Abstract
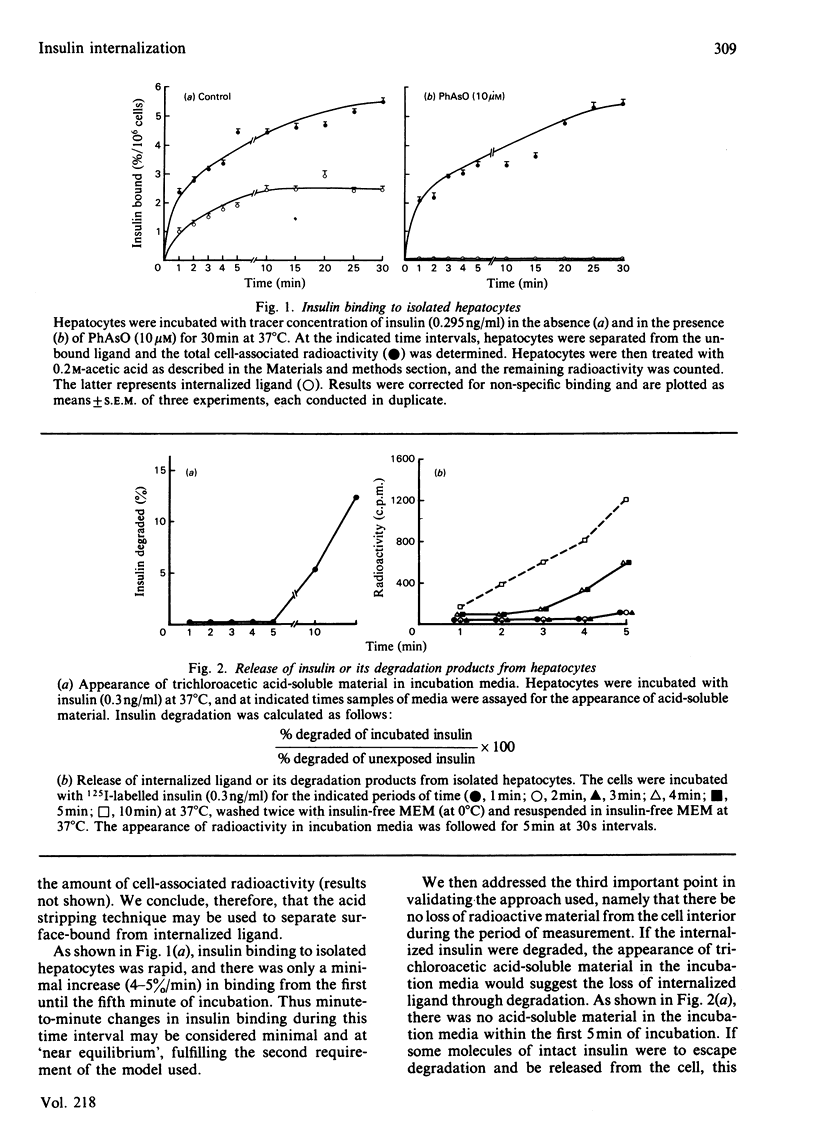
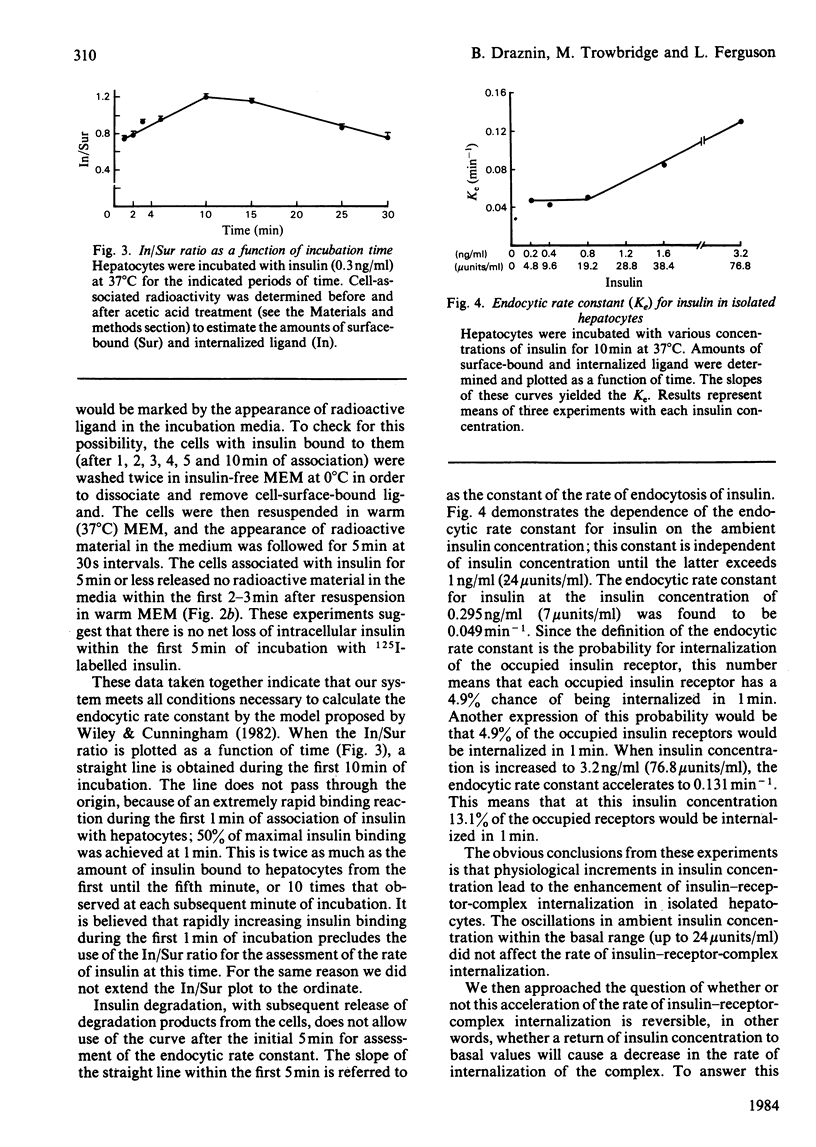
We studied internalization of 125I-labelled insulin in isolated rat hepatocytes. Using the acidification technique, we were able to dissociate the ligand from its cell-surface receptors, and thus to separate internalized from surface-bound insulin. Because during the first 5 min of incubation of 125I-labelled insulin with freshly isolated hepatocytes there is no loss of internalized label, the ratio of the amount of internalized ligand to the amount of cell-surface-bound ligand may serve as an index of insulin internalization. Within the first 10 min of insulin's interaction with hepatocytes, the plot of the above ratio as a function of time yields a straight line. The slope of this line is referred to as the endocytic rate constant (Ke) for insulin and denotes the probability with which the insulin-receptor complex is internalized in 1 min. At the insulin concentration of 0.295 ng/ml, the Ke is 0.049 min-1. It is independent of insulin concentration until the latter exceeds 1 ng/ml. At the insulin concentration of 3.2 ng/ml, the Ke accelerates to 0.131 min-1. With the Ke being the probability of insulin-receptor-complex internalization, 4.9% of occupied insulin receptors will be internalized in 1 min at an insulin concentration of 0.295 ng/ml, and 13.1% of occupied insulin receptors will be internalized in 1 min at 3.2 ng/ml. When the insulin concentration decreases from 3.2 to 0.3 ng/ml, the Ke decreases accordingly. The half-time of occupied receptor internalization is 15.4 min at the lower insulin concentration and 5.3 min at the higher insulin concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D., Jr, Prince M., Tsai P., Johnson C., Lotan R., Rubenstein A. H., Olefsky J. M. Insulin binding, internalization, and receptor regulation in cultured human fibroblasts. Am J Physiol. 1981 Sep;241(3):E251–E260. doi: 10.1152/ajpendo.1981.241.3.E251. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Guzelian P. S., Small M. E. Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology. 1978 Aug;103(2):548–553. doi: 10.1210/endo-103-2-548. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Rubin J. S. Polypeptide growth factors: some structural and mechanistic considerations. J Supramol Struct. 1980;14(2):183–199. doi: 10.1002/jss.400140207. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Muller G., Glennon J. A. Insulin processing by the liver. J Biol Chem. 1982 Jul 25;257(14):8459–8466. [PubMed] [Google Scholar]
- Draznin B., Solomons C. C., Toothaker D. R., Sussman K. E. Energy-dependent steps in insulin-hepatocyte interaction. Endocrinology. 1981 Jan;108(1):8–17. doi: 10.1210/endo-108-1-8. [DOI] [PubMed] [Google Scholar]
- Draznin B., Trowbridge M. Inhibition of intracellular proteolysis by insulin in isolated rat hepatocytes. Possible role of internalized hormone. J Biol Chem. 1982 Oct 25;257(20):11988–11993. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Fan J. Y., Orci L. Receptor mediated endocytosis of polypeptide hormones: mechanism and significance. Metabolism. 1982 Jul;31(7):664–669. doi: 10.1016/0026-0495(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P., LeCam A., Orci L. Intracellular translocation of iodine-125-labeled insulin: direct demonstration in isolated hepatocytes. Science. 1978 May 19;200(4343):782–785. doi: 10.1126/science.644321. [DOI] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Kasuga M., Kahn C. R., Hedo J. A., Van Obberghen E., Yamada K. M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6917–6921. doi: 10.1073/pnas.78.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Krupp M., Lane M. D. On the mechanism of ligand-induced down-regulation of insulin receptor level in the liver cell. J Biol Chem. 1981 Feb 25;256(4):1689–1694. [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Characterization of insulin-induced receptor loss and evidence for internalization of the insulin receptor. Diabetes. 1981 Sep;30(9):746–753. doi: 10.2337/diab.30.9.746. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M., Kao M. Surface binding and rates of internalization of 125I-insulin in adipocytes and IM-9 lymphocytes. J Biol Chem. 1982 Aug 10;257(15):8667–8673. [PubMed] [Google Scholar]
- Olefsky J. M., Marshall S., Berhanu P., Saekow M., Heidenreich K., Green A. Internalization and intracellular processing of insulin and insulin receptors in adipocytes. Metabolism. 1982 Jul;31(7):670–690. doi: 10.1016/0026-0495(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman K. E., Mehler P. S., Leitner J. W., Draznin B. Role of the secretion vesicle in the transport of receptors: modulation of somatostatin binding to pancreatic islets. Endocrinology. 1982 Jul;111(1):316–323. doi: 10.1210/endo-111-1-316. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Vigneri R., Pliam N. B., Cohen D. C., Pezzino V., Wong K. Y., Goldfine I. D. In vivo regulation of cell surface and intracellular insulin binding sites by insulin. J Biol Chem. 1978 Nov 25;253(22):8192–8197. [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]


