Abstract
Objective
Acute treatment in mild stroke patients with acute anterior circulation large vessel occlusion/stenosis (AACLVO/S) had limited evidence. Hemodynamic play an important role in neurological deterioration. We aimed to investigate predictor value of hemodynamic assessment for clinical outcome predicting and guiding individual therapeutic decisions in those patients.
Methods
We retrospectively analyze the stroke database in our stroke center. We recruited patients with mild stroke, defined by National Institutes of Health Stroke Scale (NIHSS) score ≤ 5, caused by AACLVO/S treated with just medical management (MT). They all received cerebral autoregulation (CA) assessments within 72 h after stroke onset. The primary end point was clinical outcome at 90-day after stroke.
Results
Logistic regression analysis showed that bilateral higher baseline phase difference (PD) were independent variables related to favorable 90-day outcome, (OR 0.963, 95% CI 0.936–0.991, p = 0.040; OR 0.943, 95% CI 0.970–0.997; p = 0.008, respectively). The optimal cutoff value of bilateral PD was > 34.97º and > 14.29º respectively.
Conclusion
CA evaluation can provide hemodynamic status in mild stroke patients with AACLVO/S.
Keywords: Cerebral autoregulation, Mild stroke, Large artery stenosis, Outcome
Introduction
Around half of patients presented with mild ischemic stroke in clinical practice. It was reported that the risk of stroke of 12–20% within the first 3 months after mild stroke [1]. Therefore, several studies focus on the acute treatment in patients with mild stroke. Mild stroke is commonly identified using National Institutes of Health Stroke Scale (NIHSS) scores ≤ 3 or ≤ 5 [2]. Current clinical guidelines recommend endovascular treatment (EVT) as the standard of care for acute ischemic stroke (AIS) patients due to acute anterior circulation large vessel occlusion (AACLVO), such as the occlusion/stenosis of ICA and MCA with a baseline NIHSS score of ≥ 6 presenting within 6 h from symptom onset, or within 6–24 h from symptom onset [2]. However, there is finite evidence on the efficacy and safety of EVT in mild stroke patient cohort comparing with medical management [3, 4]. The mainly debate focused on potential benefits of EVT and complications including hemorrhage transformation, which are mostly correlated with hemodynamic dysfunction.
Cerebral autoregulation (CA) is an important reserve capacity to maintain an adequate hemodynamic stable despite changes in systemic blood pressure. It was reported impaired in acute stage of ischemic stroke [5].
The aim of this retrospective observational study is to identify the predictive value of CA in medical management decision for patients with acute mild ischemic stroke in the anterior circulation.
Methods
Study design and subjects
Data were obtained from the database, which is an ongoing acute stroke registry that prospectively sources records of patients with acute stroke admitted within seven days of onset. The study was approved by the Medical Ethics Committee of Nanfang hospital Southern Medical University, and written informed consents were obtained from all participants. All the patients received CA assessment from January 2018 to November 2021. Among those patients, who meet the following criteria were recruited. 1) > 18 years of age; 2) time from onset or last seen well of the symptoms to first CA assessment ≤ 72 h; 3) ischemic stroke was attributed to 70–99% stenosis of a major ipsilateral middle cerebral artery M1, proximal M2 segment, or intracranial internal carotid artery confirmed by Computed Tomography Angiography (CTA) and diffusion-weighted MRI; 4) Pre-enrollment Modified Rankin scale (mRS) score ≤ 2; 5) Sufficient bilateral temporal bone window for insonation of the MCA; 6) The modified Rankin Scale (mRS) up to 3 months after stroke were collected during a regular clinic visit or through a structured telephone interview conducted by an appropriately trained nurse.
Treatment protocol
All patients received MT according to relevant guidelines on admission. Clopidogrel genetic screening was performed to guide the modulation of antiplatelet treatment.
CA assessment
The baseline arterial blood pressure (ABP) was measured at the brachial artery using an automatic blood pressure monitor (Omron 711). Continuous blood flow velocity of MCA at a depth of 45 mm to 60 mm was obtained with 2 MHz probes which were attached to a customized head frame (EMS-9 PB, Shenzhen, China). The phase describes the phase shift from input to output at a specific frequency. The value of phase degree (PD) was unwrapped and limited to a range of [− 1800,1800], and negative values were deleted. We adopted coherence to reflect the degree of the linear relationship between the complex values of input and output signals at different frequencies, and the value ranges from 0 to 1.
Clinical outcome
We collected clinical outcome: the score on the mRS at 3-months and recurrent stroke or TIA at 6 months after study enrollment by trained personnel who were blinded to the type of the treatment, by telephone and in the clinic. The mRS is a seven-point scale ranging from 0 (no symptoms) to 6 (death). A score of ≤ 2 indicates functional independence.
Statistical analysis
Kolmogorov–Smirnov analysis was used to test the normality of data distribution. Continuous variables were presented as mean ± standard deviation (SD), and non-normally distributed continuous variables were presented as median (interquartile range, IQR). Comparison between groups used Student’s independent t-test or Mann-Whitney U test, depending on the normality of data. Student’s paired t-test or Wilcoxon signed-rank test (non-parametric) was used for comparisons of paired samples. Categorical variables were presented as number and percentage, and compared with Pearson’s χ [6] test or Fisher’s exact test (if any expected value ≤ 5 was observed). Logistic regression models were used to investigate the association of independent variables to outcomes of patients. The receiver operation characteristics (ROC) curve was used to get the cutoff point of PD to predict the favorable outcome after MT. All statistical analyses were performed using IBM SPSS version 26 software (IBM Corporation, Somers, New York). In all analyses, a 2-tailed value of P < 0.05 was considered to indicate statistical significance.
Results
Demographics
Overall, 72 patients (mean age 59.50 ± 10.22 years; 69.44% males) fulfilled the inclusion criteria and were included in the study. Patients had a median NIHSS score of 1(0,5) on admission.
CA assessment in patients
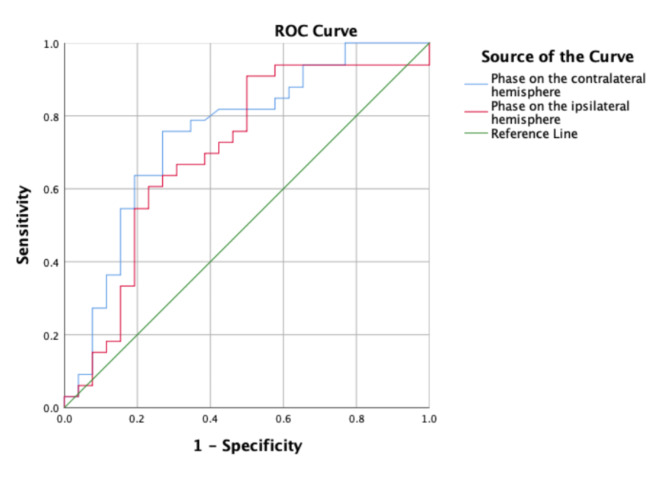
Forty-six (63.89%) patients had favorable 90-day outcome. Univariate analysis showed significantly higher LDL, CRP, Fast blood glucose, NIHSSon-admission, blood pressureon-admission, infarct volume (Table 1), and significantly lower baseline bilateral PD in patients with unfavorable outcome than those with favorable outcome (50.86 ± 23.47°vs. 29.81 ± 21.03°, 36.16 ± 22.95°vs. 18.40 ± 3.22°), all p < 0.05. Logistic regression analysis was used to identify independent predictors of 90-day outcome. After adjusting confounding factors including LDL, CRP, Fast blood glucose, NIHSSon-admission, blood pressureon-admission, the bilateral higher baseline PD were independent variables related to favorable outcome. (adjustied OR 0.962, 95% CI 0.927–0.998; p = 0.040; adjusted OR 0.917, 95% CI 0.860–0.978; p = 0.008, respectively), Table 2. The ROC curve was performed to determine the cutoff point that optimized the sensitivity and specificity associated with 90-day clinical outcomes. The optimal cutoff value of the contralateral PD for predicting favorable outcome was > 34.97º (sensitivity 75.80%, specificity 73.10%). The area under the curve of the ROC curve was 0.741, p = 0.002, (Fig. 1A). The optimal cutoff value of the ipsilateral PD for predicting favorable outcomes was > 14.29º (sensitivity 90.90%, specificity 50.00%). The area under the curve of the ROC curve was 0.743, p = 0.002. (Fig. 1). We performed 90-day follow-up. CA assessment showed that patients had lower CA value on the ipsilateral hemisphere with unfavorable outcome than those with favorable outcome 90-day after stroke, (39.36 ± 24.14°vs. 19.70 ± 17.16°, p = 0.009). Mean recurrence timepoint was 2.82 months. The detail clinical information was listed in Table 3.
Table 1.
The baseline demographic and clinical characteristics of patients
| Total (n = 72) |
Favorable outcome 46 (63.89%) |
Unfavorable outcome 26 (36.11%) |
||
|---|---|---|---|---|
| Age, years | 59.50 ± 10.22 | 58.76 ± 10.15 | 61.06 ± 10.28 | |
| Sex, Male | 50 (69.44) | 30 (65.22) | 20 (76.92) | |
| BMI, kg/m2 | 24.58 ± 2.84 | 24.42 ± 2.80 | 24.79 ± 2.91 | |
| SBP, mmHg | 142.82 ± 22.31 | 137.41 ± 22.55 | 150.15 ± 20.06# | |
| DBP, mmHg | 87.51 ± 13.93 | 85.28 ± 14.73 | 90.53 ± 12.36 | |
| Heart rate, bpm | 70.62 ± 13.80 | 70.98 ± 12.63 | 70.09 ± 15.57 | |
| Smoking status | ||||
| Current smoker | 36 (50.00) | 22 (47.83) | 14 (53.85) | |
| Alcohol | ||||
| Current drinker | 17 (23.61) | 8 (17.39) | 9 (34.62) | |
| Hypertension | 45 (62.50) | 24 (52.17) | 21 (80.77) | |
| Diabetes mellitus | 25 (34.72) | 14 (30.43) | 11 (42.31) | |
| Hyperlipidemia | 22 (30.56) | 10 (21.74) | 12 (46.15) | |
| TG, mmol/L | 1.76 ± 1.27 | 1.65 ± 1.11 | 1.92 ± 1.48 | |
| LDL, mmol/L | 2.76 ± 0.89 | 2.55 ± 0.89 | 3.06 ± 0.82# | |
| CRP, mg/L | 5.60 ± 1.20 | 6.04 ± 0.97 | 12.77 ± 3.82# | |
| Fast blood glucose, mmol/L | 7.31 ± 5.79 | 5.80 ± 1.70 | 8.36 ± 1.43# | |
| NIHSS on admission | 1 (0, 5) | 1 (0, 2) | 2 (0, 5) # | |
| Infarct volume (cm3) | 5.00 (2.30, 15.60) | 3.30 (0.86, 7.91) | 9.00 (5.00, 21.60) # | |
| MTT contralateral | 5.82 ± 1.16 | 5.57 ± 0.98 | 6.28 ± 1.40 | |
| MTT ipsilateral | 7.69 ± 3.08 | 7.63 ± 2.77 | 7.81 ± 3.75 | |
| CBF contralateral | 41.05 ± 8.75 | 40.29 ± 9.40 | 42.20 ± 7.83 | |
| CBF ipsilateral | 33.10 ± 10.26 | 33.50 ± 10.65 | 32.53 ± 9.99 | |
| CBV contralateral | 3.67 ± 0.76 | 3.66 ± 0.86 | 3.68 ± 0.59 | |
| CBV ipsilateral | 3.76 ± 0.82 | 3.92 ± 0.76 | 3.47 ± 0.88 | |
| TTP contralateral | 18.01 ± 4.57 | 17.59 ± 4.30 | 18.72 ± 5.15 | |
| TTP ipsilateral | 19.48 ± 4.89 | 19.53 ± 5.27 | 19.40 ± 4.45 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; LDL, low-density lipoprotein; CRP, C-reactive protein; NIHSS, National Institute of Health stroke scale
BMI was calculated as weight in kilograms divided by height in meters squared
Data are presented as mean ± standard deviation, or number (percentage), or median (inter- quartile range, IQR)
# indicate significant difference comparing between outcome subgroups, p < 0.05
Table 2.
Logistic regression analysis
| Group with 90-day favorable outcome |
Group with 90-day unfavorable outcome |
adjusted OR | P | |
|---|---|---|---|---|
| Baseline PDcontralateral hemisphere | 50.86 ± 23.47° | 29.81 ± 21.03° | 0.962# | 0.040# |
| Baseline PDipsilateral hemisphere | 36.16 ± 22.95° | 18.40 ± 3.22° | 0.917# | 0.008# |
PD: phase degree
# indicates significant difference comparing between outcome subgroups, p < 0.05
Fig. 1.
The ROC curve showed the optimal cutoff value of the contralateral PD for predicting favorable outcomes was > 34.97º (sensitivity 75.80%, specificity 73.10%), the ipsilateral PD for predicting favorable outcomes was > 14.29º (sensitivity 90.90%, specificity 50.00%) in MT-groupwithin90-day
Table 3.
Comparison of phase degree between different groups
| Patients with 90-day favorable outcome |
Patients with 90-day unfavorable outcome |
P | |
|---|---|---|---|
| PDcontralateral hemisphere | 50.86 ± 23.47 | 29.81 ± 21.03 | 0.040 |
| PDipsilateral hemisphere | 36.16 ± 22.95 | 18.40 ± 3.22 | 0.008 |
| PDcontralateral hemisphere 90-day after stroke | 53.43 ± 21.04 | 30.45 ± 18.68 | 0.253 |
| PDipsilateral hemisphere 90-day after stroke | 39.36 ± 24.14° | 19.70 ± 17.16° | 0.009 |
P: p-value of PD between different outcome subgroups after adjusting confounding factors
PD: phase degree
Discussion
This study was the first to explore predictive value of CA in patients with mild stroke due to acute anterior circulation large vessel occlusion. The study showed that, in patients who received medical management, bilateral higher baseline PD were independent variables related to favorable 90-day outcome. And the optimal cutoff value of the contralateral and ipsilateral PD for predicting favorable outcome was > 34.97º, > 14.29º respectively.
Several studies indicated that the hemodynamics of the stenosis is extremely complicated, and the anatomical stenosis should not be considered only, but should be combined with CA ability and collateral circulation compensation. Therefore, we assessed CA in the study. CA was a hemodynamic parameter which is the intrinsic ability of the cerebral vasculature to guarantee downstream blood supply and reflects cerebrovascular reserve capacity. Previous researches reported that CA impairments globally in ischemic stroke patients, affecting both the ipsilateral and the contralateral hemispheres [5, 7]. We got the similar finding, and we found that bilateral higher baseline CA were independently associated with favorable 90-day outcome in MT group. It means that in patients with mild stroke caused by AACLVO, if they had bilateral value of PD higher than the reference values mentioned above, MT was suggested to be selected. A possible explanation is that CA played an important role in protecting hemodynamic fluctuation under hypoperfusion condition, which is considered as the major cause of ischemic stroke. Under ischemic condition, not only the affected side CA, but also the unaffected side play the compensatory role.
Meanwhile, failure of CA is reported to be associated with secondary brain injury that may occur as an extension of the initial ischemic core, “no-reflow phenomenon”, cerebral edema, or hemorrhagic transformation [8]. All these secondary complications above play important role in outcome after acute ischemic stroke. As we know, not all patients would benefit from mechanical thrombectomy (MT).
CA monitoring utilizing TCD needed 20 min, which should be done in the acute phase before making medical management [9]. However, we believe that treatment should be individualized and that it is worthwhile to spend 20 min for a full hemodynamic assessment before making a medical decision. Intact CA during AIS has not only been shown to be responsible for a smaller infarct volume but also protects the vulnerable penumbra [10]. Further convenient CA assessment methods are needed.
The current study has several limitations. First, we recruited more male subjects than female in our study, since there are more male stroke patients than female in China, and female subjects are more likely to have insufficient bilateral temporal bone windows for insonation due to the low density of the temporal bone. Secondly, the relatively small number of patients, which affected the statistical analysis of the various groups. And we just performed 90-day and 6-month follow-up, but not long-term follow-up. Further relative data is needed. Thirdly, our result is limited by its design and lack of the control arm. However, currently, there are no prospective, randomized trials that compare endovascular management with aggressive medical therapy. Further randomized trials using the cut-off value is needed. Fourthly, more detail factors were not further analyzed. For those patient population, there are still numerous researches be performed. Lastly, we did not analyze the correlations between the size of cerebral infarctions and CA, we will perform relative analysis in future.
In conclusion, CA evaluation can provide the assessment of hemodynamic status in patients with symptomatic subacute/chronic MCA stenosis. Then help determine which patients will benefit from MT and prognostic information.
Acknowledgements
N/A.
Author contributions
Study concept and design: TG, YJ. Acquisition of data: TG, ZJJ. Statistical analysis: HJ, WQH. Drafting of the manuscript: TG, YJ. Critical revision of the manuscript for important intellectual content: TG, YJ, HJ. Imaging assessment, WQH, ZJJ. Study supervision: YJ. All the authors read and approved the final manuscript.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Nanfang Hospital. Written informed consent was obtained from all participants. The data presented were collected for the purpose of quality assurance and, thus, the identity of the individual patients was anonymous.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Medical ethics registration number
NFEC-201,910-K4.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saleem Y, Nogueira RG, Rodrigues GM, Kim S, Sharashidze V, Frankel M, et al. Acute neurological deterioration in large vessel occlusions and mild symptoms managed medically. Stroke. 2020;51:1428–34. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke. 2018;49:e46–110. [DOI] [PubMed] [Google Scholar]
- 3.Goyal N, Tsivgoulis G, Malhotra K, Ishfaq MF, Pandhi A, Frohler MT, et al. Medical management vs mechanical thrombectomy for mild strokes: an international multicenter study and systematic review and meta-analysis. JAMA Neurol. 2020;77:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarraj A, Hassan A, Savitz SI, Grotta JC, Cai C, Parsha KN, et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. 2018;49:2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian G, Ji Z, Huang K, Lin Z, Pan S, Wu Y. Dynamic cerebral autoregulation is an independent outcome predictor of acute ischemic stroke after endovascular therapy. BMC Neurol. 2020;20:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/american stroke association. Stroke. 2021;52:e364–467. [DOI] [PubMed] [Google Scholar]
- 7.Petersen NH, Ortega-Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis. 2015;39:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheriff FG, Ahmad A, Inam ME, Khatri R, Maud A, Rodriguez GJ. A systematic review on the assessment of cerebral autoregulation in patients with large vessel occlusion. Front Neurol. 2023;14:1287873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheriff F, Castro P, Kozberg M, LaRose S, Monk A, Azevedo E et al. Dynamic cerebral autoregulation post endovascular thrombectomy in acute ischemic stroke. Brain Sci. 2020;10. [DOI] [PMC free article] [PubMed]
- 10.Hecht N, Schrammel M, Neumann K, Muller MM, Dreier JP, Vajkoczy P, et al. Perfusion-dependent cerebral autoregulation impairment in hemispheric stroke. Ann Neurol. 2021;89:358–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.