Abstract
Background
Medically tailored meal (MTM) programs provide home-delivered meals to people living with serious illness and poor nutritional status. Client outcome studies have found evidence of decreased healthcare utilization and cost savings associated with MTM program participation, and inconclusive evidence of change in health measures. The purpose of this study was to use a novel observational framework to describe the client profile and change in health outcomes using routinely collected health and program data from a community-based MTM program at MANNA (Philadelphia, PA).
Methods
Clients reported their self-rated health and experiences of food insecurity and malnutrition. Healthcare providers reported clients’ body mass index, systolic blood pressure, and hemoglobin A1C. These health outcomes, measured at program intake and 3–6 months later, were linked with administrative data for 1,959 clients who completed at least two months of MTM services in 2020, 2021, and 2022.
Results
Clients exhibited substantial heterogeneity in demographics and health status at intake. Self-reported malnutrition risk decreased significantly over program duration (p < .001). Nearly one-third of clients with poor health reported improvement over time. Over 60% of clients with obesity experienced stable BMI. Clients with hypertension experienced significant improvements in systolic blood pressure (p < .001). Clients with diabetes and available data (n = 45) demonstrated significant reduction in hemoglobin A1C (p = .005).
Conclusion
We found evidence that participation in MANNA’s MTM program was associated with favorable health outcomes for clients with serious illness and nutritional risk. Community-based organizations can maximize the completeness of their data by focusing on routinely collected internal data like validated health screeners and surveys.
Keywords: Medically tailored meals, Nutrition program evaluation, Malnutrition risk, Food as medicine, Community-based organization
Introduction
The Metropolitan Area Neighborhood Nutrition Alliance (MANNA) is a non-profit, community-based organization (CBO) that delivers medically tailored meals (MTM) to clients living with serious illness in the greater Philadelphia region [1]. MTMs are prescribed as part of medical management for diet-related diseases such as diabetes, heart disease, kidney disease, cancer, and HIV/AIDS [2] and are consistent with nutritional guidelines from the American Diabetes Association [3], American Heart Association [4], National Kidney Foundation [5], and the 2020–2025 Dietary Guidelines for Americans [6]. The number of people living in the US with chronic diet-related diseases and conditions is significant. 52% of adults have at least one chronic condition, and nearly 30% have two or more [7]. Many of these conditions, including cardiovascular disease, some cancers, and type 2 diabetes, have diet-related risk factors and require specialized diets for disease management [8]. Prevalence of these conditions is higher among older adults, who are also at increased risk for malnutrition [9]. The prevalence of malnutrition among older adults in North America is estimated at 6.1% [10] and is associated with functional decline, excess morbidity, and mortality [11]. Malnutrition costs the US healthcare system an estimated $157 billion per year due to high rates of hospitalization, complications, and mortality [12].
Community-based nutrition support is needed to help individuals manage diet-related disease and offset the impact of malnutrition. However, existing public nutrition programs such as the Supplemental Nutrition Assistance Program (SNAP) and Older Americans Act Nutrition Programs do not address dietary needs related to specific disease states [13, 14]. Medically tailored meal programs can fill the gap in public programs when a specific dietary regimen is prescribed by a healthcare provider as part of disease treatment.
Access to MTM interventions is currently limited and individuals are often left to procure prescribed food and nutrition necessary for disease management on their own. Increasing universal access to MTMs requires making them a covered medical expense, similar to prescription drugs, when they are part of a treatment plan [15–18] or including them in legislation for public nutrition programs (i.e. the Agriculture Improvement Act of 2018 and the Older Americans Act) [19, 20]. Creating sustainable, evidence-based policy for equitable access to MTM interventions when necessary requires further study of associated health outcomes and impacts [21].
Only a few experimental studies have been completed and they assessed varied timeframes and MTM program models. Kaiser Permanente funded two large randomized controlled trials (RCTs) that did not observe an effect of MTMs on recently hospitalized individuals’ healthcare utilization or health outcomes. One of these studies provided individuals with heart failure (HF) or diabetes 10 weeks of MTMs upon hospital discharge and found no difference in all-cause 90-day readmissions, all-cause emergency department visits, or mortality compared to the control group; though exploratory analyses found lower 90-day readmission rates for HF patients [22]. The other study provided MTMs to patients with one or more chronic illnesses for 2 or 4 weeks after hospital discharge. The study found no differences in health outcomes (depression and anxiety), activities of daily living, nutritional health, or healthcare utilization at 60-days post-discharge, regardless of disease state [23]. A smaller RCT with people living in the community with diabetes and food insecurity showed that an MTM intervention was associated with improved dietary quality, decreased hypoglycemia, and improved quality of life (better mental health) [24].
Observational studies of MTM interventions have linked clients’ program data with their health insurance claims data in retrospective matched cohort studies. MANNA conducted a claims analysis pilot study in 2013 and showed its MTM intervention was associated with lower monthly healthcare costs and lower high-cost utilization in the months after receiving meals, indicating that MTMs may be a useful component of value-based care [25]. Two later claims analyses showed that participation in a community-based MTM program in Boston was associated with fewer hospital admissions and lower healthcare costs [26, 27]. A recent cost modeling study estimated that there is potential for significant healthcare cost savings if US adults with diet-sensitive conditions had access to MTMs through government or private health insurance [28]. These observational studies provide important insights for potential health insurance coverage, but they lack client-centered measures of social characteristics and health status that are meaningful for CBOs.
The growing literature on MTM interventions would benefit from research studies led by CBOs that focus on program evaluation within specific communities served. CBOs vary in the number of MTMs provided per week, frequency of interaction with clients, characteristics that qualify individuals for program participation, size of staff and budget, and characteristics of community settings [29]. Due to these differences, many MTM studies to date have focused on healthcare utilization as a primary outcome measure and few studies have assessed health outcomes [30]. However, gaps remain in defining the potential mechanisms that lead to changes in healthcare utilization from MTM interventions. Therefore, MTM program evaluation is a necessary step towards identifying the pathways that lead to biopsychosocial changes that potentially lead to lower healthcare utilization.
The goal of this study was to leverage routinely collected health data for outcome assessment and program evaluation. We followed a new framework to collect appropriate data and translate client records into a research dataset [31] and utilized The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) guidelines to document our methods [32]. Early analyses using this framework showed that participating in MANNA’s program was associated with lower rates of hospitalization and body mass index (BMI) stabilization among those with unintended weight loss [33]. This paper describes the methods used to conduct a pre-post observational study of the following objectives:
Describe the sociodemographic and health profile of clients participating in MANNA’s MTM program.
Estimate change in health outcomes (BMI, blood pressure, physical function, and malnutrition risk) over 2–7 months of MTM program participation.
Explore the sociodemographic and health profile of clients living with diabetes and estimate change in hemoglobin A1C.
Materials and methods
Population
The sample consisted of first-time clients ages 18 or older who started MANNA’s MTM program on or after January 1, 2020, and completed at least 2 months of the program by December 31, 2022. Clients lived in the Greater Philadelphia area. To qualify for services from MANNA, clients must have a signed referral from a health care provider indicating the presence of nutritional risk factors in the context of a serious illness [34].
Intervention
MANNA’s registered dietitians (RDs) recommended an enrollment length of either 3 months or 6 months depending on the severity of nutritional risk. Clients received 21 meals per week, designed to provide an average of 1900 calories per day and a daily macronutrient distribution of 20% protein, 30% fat, and 50% carbohydrate. Average sodium content for meals was 2 g/day and the menu was also designed to be lower in cholesterol and sugar and higher in fiber contents. Clients could receive up to three of the following modifications based on their individual health and cultural needs: kidney-friendly (2-gram potassium), diabetic/heart healthy (45–50% carbohydrate), low lactose, low fiber and mild spice, mechanical soft, pureed, high calorie and protein, no pork, no beef, and no seafood. All meals were freshly prepared by volunteers under the supervision of professional chefs and then flash-frozen and delivered weekly to clients. All clients were offered optional nutritional counseling by a MANNA RD, typically over the phone.
Data collection
Client data were collected as part of routine service enrollment and recertification. Enrollment data included sociodemographic and health information reported from the referring medical providers and phone assessments taken by clients. Recertification data included follow-up health information reported from medical providers and phone assessments with clients. Some data were stored in MANNA’s electronic database, Client Track, while other data remained on paper forms kept in the clients’ charts. This system met the operational needs of the organization but was not optimal for ongoing assessment and evaluation.
Research based on MANNA’s program data was approved by the Institutional Review Board (IRB) at the University of the Sciences (now St. Joseph’s University). MANNA’s research staff (JAH and AGC) organized and managed client chart data with Research Electronic Data Capture (REDCap), a cloud-based, HIPAA-compliant software platform hosted by Vanderbilt University [35, 36]. A team of 6 medical student research fellows (VSD, KAB, JZ, MRJ, JSE, and DA) entered data from client records into REDCap using standardized data entry procedures. Data were spot-checked by AGC and any discrepancies were reconciled and clarified with the research fellows. After data entry was complete, all outliers were identified by JMS and IRN and then either verified or corrected by JAH. We exported data to SPSS [37] for data management and statistical analysis.
Analytic sample
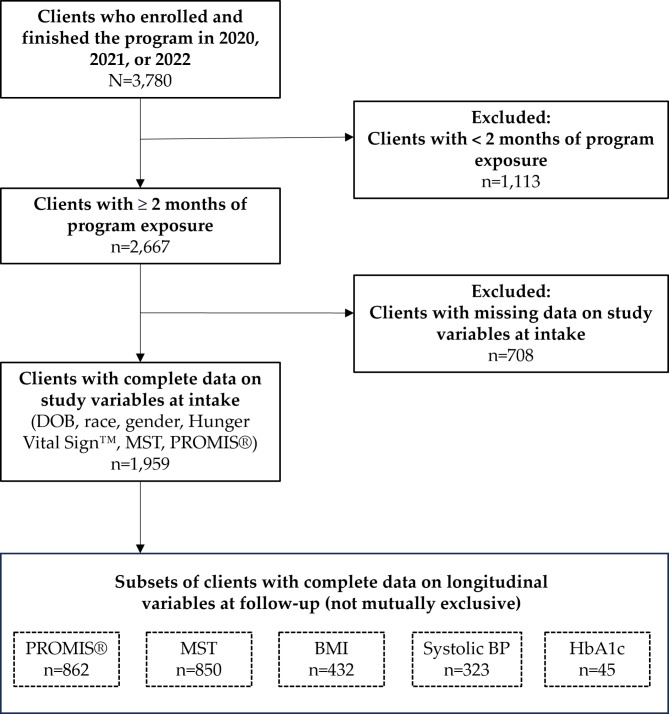
We queried Client Track to identify all 3,780 clients aged 18 years or older who enrolled in MANNA for the first time during 2020, 2021, and 2022 (Fig. 1). We excluded 1,113 clients (30%) who had less than two months of program participation (half of these clients requested to discontinue the program, 22% ended due to program noncompliance with meal delivery schedule, and 16% due to death or hospitalization). We focused on the 2,667 clients who remained on the program for at least two months– long enough to benefit from MANNA’s program model. Clients who completed at least two months were less likely to have cancer and more likely to be Black, Hispanic, and food insecure compared to those who exited the program soon after enrollment. There was a great deal of missing data in linked phone assessment and healthcare provider forms. We excluded 708 clients (26%) with missing intake data on key demographic and self-reported variables, leaving 1,959 clients with complete linked data from referral forms and client phone assessments in the analytic sample. We observed variable rates of data completeness when we linked to health outcomes data reported by healthcare providers at intake (89% of clients had complete BMI data, 58% had blood pressure values, and 40% of clients with diabetes had hemoglobin A1C values).
Fig. 1.
Flow chart of analytic sample
Linkages to follow-up data were missing at greater rates. Approximately 862 clients answered PROMIS and MST questions at both program intake (up to 60 days before starting meals) and at follow-up (2–7 months after starting meals), representing 44% of the clients with complete intake data. Fewer than one-quarter of clients had BMI reported by their healthcare provider at intake and follow-up (n = 432), and 16% had blood pressure reported at intake and follow-up (n = 323). Among clients with diabetes, just 6% had hemoglobin A1C values reported at intake and follow-up (n = 45).
Provider-reported measures
Demographic data were reported by clients’ healthcare providers and included age (categorized as 65 + vs. 18–64 years old, based on date of birth and date of program enrollment); gender (cis or trans female vs. all other gender identities), race (Black vs. other races, primarily white), and ethnicity (Hispanic vs. non-Hispanic). Providers also indicated which of the following serious illness(es) the client had: HIV/AIDS, cancer, diabetes, kidney disease, cardiovascular disease, and/or other diagnoses.
Clinical laboratory values were reported by health care providers at intake and follow-up. Body weight and height were used to calculate BMI, which was further categorized into underweight (BMI < 18.5), healthy (18.5–25), overweight (25–30), obese (30–40), and severely obese (40+). Systolic blood pressure was categorized as healthy (SBP < 120 mmHg), elevated (120–129 mmHg), hypertensive stage 1 (130–139 mmHg), and hypertensive stage 2 (140 + mmHg). Hemoglobin A1C was measured as a percentage and categorized as controlled (< 7%) and uncontrolled (≥ 7%).
Client-reported measures
Clients were contacted over the telephone during the intake process and asked several questions that measure socioeconomic status and subjective well-being. Insurance type was categorized as Medicaid vs. all other insurance types. Risk of food insecurity was determined with the Hunger Vital Sign™, which is a validated screening tool that includes two statements: “We worried whether our food would run out before we got money to buy more” and “The food we bought just didn’t last, and we didn’t have money to get more” [38, 39]. Clients were asked if the statements were never true, sometimes true, or often true, and screened positive for food insecurity if they answered “sometimes true” or “often true” to one or both statements.
Malnutrition risk was assessed with the Malnutrition Screening Tool (MST), which has been recommended as a universal screening tool based on Grade 1 evidence and generalizability [40, 41]. The MST includes the questions “Have you lost weight recently without trying?”, “If yes, how many pounds?”, and “Have you been eating poorly because of a decreased appetite?”. Clients screened as high risk for malnutrition if they reported a minimum weight loss (2–13 lbs.) in addition to appetite issues, or if they reported significant weight loss (> 13 lbs.) regardless of appetite issues (score of 2–5); scores of 0 or 1 indicate low risk of malnutrition [41].
Physical health status was measured using the two-item global physical health scale (PROMIS® Scale v1.2 – Global Health Physical 2a) from the Patient-Reported Outcome Measurement Information System (PROMIS®) [42]. This scale sums responses from two items: “In general, how would you rate your physical health?” has response categories of excellent, very good, good, fair, and poor and “To what extent are you able to carry out everyday physical activities like walking, climbing stairs, carrying groceries, or moving a chair?” has response categories of completely, mostly, moderately, a little, and not at all. The scores were uploaded to the HealthMeasures scoring service, which returns T-scores standardized to the US general population with a population mean of 50 and a standard deviation of 10 [43]. Scores can be interpreted as excellent (T-scores > 58), very good (50–58), good (42–49), fair (35–41), and poor (< 35) [44].
Longitudinal data
Follow-up data were gathered during MANNA’s recertification process. This recertification occurs after three or six months, depending on the client’s enrollment prescription. One-third of clients were reassessed at three months and two-thirds were reassessed at six months. Time-varying measures reported by clients include PROMIS and MST. Time-varying measures reported by providers include the most recent weight/BMI, blood pressure, and hemoglobin A1C values from the clients’ medical records. We used the date of clinical measurement to calculate the number of days between measurement and program start. We used the number of days to convert measures anchored by program form (e.g., intake, update 1, update 2) to measures anchored by time of program start: pre-MTM measures (up to 60 days before starting meals) and post-MTM measures (two to seven months after starting meals).
We calculated change scores for each client with follow-up data on key health outcomes, then categorized that change as decrease, stable, or increase. A change of 2 points on the PROMIS scale indicated a clinically significant change in physical health [45]. A decrease in total score from 2 + to 0 or 1 indicated a change from high risk to low risk for malnutrition [41]. A 2-unit change in BMI indicated a clinically significant change in body weight [46]. A 5 mmHg change in systolic blood pressure indicated clinically significant increase or decrease in blood pressure [47]. A decrease of 1% indicated a clinically significant improvement in hemoglobin A1C [48, 49].
Statistical analyses
We calculated percentages and frequencies to describe the distribution of categorical variables for clients in the study. We showed distributions for the full sample and for subsets with follow-up health outcome measures to examine whether subsets of clients with valid follow-up data differ from clients with missing data.
We analyzed change over time with continuous values that were not normally distributed; MST scores followed a negative binomial distribution, and PROMIS, BMI, and blood pressure exhibited positive skew at intake. We presented medians and interquartile ranges (IQR) for each outcome at intake and at follow-up to represent aggregate change over time. We used Wilcoxon signed-rank tests to test whether the medians of within-person differences between intake and follow-up were significantly different from zero. We further described individual change over time with stacked bar charts showing the percentage of clients who experienced clinically significant increase, decrease, or stability in health outcomes. We conducted these analyses for all clients, and for clients who screened as high risk when they started the program. High risk for malnutrition, hypertension, poor physical health, and obesity were defined as MST ≥ 2, systolic BP ≥ 130 mmHg, PROMIS T-score < 35, and BMI ≥ 30, respectively. We also conducted linear regressions to examine factors associated with change in outcome values, and logistic regressions to examine factors associated with likelihood of significant improvement over time. We examined change in hemoglobin A1C separately within the smaller subset of clients living with diabetes.
Results
Population characteristics
In the full client sample (n = 1,959), 53% of clients were women, 51% were seniors aged 65+, 62.5% were Black, and few were Hispanic (7.4%) (Table 1). Half of clients (52%) lived with comorbidity; the most prevalent diagnoses included cardiovascular disease (58%), diabetes (40%), cancer (31%), kidney disease (24%), and HIV/AIDS (7%). Two-thirds of clients reported food insecurity (66%) and more than half of clients were at high risk of malnutrition (56%). Clients were not well at intake, with 43% reporting poor health and 37% reporting fair health. More than 38% of clients were affected by obesity at intake and fewer than 10% were underweight. Almost half of clients with systolic blood pressure data had hypertensive values at intake.
Table 1.
Characteristics of clients at intake: percentages and frequencies
| Characteristic | Longitudinal subsets with intake and follow-up data on selected outcomes | |||
|---|---|---|---|---|
| Intake Sample | PROMIS & MSTa | BMIb | Blood pressurec | |
| n = 1,959 | n = 862 | n = 432 | n = 323 | |
| Demographics | ||||
| Age 65+ | 50.7% (994) | 52.0% (448) | 55.3% (239) | 55.1% (178) |
| Female | 53.2% (1042) | 54.2% (467) | 55.3% (239) | 54.8% (177) |
| Black | 62.5% (1225) | 63.9% (551) | 59.5% (257) | 58.8% (190) |
| Hispanic (n = 1708) | 7.4% (144) | 7.7% (66) | 6.3% (27) | 7.1% (23) |
| Medicaid (n = 1662) | 32.3% (6323 | 32.8% (283) | 30.3% (131) | 34.9% (95) |
| Diagnoses (not mutually exclusive) | ||||
| HIV/AIDS | 7.2% (141) | 6.8% (59) | 6.5% (28) | 5.9% (19) |
| Cancer | 31.0% (608) | 27.6% (238) | 34.7% (150) | 36.2% (117) |
| Diabetes | 39.9% (782) | 43.2% (372) | 40.0% (173) | 42.7% (138) |
| Kidney disease | 23.7% (465) | 24.8% (214) | 31.5% (136) | 31.6% (102) |
| CVD | 58.0% (1136) | 60.0% (517) | 56.3% (243) | 62.2% (201) |
| Nutrition | ||||
| Food insecured | 66.1% (1294) | 68.0% (586) | 65.0% (281) | 65.3% (211) |
| Malnourishede | 55.7% (1092) | 53.8% (464) | 57.6% (249) | 55.1% (178) |
| PROMIS f | ||||
| Poor | 42.8% (839) | 42.7% (368) | 41.9% (181) | 44.9% (145) |
| Fair | 37.3% (730) | 37.4% (322) | 38.9% (168) | 35.6% (115) |
| Good | 12.4% (243) | 13.0% (112) | 11.6% (50) | 12.1% (39) |
| Very good / Excellent | 7.5% (147) | 7.0% (60) | 7.6% (33) | 6.4% (25) |
| BMI Category g | ||||
| n = 1749 | n = 765 | n = 432 | n = 303 | |
| Underweight | 7.2% (126) | 7.2% (55) | 4.4% (19) | 4.3% (13) |
| Healthy | 30.5% (534) | 30.7% (235) | 33.1% (143) | 32.3% (98) |
| Overweight | 23.7% (415) | 22.6% (173) | 24.8% (107) | 24.4% (74) |
| Obese | 26.8% (468) | 26.9% (206) | 27.1% (117) | 26.1% (79) |
| Severely obese | 11.8% (206) | 12.5% (96) | 10.6% (46) | 12.9% (39) |
| Blood Pressure Category h | ||||
| n = 1137 | n = 612 | n = 314 | n = 323 | |
| Normal | 32.9% (458) | 31.2% (191) | 30.3% (95) | 30.0% (97) |
| Elevated | 20.2% (282) | 20.9% (128) | 17.8% (56) | 19.5% (63) |
| Hypertension 1 | 18.0% (251) | 19.4% (119) | 21.0% (66) | 20.7% (67) |
| Hypertension 2 | 28.9% (403) | 28.4% (174) | 30.9% (97) | 29.7% (96) |
aPROMIS Scale v1.2 – Global Health Physical 2a and the Malnutrition Screening Tool (MST) collected by MANNA staff. bBody Mass Index calculated with height and weight provided by health care provider. cSystolic blood pressure provided by health care provider. dClients who responded “sometimes true” or “often true” to one or both statements of the Hunger Vital Sign. eMST score ≥ 2. fPROMIS T-score categories: excellent (T-score > 58), very good (50–58), good (42–49), fair (35–41), and poor (< 35). gBMI categories: underweight (BMI < 18.5), healthy (18.5–25), overweight (25–30), obese (30–40), and severely obese (40+). hSystolic blood pressure categories: healthy (BP < 120 mmHg), elevated (120–130 mmHg), hypertensive stage 1 (130–140 mmHg), and hypertensive stage 2 (140 + mmHg)
The subsets of clients with valid health outcomes at both intake and follow-up were smaller and more selected than the full sample (Table 1). When we focused on the outcomes collected by MANNA staff (PROMIS and MST), the subset with follow-up data represented 44% of the intake sample and was similar in sociodemographic characteristics and disease prevalence (n = 862). When we focused on the outcomes collected from external health care providers, subsets with follow-up data represented just 16–23% of the intake sample. The clients with provider-reported clinical values (BMI and blood pressure) at intake and follow-up were older and more likely to be living with cancer or kidney disease compared to the intake sample.
Change in health over time
Among all clients, there was no significant difference in BMI value or systolic blood pressure between intake and follow-up; a slight decrease in PROMIS score was marginally significant (Table 2). The median MST score decreased from 2 to 1 at follow-up, which showed a statistically and clinically significant change from median high risk to median low risk. When analyses were focused on clients with high risk at intake, aggregate change was more apparent. Median MST score decreased from 3 to 1, and median systolic blood pressure decreased from 142 to 135 mmHg. Median BMI and PROMIS scores did not differ between intake and follow-up, but related-samples Wilcoxon signed rank test showed evidence of a slight decrease in physical health scores within individuals.
Table 2.
Change over time: all clients and high risk subset
| Outcome Variable | Aggregate Change | Within-person Change | |
|---|---|---|---|
| Median (IQR) at intakei | Median (IQR) at follow-up | Wilcoxon Signed Rank Test | |
| All Clients | |||
| PROMIS (n = 862, range 23–63)a | 37 (33–41) | 37 (33–41) | W= -1.99, p = .046 |
| MST (n = 850, range 0–5)b | 2 (0–3) | 1 (0–2) | W= -10.08, p < .001 |
| BMI (n = 432, range 12–63)c | 27 (22–34) | 27 (22–33) | W= -1.48, p = .138 |
| BP (n = 323, range 74–215)d | 130 (118–142) | 128 (116–144) | W= -0.47, p = .638 |
| High Risk Subsets | |||
| PROMIS among clients with poor health (n = 368)e | 33 (28–33) | 33 (28–37) | W = 6.9, p < .001 |
| MST among clients with malnutrition (n = 459)f | 3 (2–3) | 1 (0–2) | W= -15.230, p < .001 |
| BMI among clients with obesity (n = 163)g | 36 (33–41) | 36 (31–41) | W= -3.29, p < .001 |
| BP among clients with hypertension (n = 163)h | 142 (134–156) | 135 (122–149) | W= -5.08, p < .001 |
aPROMIS Scale v1.2 – Global Health Physical 2a. bMalnutrition Screening Tool. cBody mass index. dSystolic blood pressure in mmHg. ePROMIS T-score < 35. fMST ≥ 2. gBMI ≥ 30. hSystolic BP ≥ 130 mmHg. iIQR = inter-quartile range
Within-person results for the full sample (Fig. 2) showed that 30.5% of clients reported a statistically significant and clinically meaningful decrease in MST score from intake to follow-up. BMI values were stable over time for 70.6% of clients. Individuals experienced greater variability in blood pressure and physical health, with only 18.5% reporting stable blood pressure and 27.4% reporting stable physical health. Blood pressure increase was equally likely as blood pressure decrease, while physical health decline was slightly more likely than improvement (38.1% vs. 34.6%).
Fig. 2.
Within-person change over time: full sample. Thresholds for clinically significant change over time differ by measure: 2-point change in PROMIS; MST change from ≥ 2 to < 2; 2-point change in BMI; 5 mmHg change in systolic BP
When analyses were restricted to the clients who demonstrated the highest levels of risk at intake, we saw significant improvement across all health outcomes (Fig. 3). Almost half of clients with poor health at intake reported an increase of at least 2 points in PROMIS score, although most clients remained in the poor health category at follow-up. More than half of clients who started the program at high risk for malnutrition experienced a significant decrease in malnutrition risk (change from ≥ 2 to < 2). More than one-quarter of clients with obesity at intake reported a decrease of at least 2 BMI units, and 63% reported stability in BMI. Finally, 62% of clients with hypertension experienced at least 5 mmHg decrease in systolic blood pressure from intake to follow-up. Regression analyses confirmed that level of risk at intake was the factor most strongly associated with change over time – not physical health, sociodemographic characteristics, nor food insecurity – whereas cancer diagnosis was associated with increase in malnutrition risk over time (Appendix A).
Fig. 3.
Within-person change over time: high risk subset. High risk = PROMIS T-score < 35, MST ≥ 2, BMI ≥ 30, Systolic BP ≥ 130 mmHg. Thresholds for clinically significant change over time differ by measure: 2-point change in PROMIS; MST change from ≥ 2 to < 2; 2-point change in BMI; 5 mmHg change in systolic BP
Subsample with diabetes
Clients with diabetes are of special interest for MTM programs and represent 40% of MANNA’s client population. Clients with diabetes were more likely to be older, female, and Black compared to the full client population (n = 782; Table 3). Almost three-quarters of clients with diabetes lived with comorbid cardiovascular disease, and almost one-third lived with kidney disease. Half of clients with diabetes also lived with obesity and half had hypertensive blood pressure, showing greater prevalence compared to the full sample. Three-quarters of clients with diabetes did not have well-managed blood sugar as measured by hemoglobin A1C, with values ≥ 7%. Compared to the full sample, they were less likely to be at risk of malnutrition and reported slightly better health.
Table 3.
Characteristics of clients with diabetes at intake: percentages and frequencies
| Characteristic | Intake Sample | Longitudinal Subset with HbA1ca |
|---|---|---|
| n = 782 | n = 45 | |
| Demographics | ||
| Age 65+ | 51.7% (404) | 60.0% (27) |
| Female | 52.2% (408) | 62.2% (28) |
| Black | 66.4% (519) | 75.6% (34) |
| Hispanic | 9.0% (691) | 5.7% (2) |
| Medicaid | 33.9% (265) | 38.1% (16) |
| Diagnoses (not mutually exclusive) | ||
| Diabetes | 100% (782) | 100% (47) |
| HIV/AIDS | 4.3% (34) | 6.7% (3) |
| Cancer | 15.6% (122) | 15.6% (7) |
| Kidney disease | 32.5% (254) | 48.9% (22) |
| CVD | 72.4% (566) | 73.3% (33) |
| Nutrition | ||
| Food insecureb | 68.4% (535) | 84.4% (38) |
| Malnourishedc | 50.3% (393) | 42.2% (19) |
aHemoglobin A1C value provided by a healthcare provider at intake and follow-up. bClients who responded “sometimes true” or “often true” to one or both statements of the Hunger Vital Sign. cMST score ≥ 2
Hemoglobin A1C values were collected at intake (up to 90 days before program start) and follow up (2–7 months after program start). The sample of clients with diabetes and longitudinal hemoglobin A1C values was small and selected, representing just 6% of the clients with diabetes in the sample (n = 45, Table 3). These clients were even more likely to be older, female, and Black. They were also more likely to have kidney disease. Among this selected group of clients with diabetes and follow-up data, we observed a significant within-person decrease in hemoglobin A1C, with the group median decreasing from 8.3 to 7.7%. Risk of malnutrition was relatively low among clients with diabetes, and decreased over time (Table 4).
Table 4.
Change over time: clients with diabetes
| Outcome Variable | Aggregate Change | Within-person Change | |
|---|---|---|---|
| Median (IQR) at intakef | Median (IQR) at follow-up | Wilcoxon Signed Rank Test | |
| PROMISa (n = 372, range 23–50)a | 37 (33–41) | 37 (33–41) | W= -1.05, p = .296 |
| MSTb (n = 363, range 0–5)b | 1 (0–3) | 1 (0–2) | W= -6.20, p < .001 |
| BMIc (n = 173, range 16–78)c | 29 (24–36) | 29 (24–36) | W = − 0.06, p = .955 |
| BPd (n = 138, range 89–181)d | 131.5 (120–145) | 130.5 (120–148) | W = 0.02, p = .979 |
| Hemoglobin A1Ce (n = 45, range 5–16) | 8.3 (6.7–10.5) | 7.7 (6.0-9.3) | W= -2.79, p = .005 |
aPROMIS Scale v1.2 – Global Health Physical 2a. bMalnutrition Screening Tool. cBody mass index. dSystolic blood pressure in mmHg. eHemoglobin A1C, %. fIQR = inter-quartile range
Discussion
This study analyzed MANNA program records to evaluate whether participation in a medically tailored meal (MTM) program was associated with change in client health outcomes. The primary goal of MANNA’s MTM program is to enroll as many eligible clients as possible, as opposed to focusing on research. However, this study shows that rigorous assessment and evaluation of routinely collected health data is possible within normal operations of community-based organizations. This mirrors the research that can be achieved with electronic medical records in health care settings [50]. This framework may be feasible for similar MTM organizations seeking to conduct health outcomes evaluation within existing program structures and processes. Partnerships with local academic institutions or interested researchers can provide additional research capacity in the form of statistical consultation, student engagement, and IRB monitoring for responsible conduct of research and protection of human subjects when needed, as illustrated in this evaluation project.
The first objective was to describe the clients who use MTM services in the Greater Philadelphia area. Given that serious illness and nutritional risk were criteria for program eligibility, it was not surprising that many – but not all – of MANNA’s clients experienced substantially worse health than the general population [51]. Half of clients were living with comorbidity, 80% reported fair or poor health, and we observed disproportionate rates of diabetes, hypertension, and obesity. In addition, 66% of clients reported that they ran out of food or worried about it. This prevalence of food insecurity is substantially greater than the already high rates observed in the Greater Philadelphia Area [52, 53]. We did not observe direct associations between food insecurity and health outcomes; future studies should investigate how food insecurity interacts with serious illness to influence clients’ need for and response to MTM services.
The second objective was to measure change in client outcomes after several months of MTM services. Subjective client-reported outcomes measuring physical health (PROMIS) and malnutrition risk (MST) were the most robust client outcomes. MANNA staff conducted telephone interviews and collected data from 73% of the MTM program participants at intake and from 43% at follow-up. The subsets with follow-up data were similar to the full sample, suggesting that these results are not biased toward specific clients with follow-up data. Overall, clients’ physical health declined over time, but we observed improvement for clients who entered the program with poor health. These results corroborate studies focused on MTM interventions for clients with serious illnesses. A pilot RCT with HF patients who received MTMs showed a trend toward improved clinical scores in the Kansas City Cardiomyopathy Questionnaire and lower healthcare utilization (less 30-day HF readmissions and days hospitalized) [54]. Promising health outcomes including improved quality of life were also observed in a pilot RCT that provided MTMs to patients with cirrhosis [55].
Clinical measures reported by health care providers (BMI and blood pressure) were missing at higher rates, and we found evidence of sample selection– clients with provider-reported clinical values were older and more likely to have cancer or kidney disease, indicating that they may have had more contact with healthcare providers. Accordingly, our interpretations are cautious. We observed a high prevalence of stability in BMI, which is consistent with previous reports that BMI remained stable after 6 months of MTM services [33], and that for heart failure clients who were at high risk of hospital admission and food insecurity, MTM program participation for 3 months was not associated with substantial change in BMI or blood pressure [56]. Stability of BMI may support better health outcomes for clients with diagnoses often associated with malnutrition and muscle wasting such as cancer, kidney disease, and heart failure [57].
Results consistently showed that high risk at intake was the strongest predictor of change over time. Our data indicated that a significant number of clients who entered our program with hypertension dropped their blood pressure to pre-hypertensive values by program end. Lowering blood pressure in individuals decreases their risk of cardiovascular events such as heart attack and stroke [47]. Changes in PROMIS and BMI in high-risk groups were statistically significant and while the median of both did not change, over two-thirds of high risk clients maintained a stable BMI and 60% of clients improved or maintained PROMIS scores. This suggests that MTM programs may help clients stabilize their health and improve quality of life with serious illness, even if they will not become “well”. This concept is supported by a mixed-method RCT that included qualitative interviews with people living in the community with diabetes and food insecurity. Results indicated that access to MTMs improved quality of life and ability to manage diabetes by modeling what clients should eat, decreasing economic barriers to diabetes medications and supplies, and lowering stress associated with complicated dietary compliance [58].
The strongest results were related to risk of malnutrition, with more than half of malnourished clients shifting from high risk at intake to low risk at follow-up. This result builds upon previous studies that have shown improved nutritional status with home delivered meals [59]. The downstream effects of malnutrition – functional decline and higher rates of hospitalization, institutionalization, and mortality [60] – create an estimated $157 billion annual burden for the US economy [12]. By directly providing meals to clients, MANNA and other MTM organizations can decrease risk of malnutrition, positively impact related health outcomes, and reduce healthcare costs [61]. Decreasing malnutrition risk among clients with complex illness is an important, measurable, and achievable goal for MTM organizations.
The third objective focused on clients with diabetes and available blood glucose measures. 39% of clients with clinical data showed a 1% point improvement in hemoglobin A1C, which is within the range (0.3-2%) that is considered achievable with diet [3]. Median hemoglobin A1C values significantly decreased from 8.3 at intake to 7.7 at follow-up. For this sample of clients with complex risk factors and comorbidities, keeping values below 8% may be a treatment goal [62] to lower incidence of cardiovascular events and decrease healthcare costs [63]. This study corroborates the many previous studies that have shown a positive association between MTM program participation and hemoglobin A1C values [24, 64] and fits conclusions from other outcomes of this study – that MTM program participation may help clients better manage their illness.
The unavoidable limitation of this study design is the large amount of missing data because MANNA must rely on health data reported from providers. It is unknown whether the missing outcomes were measured and not provided to MANNA, or if they were not measured at all. Many routine medical and specialty care visits were delayed or forgone due to COVID transmission rates in 2020 and 2021; this likely impacted providers’ ability to report recent clinical and lab values [65]. Additional disease-specific laboratory values were collected for clients with specific illnesses including HIV/AIDS (viral load, CD4), renal disease (GFR, BUN, creatinine, potassium, phosphorus, albumin), and cardiovascular disease (LDL-C, HDL-C, triglycerides). However, the sample sizes were not sufficient for analyses. Given the high level of effort required to collect clinical data from providers and high levels of missing data, we suggest that other community-based MTM organizations consider alternative ways to obtain these outcomes in their assessment plans. Having direct access to health records [56] or data-sharing agreements with local or regional health information exchange (HIE) networks to access clinical and laboratory values are opportunities for more robust program evaluation. These approaches could also provide data on prescription medication use, which was not measured in this study.
CBOs can minimize bias from missing data in their program evaluations by focusing on client-reported outcomes that are easy to collect over the phone as part of normal service provision (i.e. during enrollment and recertification processes). The Hunger Vital Sign™ [38] and Malnutrition Screening Tool [40] are highly relevant to nutritional needs and easy to administer and interpret. The PROMIS tool (PROMIS® Scale v1.2 – Global Health Physical 2a) [42] was useful to understand the physical health status of clients. Additional PROMIS tools should be used in the future to measure changes in psychosocial outcomes, such as mental or social health, or other health-related quality of life outcomes, such as fatigue, energy, and functional capacity [66]. Organizations should also explore digital health monitors, tracking devices, and nutrition diaries that would allow clients to report their own health measures without need to travel to a healthcare appointment [67].
Finally, some study design elements are not ideal because they are driven by the organization’s function, not research. Access to a control group and long-term follow-up data would improve analyses of the impact of MTM programs. The change over time that we measured in our observational data could be “regression towards the mean,” whereby natural stabilization and more normal distribution at follow-up could be misinterpreted as an intervention effect [68]. This was illustrated in a recent RCT examining the impact of a grocery delivery intervention, in which decreasing hemoglobin A1C levels was observed among both the intervention and control groups [69]. The decline that we observed in malnutrition risk may represent recovery from a recent health event that prompted program referral, not necessarily the meals or the program itself. Similarly, the subtle aggregate decline in PROMIS scores may reflect the progression of serious illness and the older age of many MANNA clients, regardless of program involvement. Partnerships with local or regional HIE networks can also help to construct synthetic matched control groups when RCTs and waitlist studies are not feasible for CBOs.
Conclusions
Participating in MANNA’s MTM program is associated with significant decreases in malnutrition risk. It is also associated with significant improvement in blood pressure for clients who begin the program with stage 2 hypertension, and with reduced hemoglobin A1C for individuals with diabetes. BMI and PROMIS physical health scores remained stable or improved for most clients. Results indicate that MTMs are a valuable adjunct to medical care for improved management of nutrition-related conditions and/or malnutrition in community-dwelling older adults with complex illnesses. Future studies that evaluate health outcomes of functioning MTM programs would benefit from methodology that addresses missing data and a comparison group.
Acknowledgements
Not applicable.
Appendix A
Table A1.
Linear regression predicting change in score
| PROMIS Change | MST Change | BMI Change | SBP Change | |
|---|---|---|---|---|
| Age 65+ | ns | ns | ns | ns |
| Female gender | ns | ns | ns | ns |
| Black race | ns | ns | ns | ns |
| Hispanic ethnicity | ns | ns | ns | ns |
| Medicaid insurance | ns | ns | ns | ns |
| Food insecurity | ns | ns | ns | ns |
| Poor health | B = 5.66*** | ns | ns | ns |
| High risk of malnutrition | ns | B= -1.94*** | ns | ns |
| Obese BMI | -- | -- | ns | -- |
| Hypertensive SBP | -- | -- | -- | B= -20.63*** |
| HIV/AIDS Dx | ns | ns | ns | ns |
| CVD Dx | ns | ns | ns | ns |
| Kidney Dx | ns | ns | ns | ns |
| Diabetes Dx | ns | ns | ns | ns |
| Cancer Dx | ns | B = 0.29* | ns | ns |
| Constant | -1.2 | 0.20* | 0.62 | 9.2 |
| R2 | 0.154 | 0.37 | -0.215 | 0.46 |
| model sig. | F = 8.52*** | F = 28.2*** | F = 0.96, p = .496 | F = 4.27*** |
| n | 621 | 610 | 293 | 235 |
Note. PROMIS = Scale v1.2 – Global Health Physical 2a. MST = Malnutrition Screening Tool. BMI = body mass index. SBP = systolic blood pressure. Dx = diagnosis. B = unstandardized beta. ns = not significant. -- = not included in the model. R2 = model fit. F = test statistic. n = sample size. *p < .01. ***p < .001
Table A2.
Logistic regression predicting improvement
| PROMIS Improve | MST Improve | BMI Improve | SBP Improve | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age 65+ | ns | ns | ns | ns |
| Female gender | ns | ns | ns | ns |
| Black race | ns | ns | ns | ns |
| Hispanic ethnicity | ns | ns | ns | ns |
| Medicaid insurance | ns | ns | ns | ns |
| Food insecurity | ns | ns | ns | ns |
| Poor health | 3.82 (2.66–5.50) | ns | ns | ns |
| High risk of malnutrition | ns | 16.95 (11.15–25.77) | ns | ns |
| Obese BMI | -- | -- | 3.21 (1.58–6.56) | -- |
| Hypertensive SBP | -- | -- | -- | 7.50 (3.98–14.10) |
| HIV/AIDS Dx | ns | ns | ns | ns |
| CVD Dx | ns | ns | ns | ns |
| Kidney Dx | ns | ns | ns | ns |
| Diabetes Dx | ns | ns | ns | ns |
| Cancer Dx | ns | ns | ns | ns |
| Nagelkerke R2 | 0.14 | 0.57 | 0.14 | 0.26 |
| H&L test | X2 = 17.54, p = .025 | X2 = 4.08, p = .850 | X2 = 3.54, p = .896 | X2 = 7.54, p = .480 |
| n | 621 | 609 | 293 | 235 |
Note. PROMIS = Scale v1.2 – Global Health Physical 2a. MST = Malnutrition Screening Tool. BMI = body mass index. SBP = systolic blood pressure. Dx = diagnosis. OR = odds ratio. CI = confidence interval. ns = not significant. -- = not included in the model. R2 = model fit. n = sample size. X2 = chi-square value. H&L = Hosmer and Lemeshow test
Author contributions
Conceptualization, J.A.H. and A.G.C.; methodology, J.A.H., A.G.C. and J.M.S.; validation, J.A.H., A.G.C., J.M.S., and I.R.N.; formal analysis, J.M.S.; investigation, J.A.H., A.G.C., V.S.D., K.A.B., J.S.E., M.R.J., J.Z., D.A. and I.R.N.; resources, J.A.H., A.G.C. and J.M.S.; data curation, A.G.C. and J.M.S.; writing—original draft preparation, J.A.H., A.G.C., and J.M.S.; writing—review and editing, J.A.H., J.M.S., A.G.C., S.D., N.L., T.C., V.S.D., K.A.B., J.S.E., M.R.J., J.Z., D.A. and I.R.N.; visualization, J.A.H., A.G.C., and J.M.S.; supervision, J.A.H., A.G.C., and J.M.S.; project administration, J.A.H. and A.G.C. All authors read and approved the manuscript.
Funding
This research received no external funding.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due patient privacy protections but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Saint Joseph’s University (1537925-6, May 1, 2023). Patient consent was waived because the research involved no more than minimal risk to the subjects and the waiver did not adversely affect the rights and welfare of the subjects. MANNA’s clients are informed of potential use of client information for research in the Notice of Privacy Practices.
Consent for publication
Not applicable.
Competing interests
The authors J.A.H. and A.G.C. are employed by MANNA. The authors V.S.D., K.A.B., J.S.E., M.R.J., J.Z., and D.A. each received a Reid B. Reames Fellowship and modest stipend from MANNA for participation in research projects. The author J.M.S. has received payment from MANNA as a statistical consultant. The author I.R.N. has no conflicts of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica M. Sautter, Email: JSautter1@sju.edu
Jule Anne Henstenburg, Email: JHenstenburg@mannapa.org.
References
- 1.MANNA [Internet]. [cited 2024 Feb 16]. Our Mission. https://mannapa.org/about/power-of-food-as-medicine/
- 2.Cleveland Clinic [Internet]. 2024 [cited 2024 Mar 4]. Medical Nutrition Therapy. https://my.clevelandclinic.org/health/treatments/medical-nutrition-therapy-mnt
- 3.American Diabetes Association Professional Practice Committee. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care. 2023;47(Supplement_1):S77–110. [DOI] [PMC free article] [PubMed]
- 4.Lichtenstein AH, Appel LJ, Vadiveloo M, Hu FB, Kris-Etherton PM, Rebholz CM et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2021 Dec 7 [cited 2024 Apr 2];144(23). https://www.ahajournals.org/doi/10.1161/CIR.0000000000001031 [DOI] [PubMed]
- 5.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3):S1–107. [DOI] [PubMed] [Google Scholar]
- 6.Dietary, Guidelines for Americans. 2020–2025 [Internet]. US Department of Agriculture and US Department of Health and Human Services; 2020 Dec [cited 2024 Mar 4]. Report No.: 9th Edition. Available from: dietaryguidelines.gov.
- 7.Boersma P. Prevalence of Multiple Chronic Conditions Among US Adults, 2018. Prev Chronic Dis [Internet]. 2020 [cited 2024 Jun 11];17. https://www.cdc.gov/pcd/issues/2020/20_0130.htm [DOI] [PMC free article] [PubMed]
- 8.Gropper SS. The Role of Nutrition in Chronic Disease. Nutrients. 2023;15(3):664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr Edinb Scotl. 2008;27(1):5–15. [DOI] [PubMed] [Google Scholar]
- 10.Crichton M, Craven D, Mackay H, Marx W, de van der Schueren M, Marshall S. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: associations with geographical region and sex. Age Ageing. 2019;48(1):38–48. [DOI] [PubMed] [Google Scholar]
- 11.Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enter Nutr. 2014;38(2):186–95. [DOI] [PubMed] [Google Scholar]
- 12.Snider JT, Linthicum MT, Wu Y, LaVallee C, Lakdawalla DN, Hegazi R et al. Economic Burden of Community-Based Disease‐Associated Malnutrition in the United States. J Parenter Enter Nutr [Internet]. 2014 Nov [cited 2024 Apr 2];38(2S). https://aspenjournals.onlinelibrary.wiley.com/doi/10.1177/0148607114550000 [DOI] [PubMed]
- 13.Supplemental Nutrition Assistance Program (SNAP). | Food and Nutrition Service [Internet]. [cited 2024 Jun 12]. https://www.fns.usda.gov/snap/supplemental-nutrition-assistance-program
- 14.Nutrition Services | ACL. Administration for Community Living [Internet]. [cited 2024 Jun 12]. http://acl.gov/programs/health-wellness/nutrition-services
- 15.Policy Priorities |. Food is Medicine Coalition [Internet]. [cited 2024 Jun 12]. https://fimcoalition.org/policy/policy-priorities/
- 16.Berkowitz SA, Terranova J. Medically Tailored Meals to Address the Health Consequences of Food Insecurity. N Engl J Med. 2024;390(9):775–6. [DOI] [PubMed] [Google Scholar]
- 17.Food is Medicine [Internet]. The Rockefeller Foundation. [cited 2024 Apr 2]. https://www.rockefellerfoundation.org/initiative/food-is-medicine/
- 18.Food Is Medicine. @ Tufts University [Internet]. [cited 2024 Mar 7]. What is Food is Medicine? https://tuftsfoodismedicine.org/
- 19.USDA ERS –. 2024 Farm Bill [Internet]. [cited 2024 Sep 17]. https://www.ers.usda.gov/topics/farm-bill/2024-farm-bill/
- 20.Older Americans Act. | ACL Administration for Community Living [Internet]. [cited 2024 Sep 17]. http://acl.gov/about-acl/authorizing-statutes/older-americans-act
- 21.Kurt Hager C, Kummer A, Lewin-Zwerdling Z, Li. Food is Medicine Research Action Plan [Internet]. Aspen Institute; 2024 Apr [cited 2024 Jun 12]. https://aspenfood.org/food-is-medicine/
- 22.Go AS, Tan TC, Horiuchi KM, Laws D, Ambrosy AP, Lee KK, et al. Effect of Medically Tailored Meals on Clinical Outcomes in Recently Hospitalized High-Risk Adults. Med Care. 2022;60(10):750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boxer R, Drace ML, Kelly C, Robinson R, Schwartz P, Ausiello J, et al. Comparing two durations of medically tailored meals posthospitalization: A randomized clinical trial. J Hosp Med. 2023;18(7):576–87. [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz SA, Delahanty LM, Terranova J, Steiner B, Ruazol MP, Singh R, et al. Medically Tailored Meal Delivery for Diabetes Patients with Food Insecurity: a Randomized Cross-over Trial. J Gen Intern Med. 2019;34(3):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurvey J, Rand K, Daugherty S, Dinger C, Schmeling J, Laverty N. Examining health care costs among MANNA clients and a comparison group. J Prim Care Community Health. 2013;4(4):311–7. [DOI] [PubMed] [Google Scholar]
- 26.Berkowitz SA, Terranova J, Hill C, Ajayi T, Linsky T, Tishler LW, et al. Meal Delivery Programs Reduce The Use Of Costly Health Care In Dually Eligible Medicare And Medicaid Beneficiaries. Health Aff (Millwood). 2018;37(4):535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkowitz SA, Terranova J, Randall L, Cranston K, Waters DB, Hsu J. Association Between Receipt of a Medically Tailored Meal Program and Health Care Use. JAMA Intern Med. 2019;179(6):786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hager K, Cudhea FP, Wong JB, Berkowitz SA, Downer S, Lauren BN, et al. Association of National Expansion of Insurance Coverage of Medically Tailored Meals With Estimated Hospitalizations and Health Care Expenditures in the US. JAMA Netw Open. 2022;5(10):e2236898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Food is Medicine Coalition [Internet]. [cited 2024 Apr 2]. Our Model. https://fimcoalition.org/about-fimc/our-model/
- 30.Mozaffarian D, Aspry KE, Garfield K, Kris-Etherton P, Seligman H, Velarde GP, et al. Food Is Medicine Strategies for Nutrition Security and Cardiometabolic Health Equity: JACC State-of-the-Art Review. J Am Coll Cardiol. 2024;83(8):843–64. [DOI] [PubMed] [Google Scholar]
- 31.Jule Anne Henstenburg. Developing a framework to assess outcomes and impacts of medically-tailored food and nutrition services offered by the Metropolitan Area Neighborhood Nutrition Alliance (MANNA). [Philadelphia, PA]: University of the Sciences; 2018. [Google Scholar]
- 32.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henstenburg JA, Parvanta C, Pontiggia L, Daugherty S, Laverty N. Food Is Medicine: Providing Medically Tailored Meals to Community Members with Disease-associated Nutritional Risk Supports Stable BMI and Decreased Hospitalization (P12-005-19). Curr Dev Nutr. 2019;3(Suppl 1):nzz035. P12-005-19.
- 34.MANNA [Internet]. [cited 2024 Aug 22]. Apply for MANNA Services. https://mannapa.org/services/apply-for-manna-services/
- 35.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inf. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2021. [Google Scholar]
- 38.Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and Validity of a 2-Item Screen to Identify Families at Risk for Food Insecurity. Pediatrics. 2010;126(1):e26–32. [DOI] [PubMed] [Google Scholar]
- 39.Gundersen C, Engelhard EE, Crumbaugh AS, Seligman HK. Brief assessment of food insecurity accurately identifies high-risk US adults. Public Health Nutr. 2017;20(8):1367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458–64. [DOI] [PubMed] [Google Scholar]
- 41.Skipper A, Coltman A, Tomesko J, Charney P, Porcari J, Piemonte TA, et al. Position of the Academy of Nutrition and Dietetics: Malnutrition (Undernutrition) Screening Tools for All Adults. J Acad Nutr Diet. 2020;120(4):709–13. [DOI] [PubMed] [Google Scholar]
- 42.Hays RD, Schalet BD, Spritzer KL, Cella D. Two-item PROMIS® global physical and mental health scales. J Patient-Rep Outcomes. 2017;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HealthMeasures [Internet]. [cited 2024 Apr 2]. PROMIS. https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis
- 44.Hays RD, Spritzer KL, Thompson WW, Cella D. U.S. General Population Estimate for Excellent to Poor Self-Rated Health Item. J Gen Intern Med. 2015;30(10):1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terwee CB, Peipert JD, Chapman R, Lai JS, Terluin B, Cella D, et al. Minimal important change (MIC): a conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual Life Res. 2021;30(10):2729–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panza E, Kip KE, Venkatakrishnan K, Marroquin OC, Wing RR. Changes in body weight and glycemic control in association with COVID-19 Shutdown among 23,000 adults with type 2 diabetes. Acta Diabetol. 2023;60(6):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canoy D, Nazarzadeh M, Copland E, Bidel Z, Rao S, Li Y, et al. How Much Lowering of Blood Pressure Is Required to Prevent Cardiovascular Disease in Patients With and Without Previous Cardiovascular Disease? Curr Cardiol Rep. 2022;24(7):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, et al. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J Acad Nutr Diet. 2017;117(10):1659–79. [DOI] [PubMed] [Google Scholar]
- 49.Academy of Nutrition and Dietetics. Evidence Analysis Library [Internet]. [cited 2024 Apr 2]. DM: Effectiveness of MNT Provided by an RD/RDN. http://www.andeal.org/topic.cfm?menu=5305&pcat=5491&cat=5161
- 50.Nguyen J, McNaughton C, Sautter J. Documenting limited health literacy in a clinical setting. PEC Innov. 2022;1:100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.2021 Health of the City. Philadelphia’s Community Health Assessment. City of Philadelphia Department of Public Health; 2021.
- 52.Differentials and predictors of food insecurity among Federally Qualified. Health Center target populations in Philadelphia: a cross-sectional study | BMC Public Health | Full Text [Internet]. [cited 2024 Jun 13]. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-16208-3 [DOI] [PMC free article] [PubMed]
- 53.Food Insecurity Rates for Our Service. Area - Philabundance [Internet]. [cited 2024 Jun 13]. https://www.philabundance.org/food-insecurity-rates-for-our-service-area/
- 54.Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ Heart Fail. 2018;11(8):e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tapper EB, Baki J, Nikirk S, Hummel S, Asrani SK, Lok AS. Medically tailored meals for the management of symptomatic ascites: the SALTYFOOD pilot randomized clinical trial. Gastroenterol Rep. 2020;8(6):453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belak L, Owens C, Smith M, Calloway E, Samnadda L, Egwuogu H, et al. The impact of medically tailored meals and nutrition therapy on biometric and dietary outcomes among food-insecure patients with congestive heart failure: a matched cohort study. BMC Nutr. 2022;8(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berkowitz SA, Shahid NN, Terranova J, Steiner B, Ruazol MP, Singh R, et al. I was able to eat what I am supposed to eat-- patient reflections on a medically-tailored meal intervention: a qualitative analysis. BMC Endocr Disord. 2020;20(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleury S, Tronchon P, Rota J, Meunier C, Mardiros O, Van Wymelbeke-Delannoy V et al. The Nutritional Issue of Older People Receiving Home-Delivered Meals: A Systematic Review. Front Nutr [Internet]. 2021 Mar 4 [cited 2024 Jun 13];8. https://www.frontiersin.org/articles/10.3389/fnut.2021.629580 [DOI] [PMC free article] [PubMed]
- 60.Norman K, Haß U, Pirlich M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients. 2021;13(8):2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guide to Evidence for Health-Related Social Needs Interventions. 2022 Update | Playbook [Internet]. 2022 [cited 2024 Jun 13]. https://bettercareplaybook.org/resources/guide-evidence-health-related-social-needs-interventions-2022-update
- 62.American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Supplement_1):S111–25. [DOI] [PMC free article] [PubMed]
- 63.Bansal M, Shah M, Reilly B, Willman S, Gill M, Kaufman FR. Impact of Reducing Glycated Hemoglobin on Healthcare Costs Among a Population with Uncontrolled Diabetes. Appl Health Econ Health Policy. 2018;16(5):675–84. [DOI] [PubMed] [Google Scholar]
- 64.Gao Y, Yang A, Zurbau A, Gucciardi E. The Effect of Food is Medicine Interventions on Diabetes-related Health Outcomes Among Low-income and Food-insecure Individuals: A Systematic Review and Meta-analysis. Can J Diabetes. 2023;47(2):143–52. [DOI] [PubMed] [Google Scholar]
- 65.Hernandez J, Batio S, Lovett RM, Wolf MS, Bailey SC. Missed Healthcare Visits During the COVID-19 Pandemic: A Longitudinal Study. J Prim Care Community Health. 2024;15:21501319241233869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.List of Adult Measures [Internet]. [cited 2024 Jun 13]. https://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis/list-of-adult-measures
- 67.Challenges. and opportunities for better nutrition science—an essay by Tim Spector and Christopher Gardner | The BMJ [Internet]. [cited 2024 Jun 13]. https://www.bmj.com/content/369/bmj.m2470 [DOI] [PMC free article] [PubMed]
- 68.Thomas DM, Clark N, Turner D, Siu C, Halliday TM, Hannon BA, et al. Best (but oft-forgotten) practices: identifying and accounting for regression to the mean in nutrition and obesity research. Am J Clin Nutr. 2020;111(2):256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyle J, Alsan M, Skelley N, Lu Y, Cawley J. Effect of an Intensive Food-as-Medicine Program on Health and Health Care Use: A Randomized Clinical Trial. JAMA Intern Med. 2024;184(2):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due patient privacy protections but are available from the corresponding author on reasonable request.