Abstract
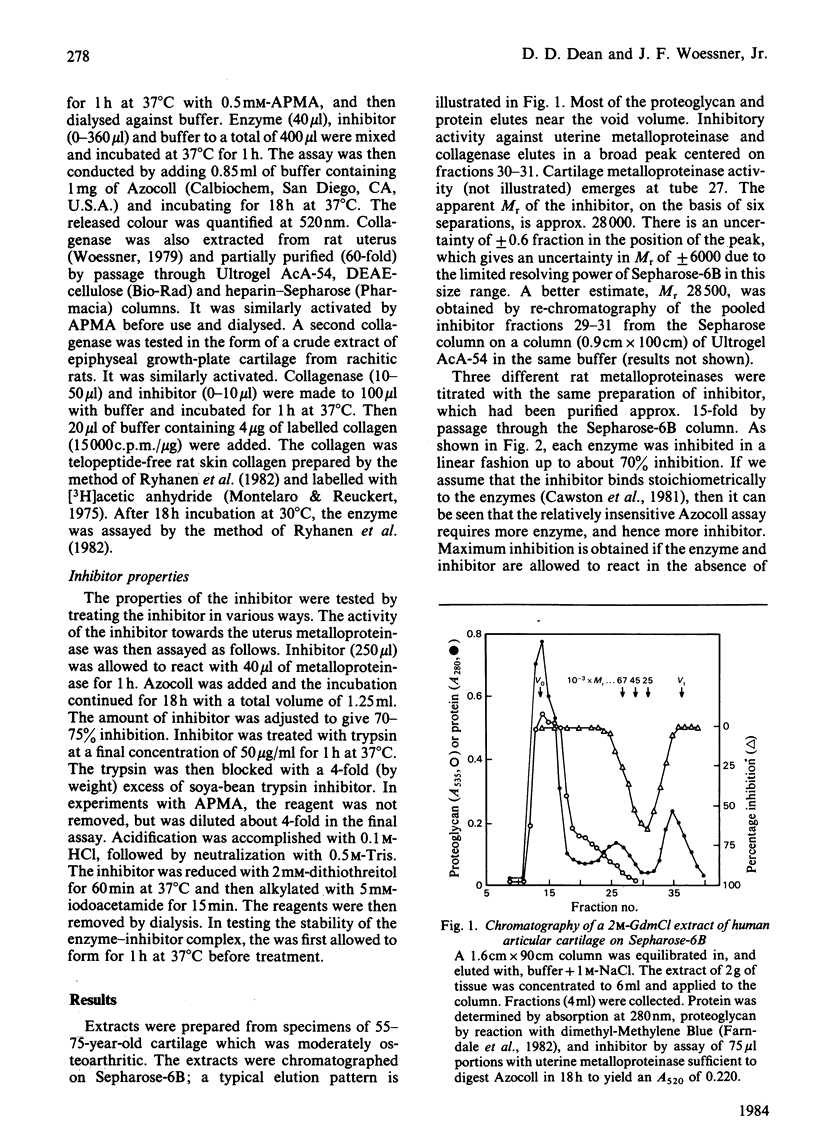
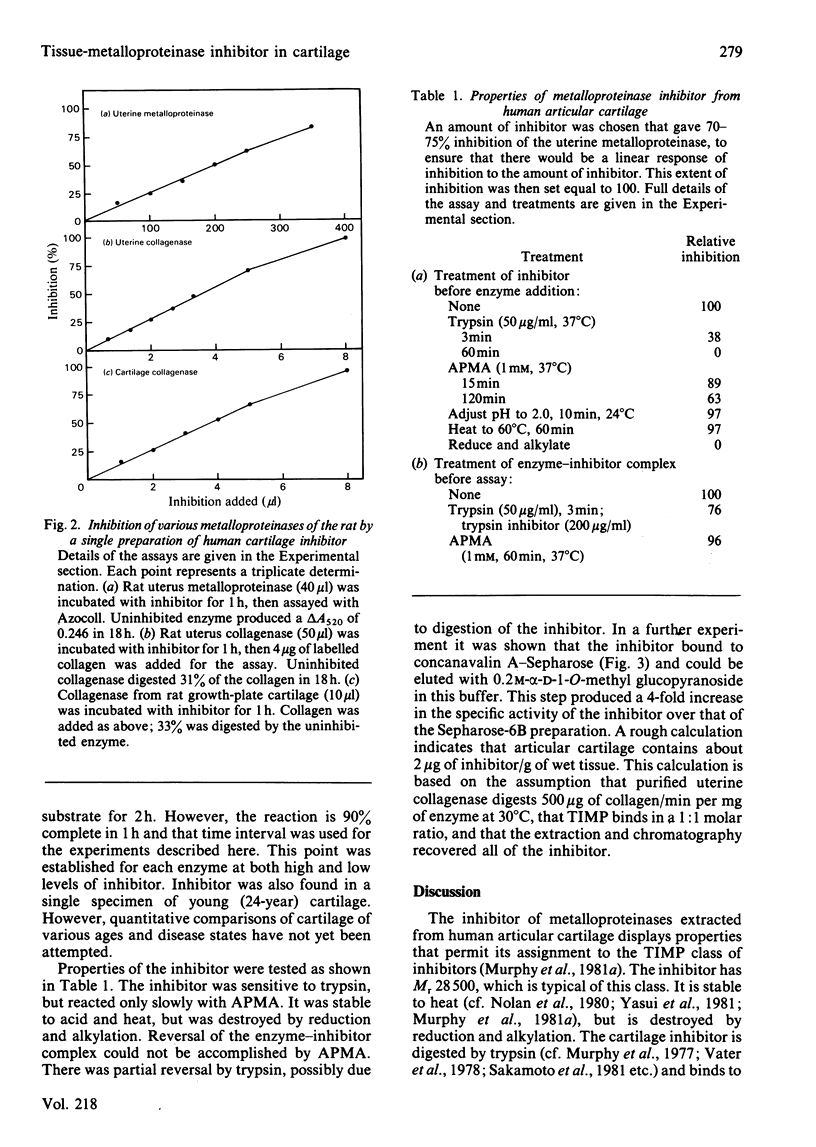
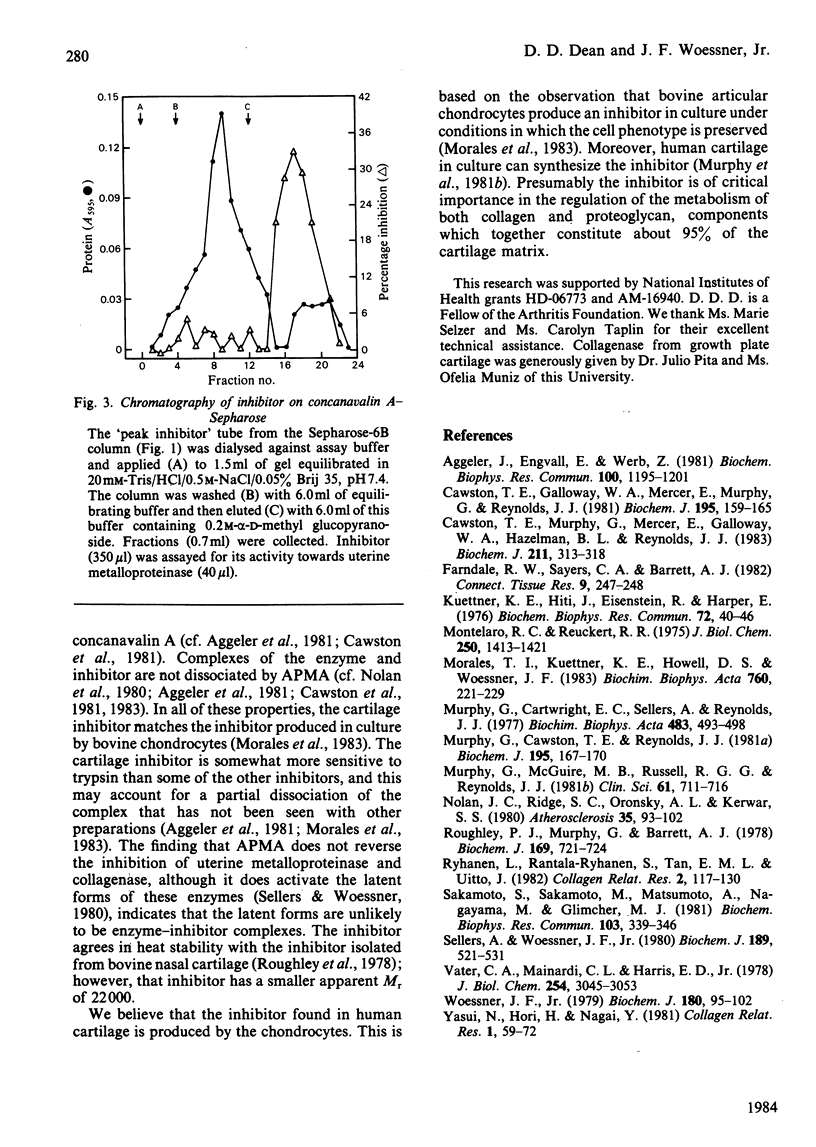
When human articular cartilage is extracted with 2M-guanidinium hydrochloride at pH 7.5, an inhibitor is obtained that blocks the activity of three metalloproteinases, including collagenase. Molecular-sieve chromatography of the inhibitor gives an Mr value for the inhibitor of 28 500. The inhibitor is stable to heat (60 degrees C, 1h) and acid (pH2, 24 degrees C, 10 min). It is destroyed by trypsin and by reduction and alkylation. It is slowly inactivated by aminophenylmercuric acetate. It binds to concanavalin A-Sepharose and is eluted with alpha-D-1-O-methyl glucopyranoside. Complexes of enzyme and inhibitor are not re-activated by aminophenylmercuric acetate and only partially so by high levels of trypsin. These properties indicate that this inhibitor is a member of the TIMP (tissue inhibitor of metalloproteinases) class. Such an inhibitor, previously found in tissue culture and amniotic fluid, is now shown to be directly extractable from tissue.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Engvall E., Werb Z. An irreversible tissue inhibitor of collagenase in human amniotic fluid: characterization and separation from fibronectin. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1195–1201. doi: 10.1016/0006-291x(81)91950-1. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Murphy G., Mercer E., Galloway W. A., Hazleman B. L., Reynolds J. J. The interaction of purified rabbit bone collagenase with purified rabbit bone metalloproteinase inhibitor. Biochem J. 1983 May 1;211(2):313–318. doi: 10.1042/bj2110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Rueckert R. R. Radiolabeling of proteins and viruses in vitro by acetylation with radioactive acetic anhydride. J Biol Chem. 1975 Feb 25;250(4):1413–1421. [PubMed] [Google Scholar]
- Morales T. I., Kuettner K. E., Howell D. S., Woessner J. F. Characterization of the metalloproteinase inhibitor produced by bovine articular chondrocyte cultures. Biochim Biophys Acta. 1983 Oct 18;760(2):221–229. doi: 10.1016/0304-4165(83)90167-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cartwright E. C., Sellers A., Reynolds J. J. The detection and characterisation of collagenase inhibitors from rabbit tissues in culture. Biochim Biophys Acta. 1977 Aug 11;483(2):493–498. doi: 10.1016/0005-2744(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., McGuire M. B., Russell R. G., Reynolds J. J. Characterization of collagenase, other metallo-proteinases and an inhibitor (TIMP) produced by human synovium and cartilage in culture. Clin Sci (Lond) 1981 Dec;61(6):711–716. doi: 10.1042/cs0610711. [DOI] [PubMed] [Google Scholar]
- Nolan J. C., Ridge S. C., Oronsky A. L., Kerwar S. S. Purification and properties of a collagenase inhibitor from cultures of bovine aorta. Atherosclerosis. 1980 Jan;35(1):93–102. doi: 10.1016/0021-9150(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Murphy G., Barrett A. J. Proteinase inhibitors of bovine nasal cartilage. Biochem J. 1978 Mar 1;169(3):721–724. doi: 10.1042/bj1690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryhänen L., Rantala-Ryhänen S., Tan E. M., Uitto J. Assay of collagenase activity by a rapid, sensitive, and specific method. Coll Relat Res. 1982 Mar;2(2):117–130. doi: 10.1016/s0174-173x(82)80028-9. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M., Matsumoto A., Nagayama M., Glimcher M. J. Chick bone collagenase inhibitor and latency of collagenase. Biochem Biophys Res Commun. 1981 Nov 16;103(1):339–346. doi: 10.1016/0006-291x(81)91698-3. [DOI] [PubMed] [Google Scholar]
- Sellers A., Woessner J. F., Jr The extraction of a neutral metalloproteinase from the involuting rat uterus, and its action on cartilage proteoglycan. Biochem J. 1980 Sep 1;189(3):521–531. doi: 10.1042/bj1890521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Inhibitor of human collagenase from cultures of human tendon. J Biol Chem. 1979 Apr 25;254(8):3045–3053. [PubMed] [Google Scholar]
- Woessner J. F., Jr Total, latent and active collagenase during the course of post-partum involution of the rat uterus. Effect of oestradiol. Biochem J. 1979 Apr 15;180(1):95–102. doi: 10.1042/bj1800095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui N., Hori H., Nagai Y. Production of collagenase inhibitor by the growth cartilage of embryonic chick bone: isolation and partial characterization. Coll Relat Res. 1981;1(1):59–72. doi: 10.1016/s0174-173x(80)80008-2. [DOI] [PubMed] [Google Scholar]



