial fibrillation in the general population: the Copenhagen City heart study. JACC Cardiovasc Imaging. 2019;12:981–9. [DOI] [PubMed] [Google Scholar]
- 4.Hauser R, Nielsen AB, Skaarup KG, Lassen MCH, Duus LS, Johansen ND, Sengeløv M, Marott JL, Jensen G, Schnohr P, et al. Left atrial strain predicts incident atrial fibrillation in the general population: the Copenhagen City Heart Study. Eur Heart J Cardiovasc Imaging. 2021;23:52–60. [DOI] [PubMed] [Google Scholar]
- 5.Morrone D, Arbucci R, Wierzbowska-Drabik K, Ciampi Q, Peteiro J, Agoston G, Varga A, Camarozano AC, Boshchenko A, Ryabova T, et al. Feasibility and functional correlates of left atrial volume changes during stress echocardiography in chronic coronary syndromes. Int J Cardiovasc Imaging. 2021;37:953–64. [DOI] [PubMed] [Google Scholar]
- 6.Rausch K, Shiino K, Putrino A, Lam AK, Scalia GM, Chan J. Reproducibility of global left atrial strain and strain rate between novice and expert using multi-vendor analysis software. Int J Cardiovasc Imaging. 2019;35(3):419–26. [DOI] [PubMed] [Google Scholar]
- 7.Picano E, Ciampi Q, Citro R, D’Andrea A, Scali MC, Cortigiani L, Olivotto I, Mori F, Galderisi M, Costantino MF, et al. Stress echo 2020: the international stress echo study in ischemic and non-ischemic heart disease. Cardiovasc Ultrasound. 2017;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picano E, Ciampi Q, Cortigiani L, Arruda-Olson AM, Borguezan-Daros C, de Castro E Silva Pretto JL, Cocchia R, Bossone E, Merli E, Kane GC, et al. Stress Echo 2030: the Novel ABCDE-(FGLPR) protocol to define the future of imaging. J Clin Med. 2021;10:3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picano E, Ciampi Q, Arbucci R, Cortigiani L, Zagatina A, Celutkiene J, Bartolacelli Y, Kane GC, Lowenstein J, Pellikka P. Stress Echo 2030: the new ABCDE protocol defining the future of cardiac imaging. Eur Heart J Suppl. 2023;25(Suppl. C):C63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. Erratum in: Eur Heart J Cardiovasc Imaging. 2016;17:412. Erratum in: Eur Heart J Cardiovasc Imaging. 2016;17:969. [DOI] [PubMed] [Google Scholar]
- 11.Picano E, Zagatina A, Wierzbowska-Drabik K, BorguezanDaros C, D’Andrea A, Ciampi Q. Sustainability and versatility of the ABCDE protocol for stress echocardiography. J Clin Med. 2020;9:3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:1–41. [DOI] [PubMed] [Google Scholar]
- 13.Ciampi Q, Picano E, Paterni M, Daros CB, Simova I, de Castro E Silva Pretto JL, Scali MC, Gaibazzi N, Severino S, Djordjevic-Dikic A, et al. Quality control of regional wall motion analysis in stress Echo 2020. Int J Cardiol. 2017;249:479–85. [DOI] [PubMed] [Google Scholar]
- 14.Scali MC, Ciampi Q, Picano E, Bossone E, Ferrara F, Citro R, Colonna P, Costantino MF, Cortigiani L, Andrea A, et al. Quality control of B-lines analysis in stress Echo 2020. Cardiovasc Ultrasound. 2018;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picano E, Pierard L, Peteiro J, Djordjevic-Dikic A, Sade LE, Cortigiani L, Van De Heyning CM, Celutkiene J, Gaibazzi N, Ciampi Q, et al. The Clinical use of stress echocardiography in chronic coronary syndromes and beyond coronary artery disease: a clinical consensus statement from the European association of cardiovascular imaging of the ESC. Eur Heart J Cardiovasc Imaging. 2024;25:e65–90. [DOI] [PubMed] [Google Scholar]
- 16.Badano LP, Kolias Tj, Muraru D, Abraham Tp, Aurigemma G, Edvardsen T, D’Hooge J, Donal E, Fraser AG, Marwick T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;9:591–600. [DOI] [PubMed] [Google Scholar]
- 17.Yaghi S, Boehme AK, Hazan R, Hod EA, Canaan A, Andrews HF, Kamel H, Marshall RS, Elkind MS. Atrial cardiopathy and cryptogenic stroke: a cross-sectional pilot study. J Stroke Cerebrovasc Dis. 2016;25:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaghi S, Kamel H, Elkind MSV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert Rev Cardiovasc Ther. 2017;15:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung M, van Rosendael PJ, van der Bijl P, Regeer MV, van Wijngaarden SE, Leung DY, Delgado V, Marsan NA, Ng ACT, Bax JJ. The value of serial echocardiography in risk assessment of patients with paroxysmal atrial fibrillation. Int J Cardiovasc Imaging. 2024;40:499–508. [DOI] [PubMed] [Google Scholar]
- 20.Maheshwari A, Norby FL, Inciardi RM, Wang W, Zhang MJ, Soliman EZ, Alonso A, Johansen MC, Gottesman RF, Solomon SD, et al. Left atrial mechanical dysfunction and the risk for ischemic stroke in people without prevalent atrial fibrillation or stroke: a prospective cohort study. Ann Intern Med. 2023;176:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariyaratnam JP, Mishima RS, McNamee O, Emami M, Thiyagarajah A, Fitzgerald JL, Gallagher C, Sanders P, Elliott AD. Exercise echocardiography to assess left atrial function in patients with symptomatic AF. Int J Cardiol Heart Vasc. 2023;21(50):101324. 10.1016/j.ijcha.2023.101324. PMID: 38204984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zagatina A, Rivadeneira Ruiz M, Ciampi Q, Wierzbowska-Drabik K, Kasprzak J, Kalinina E, Begidova I, Peteiro J, Arbucci R, Marconi S, Stress Echo 2030 Study Group, et al. Rest and stress left atrial dysfunction in patients with atrial fibrillation. J Clin Med. 2023;12:5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wierzbowska-Drabik K, Kasprzak JD, Haberka M, Peteiro J, Re F, D’Alfonso MG, Mori F, Palinkas ED, Agoston G, Varga A, et al. Left atrial volume changes during exercise stress echocardiography in heart failure and hypertrophic cardiomyopathy. Hellenic J Cardiol. 2022;67:9–18. [DOI] [PubMed] [Google Scholar]
- 24.Romero Z, Arbucci R, Sevilla D, Rousse MG, Lowenstein D, Rodriguez I, Ugalde GN, Lowenstein J. The Reservoir Function. Functional evaluation of the left atrium by two-dimensional strain during rest and exercise stress. Rev Argentina Cardiol. 2017;85:520–6. [Google Scholar]
- 25.Maffeis C, Rossi A, Cannata L, Zocco C, Belyavskiy E, Radhakrishnan AK, Feuerstein A, Morris DA, Pieske-Kraigher E, Pieske B, et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. Heart Fail. 2022;9:842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zegkos T, Kamperidis V, Ntelios D, Gossios T, Parcharidou D, Tziomalos G, Papanastasiou CA, Boutou AΚ, Katranas S, Rouskas P, et al. Left atrial myopathy is associated with exercise incapacity and ventilatory inefficiency in hypertrophic cardiomyopathy. Heart Lung Circ. 2023;32:215–23. [DOI] [PubMed] [Google Scholar]
- 27.Gautier A, Picard F, Ducrocq G, Elbez Y, Fox KM, Ferrari R, Ford I, Tardif JC, Tendera M, Steg PG, CLARIFY investigators. New-onset atrial fibrillation and chronic coronary syndrome in the CLARIFY registry. Eur Heart J. 2024;45:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatala R, Hlivák P. Atrial fibrillation in chronic coronary syndromes: a neglected challenge. Eur Heart J. 2024;45:376–8. [DOI] [PubMed] [Google Scholar]
- 29.Prota C, Cortigiani L, Campagnano E, Wierzbowska-Drabik K, Kasprzak JD, Colonna P, Merli E, Manganelli F, Gaibazzi N, D’Andrea A, et al. Left atrial volume, function and B-lines during vasodilator stress echocardiography. Explor Cardiol. 2024;3:9–18. [Google Scholar]
- 30.Yuan N, Duffy G, Dhruva SS, Oesterle A, Pellegrini CN, Theurer J, Vali M, Heidenreich PA, Keyhani S, Ouyang D. Deep learning of electrocardiograms in sinus rhythm from US veterans to predict atrial fibrillation. JAMA Cardiol. 2023;8:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.