Abstract
Aim
Studies have shown that renal hypertrophy seen in experimental hyperthyroidism induced by thyroxine (T4) is due to angiotensin (Ang) II. However, other renal effects of Ang II in experimental hyperthyroidism have not been investigated. In addition, Ang 1–7 is believed to be protective against renal injury, but its possible role in thyroxine-induced renal injury is not known. The aim of this study is to elaborate the role of Ang II in thyroxine-induced renal injury and the possible protective role of Ang 1–7. We hypothesize that Ang 1–7 will be as protective against thyroxine-induced renal injury as the use of an ACE inhibitor or an Ang II receptor blocker.
Methods
Adult Sprague Dawley rats were used in this study and were divided into 5 groups: (1) Control (treated with vehicle), (2) Treated with thyroxine (T4, 100 µg/kg), (3) Treated with T4 and Ang 1–7 (500 µg/kg), (4) Treated with T4 and captopril (20 mg/kg), and (5) Treated with T4 and losartan (10 mg/kg). Parameters tested after fourteen days of treatment were creatinine clearance, protein excretion rate, glomerular volume, renal ACE1 and ACE2 protein expression. Data were compared using One-way-ANOVA followed by Tukey’s HSD post hoc test.
Results
Thyroxine caused glomerular hypertrophy and proteinuria but had no effect on glomerular filtration rate (GFR). Glomerular hypertrophy was prevented by losartan and captopril, but not by Ang 1–7. Captopril and losartan had no effect on GFR; however, Ang 1–7 caused an increase in GFR in T4-treated rats. The increase in protein excretion rate was prevented by losartan but not by captopril or Ang 1–7. Renal expression of ACE1 protein was not altered in any of the treatment groups except in captopril treated rats were ACE1 expression was increased. Renal ACE2 protein expression was only increased in T4-losartan-treated rats and not affected by any of the other treatments.
Conclusion
We conclude that losartan was more protective than captopril against thyroxine-induced renal changes while Ang 1–7 offered no protection.
Keywords: Thyroid, Thyroxine, Kidney, Ang 1–7, Losartan
Introduction
Treatment of experimental animals with thyroxine (T4) has been shown to reproduce the effects of hyperthyroidism such as pulmonary hypertension [1, 2], cardiac hypertrophy, hypertension [3, 4], and weight loss [3]. Renal hypertrophy [5–7], glomerular growth and mesangial expansion have also been observed in T4-treated rats [8].
Previous studies in rats have shown that thyroxine-induced cardiac hypertrophy involves activation of cardiac renin-angiotensin system (RAS) [9]. All components of RAS are expressed in the kidney [6, 10] and Ang II is locally produced. Studies have shown that thyroxine caused renal hypertrophy and increased renal Ang II [5] and angiotensin converting enzyme (ACE) activity and mRNA expression [6]. Treatment with losartan, a selective angiotensin receptor 1 (AT1R)-receptor antagonist, reduced renal hypertrophy, indicating a significant role for Ang II in thyroxine-induced renal growth. However, the role of Ang II in thyroxine-induced renal functional changes was not explored.
In the classical pathway, Ang II is produced from angiotensinogen mainly by angiotensin converting enzyme (ACE1). In this pathway, renin converts angiotensinogen to angiotensin I which is then converted to Ang II by ACE1. Ang II can also be produced from non-ACE pathways involving chymases [11]. In addition to the classical/alternate pathways of Ang II generation in the kidney, another pathway leading to the generation of Ang 1–7 has also been reported where ACE2 converts Ang II into Ang 1–7 [12]. Even though Ang II is the main substrate for the generation of Ang 1–7, it has been reported that Ang 1–7 can also be generated by a direct action of neprilysin, a neuropeptidase on angiotensin I [11]. Ang 1–7 is an endogenous ligand for the G protein-coupled receptor Mas (MasR) [13] (Santos et al., 2003) and all the components of ACE2-angiotensin-(1–7)- MasR axis are expressed in the kidney [14].
Ang 1–7 plays an opposing role to Ang II and hence could play a protective role against renal injury [15, 16]. The possible role of Ang 1–7 in hyperthyroidism-induced hypertrophy was studied in the rat heart, where cardiac ACE2 activity and protein levels of Ang 1–7 and MasR were increased [17]. Senger et al. (2017) [18] showed that transgenic rats overexpressing Ang 1–7 do not develop cardiac hypertrophy when treated with thyroxine; and when the rats were treated with A779, a MasR blocker, cardiac hypertrophy developed. These studies show that hyperthyroidism is associated with an upregulation of Ang 1–7, ACE2 activity and MasR in the cardiac muscle providing a counter-regulatory system against thyroxine-induced cardiac hypertrophy. The possible protective effect of Ang 1–7 has been studied in renal injury associated with diabetes where some studies show protection against renal injury [19–21], and others show more exacerbation or lack of protection [22–24]. However, the role of ACE2-Ang 1-7-MasR axis in thyroxine-induced renal changes is not known. The aim of this study is to further elaborate the contribution of Ang II to renal changes in experimental hyperthyroidism and to identify the possible protective role of Ang 1–7 against these changes. Therefore, we hypothesize that Ang 1–7 will be as protective as an ACE inhibitor or an Ang II receptor blocker against thyroxine-induced renal functional and structural changes.
Methods
Animals
Adult male Sprague-Dawley rats weighing about 200–250 g were used in this study. The rats were placed in a room with a 12:12 light dark cycle and kept at 22.3 ± 0.3oC and 31.2 ± 0.8% humidity. The rats had free access to water and standard chow (801151, Special Diets Services, UK). All animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals and all experimental protocols used in this study were approved by the Health Sciences Research Ethics committee, Health Sciences Centre. Hyperthyroidism was induced by treating the rats with thyroxine (100 µg/kg) intraperitoneally daily for 14 days. Thyroxine-treated rats were randomly divided into 4 groups (n = 6–8 per group): (1) Treated with thyroxine (T4), (2) Treated with T4 + the ACE1 inhibitor captopril (20 mg/Kg i.p.), (3) Treated with T4 + the AT1R blocker losartan (10 mg/Kg, i.p.), (4) Treated with T4 + Ang 1–7 (500 µg/Kg, i.p.) [20, 25]. The control group (C, n = 6–8) was treated with saline.
Data collection
The rats were euthanized at day 15 post treatment. Twenty-four hours before sacrifice, rats were placed into metabolic cages, and 24 hours’ urine samples were collected, and urine volume (V, ml/24hrs) was measured. The rats were then anesthetized with urethane (94300, Sigma-Aldrich, USA) at a dose of 1.3–1.5 g/kg, i.p. [26] then blood samples were collected from the right ventricle, centrifuged at 3000 RPM for 5 min and stored at -80 °C. Both kidneys were removed. The right kidney was removed, then a 5 mm-thick transverse section was cut and placed in 4% paraformaldehyde then fixed in paraffin wax for morphometric studies. The left kidney was snap frozen in liquid nitrogen and stored at -80oC to be used for Western blotting.
Assessment of renal function
Creatinine clearance as an estimate of glomerular filtration rate
Creatinine concentration in serum and 24-hour urine samples was measured using Colorimetric Rat Creatinine kit (#80340 by CrystalChem, USA). Creatinine clearance (ml/min) was calculated as (UCr X V)/PCr where UCr is the concentration of creatinine in urine (mg/ml), V = urine flow rate (ml/min) and PCr = Concentration of creatinine in serum in mg/ml. Clearance was then corrected to grams of body weight.
Protein excretion rate
Urinary protein concentration was measured using Coomassie Plus (Bradford) assay kit (# PI23200, ThermoFisher Scientific, USA). Protein excretion rate (PER) was calculated as: PER = Uprot X V, where Uprot= Concentration of proteins in mg /24-hour urine samples, V = Urine flow rate in ml/24 hours.
Assessment of glomerular morphometry
Four µm-thick paraffin sections were cut and mounted on APES-coated slides for morphometric studies. To assess the glomerular area and mesangial matrix area the sections were stained with periodic acid Schiff stain [27]. The images were viewed and captured using Axio microscope, then image analysis was performed using ImageJ software. Fifteen to twenty glomeruli were studied per rat. Glomerular volume was calculated from the glomerular tuft area as described earlier [27].
Measurement of Ang II and Ang 1–7 in sera and kidneys of control and thyroxine-treated rats
Levels of Ang II in serum were determined using ELISA kit (# E-EL-R1430, ElabScience, USA) and, Ang 1–7 was measured in serum using ELISA kit (#OKEH02599, Aviva Systems Biology, USA). As for the kidney samples, they were homogenized in sodium phosphate buffer (1 g/10 ml) and protease inhibitors with stainless steel homogenization beads (#E-8045, Next Advance, USA). Homogenization of tissues was performed at 12,000 rpm for 4 min. The homogenates were then centrifuged at 5000 rpm for 30 min at 4oC and the supernatant was used without dilution for the determination of Ang II and Ang 1–7 as described by the manufacturer.
The effect of thyroxine treatment on renal RAS components
The effect of T4, T4-Ang 1-, T4-Captopril, and T4-losartan on renal expression of ACE1 and ACE2 using Western blotting
Western blotting was used to assess the renal protein expression of ACE 1 and ACE2 in all treatment groups. Renal tissue was homogenized in ice-cold tris homogenization buffer with protease inhibitors (Pierce Protease inhibitor tablets, SA 2286912, ThermoFisher Scientific, USA) using stainless steel homogenization beads (E-8045, Next Advance, USA). Primary antibodies used were rabbit monoclonal anti-ACE1 antibody (ab254222, Abcam, USA) at 1:250 dilution and rabbit polyclonal anti-rat ACE2 antibody (C717324, LifeSpan Biosciences, USA) at 1:200 dilution.
The effect of thyroxine treatment on protein expression of AT1R, AT2R and MasR in renal membranes using Western blotting
Western blotting was used to assess the protein expression of AT1R, AT2R and MasR in renal membrane fractions from control and thyroxine-treated rats. To prepare membrane fractions, renal homogenates were centrifuged for 10 min at 4oC at 6000xg. Then the supernatant was centrifuged at 4oC at 150000xg for 60 min and the pellet was used for Western blotting [28].
Primary antibodies used were rabbit polyclonal AT1R antibody (ab18801, Abcam, USA), at a 1:500 dilution, AT2R (ab19134, Abcam, USA) at a 1:200 dilution, anti-MAS1L antibody (ab200685, Abcam, USA) at 1:250 dilution.
Western blotting protocol
Samples were incubated with X1 Laemmli buffer (#161–0747, BioRad, USA) and homogenization buffer at 37oC for 35 min. Fifty µg of proteins was loaded on stain-free gel (Mini-protein tgx stain-free gels: 4568096, BioRad, USA) and electrophoresis was run at 200 V for 30–40 min after which the proteins were transferred to nitrocellulose transfer membranes (trans-blot turbo transfer pack cat no: 1704158, BioRad) at 25 V and 1.0 Amp for 30 min and visualized on ChemiDoc MP imaging system from BioRad to measure total proteins loaded per sample. After washing, the membranes were incubated with primary antibodies at 4oC overnight.
The membranes were then blocked using 1x Tris buffered saline (TBS) (1% casein blocker: #161078, BioRad, USA). Secondary antibodies, rabbit polyclonal antibody for ACE1, ACE2, AT1R, AT2R and guineapig polyclonal antibody for MasR, were then added at a dilution (1:10,000). Negative controls with elimination of the primary antibody were run for every western blot. Heart tissue was used as a positive control where specified. The bands were visualized using chemiluminescence on Chemidoc Imager, then analyzed using Image Lab software from BioRad. The expression of each protein was taken as a ratio of band density of the protein of interest to the cumulative densities of all proteins separated in the gel [29].
The effect of thyroxine on renal expression of nephrin using western blotting
The effect of thyroxine treatment on the expression of nephrin, a protein expressed in podocytes and an important constituent of the glomerular filtration barrier, was studied in renal membrane fractions using the primary antibody anti-nephrin antibody (ab216341, Abcam, USA) at a dilution of 1:500 following the same sample preparation and Western blotting protocol explained previously.
Statistical analyses
Data was expressed as mean ± SEM. Data was compared using one-way ANOVA followed by Tukey’s HS post hoc test. When comparing thyroxine-treated with controls, unpaired student t-test was used. Significance is considered when p value is less than 0.05.
Results
The effects of T4, T4-Ang 1–7, T4-Captopril, and T4-losartan on renal function
The effects of T4, T4-Ang 1–7, T4-Captopril, and T4-losartan on creatinine clearance
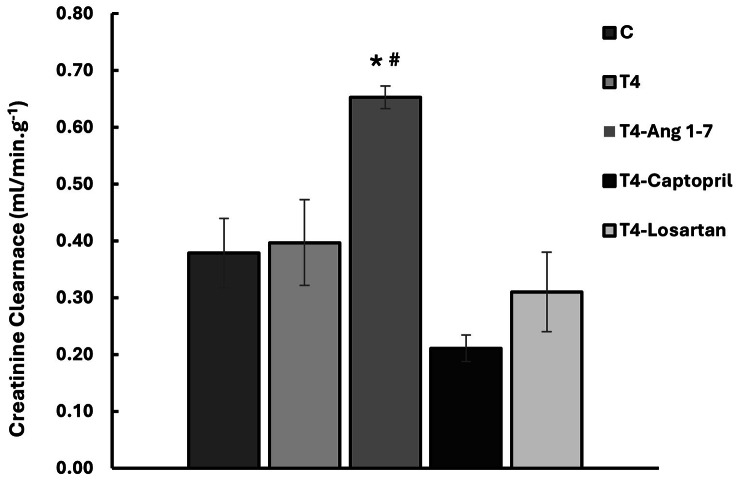
Thyroxine had no effect on creatinine clearance. Similarly, losartan and captopril had no effect on creatinine clearance in T4-treated rats. However, Ang 1–7 caused a significant increase in creatinine clearance in T4-treated rats (p < 0.05, Fig. 1).
Fig. 1.
The effect of thyroxine on creatinine clearance. C = Control, T4 = Thyroxine. Results are expressed as mean ± SE. *p < 0.05 when compared to the control group, #p < 0.05, when compared to T4-treated group (One-way ANOVA followed by Tukey’s HSD post hoc test)
The effects of T4, T4-Ang 1–7, T4-Captopril, and T4-losartan on protein excretion rate
Thyroxine caused a significant increase in protein excretion rate (p < 0.01, Table 1). Treatment with losartan decreased protein excretion rate to values not different from controls and significantly lower than T4-treated (p < 0.01). However, protein excretion rates in captopril and Ang 1–7 treated rats were not different from thyroxine-treated rats (Table 1).
Table 1.
The effect of T4,T4-Ang 1–7, T4-Captopril, T4-Losartan on protein excretion rate: differences between the groups are tested using one-way-ANOVA followed by Tukey’s HSD post hoc test). Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01 when compared to the control group. #p < 0.05, when compared to T4-treated group. C = control, T4 = thyroxine. Up = urinary protein concentration, PER = protein excretion rate
| Group | Urine Volume (ml/day) | Up (mg/ml) |
PER (mg/24hrs) |
|---|---|---|---|
| C (n = 6) | 11.7 ± 2.4 | 0.8 ± 0.1 | 11.1 ± 1.0 |
| T4 (n = 8) | 16.7 ± 1.6 | 2.4 ± 0.2* | 39.6 ± 5.8** |
| T4-Ang 1–7 A (n = 6) | 15.8 ± 2.9 | 2.3 ± 0.4* | 31.8 ± 5.3* |
| T4-Captopril (n = 8) | 29.1 ± 3.3*# | 1.1 ± 0.3# | 26.1 ± 3.3 |
| T4-Losartan (n = 8) | 16.9 ± 2.5 | 1.6 ± 0.4 | 21.9 ± 3.1# |
The effect of effects of T4, T4-Ang 1–7, T4-Captopril, and T4-losartan on glomerular volume
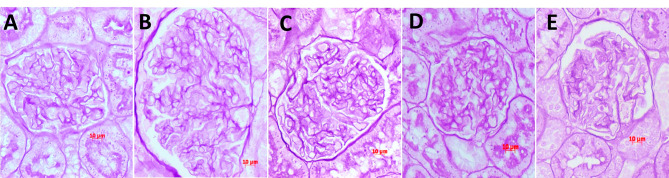
Thyroxine caused a significant increase in glomerular tuft area (p < 0.01) and glomerular volume (p < 0.01) (Fig. 2; Table 2), which was prevented by captopril and losartan. However, the glomerular tuft area and volume in the group treated with Ang 1–7 were not different from thyroxine-treated rats (Table 2). There was no mesangial matrix expansion with thyroxine treatment (Table 2). Captopril caused a slight but significant increase in the ratio of mesangial area to glomerular area.
Fig. 2.
Glomerular morphometric changes in control (A), T4-treated rats (B), T4-Ang 1–7 (C), T4-Captopril (D) and T4-losartan (E). There was a significant increase in glomerular tuft area (GTA) but no change in mesangial matrix area (MMA) or MMA/GTA with T4 treatment. Glomerular volume was not different from controls after captopril or losartan treatments, however in Ang 1-7 treated rats glomerular volume was not different from T4-treated rats. PAS stain, X 40
Table 2.
The effect of T4,T4-Ang 1–7, T4-Captopril, T4-Losartan on glomerular morphometry. Differences between the groups are tested using one-way-ANOVA followed by Tukey’s HSD post hoc test. Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01 when compared to the control group. #p < 0.05, when compared to T4-treated group. C = control, T4 = thyroxine
| Group | Glomerular tuft area (µm2) | Glomerular tuft volume (µm3 × 103) |
Mesangial matrix area (µm2) |
Mesangial area/ Glomerular tuft area |
|---|---|---|---|---|
| C (n = 6) | 6617.19 ± 216 | 690.269 ± 34.2 | 408.38 ± 41.2 | 6.11 ± 0.5 |
| T4 (n = 6) | 8126.29 ± 340** | 937.63 ± 63.2** | 496.48 ± 34.2 | 6.08 ± 0.3 |
| T4-Ang 1–7 (n = 6) | 7514.55 ± 198 | 829.36 ± 33.2 | 489.58 ± 14.8 | 6.39 ± 6.4 |
| T4-Captopril (n = 6) | 6861.94 ± 155# | 727.05 ± 27.8# | 511.30 ± 25.3 | 7.44 ± 0.2* |
| T4-Losartan (n = 6) | 6923.20 ± 220# | 734.478 ± 353# | 485.55 ± 19.0 | 7.12 ± 0.2 |
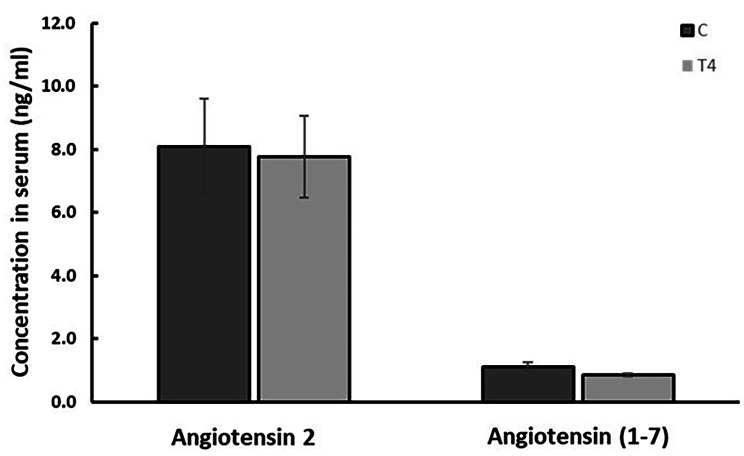
The effect of thyroxine on serum levels of Ang II and Ang 1–7
Treatment with thyroxine had no effect on levels of Ang II or Ang 1–7 in rat serum (Fig. 3). Ang II and Ang 1–7 could not be detected in the renal homogenates probably because levels are lower than the minimum detection level of 15.63 pg/ml for Ang II and 7.8pg/ml for Ang 1–7 kits.
Fig. 3.
The effect thyroxine on Ang II and Ang 1–7 levels in rat serum. C = Control, T4 = Thyroxine-treated. Results are expressed as mean ± SEM and were compared using unpaired student t test
The effect of effects of T4, T4-Ang 1–7, T4-Captopril, and T4-losartan on renal expression of ACE
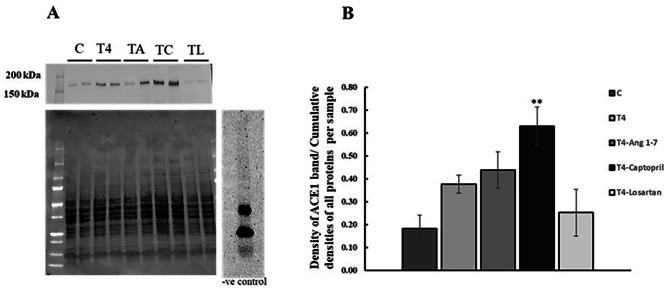
ACE1 bands were detected at the expected size (Fig. 4). Treatment with thyroxine did not affect renal ACE1 protein expression. Similarly, ACE1 protein expression with Ang 1–7 and losartan treatments was not different from control. However, treatment with the ACE1 inhibitor captopril significantly increased renal expression of ACE1 (p < 0.01, Fig. 4).
Fig. 4.
The effect of all treatments on renal expression of ACE1 protein. (A) Representative Western blot of ACE1 protein expression in renal homogenates, total protein, and negative control. Observed band size is a round 180kDa. (B) Western blot analysis and quantification of ACE1 protein expression. Results are expressed as mean ± SEM, n = 6 for all groups. C = control, T4 = Thyroxine. **p < 0.01 when compared to control (One-way ANOVA followed by Tukey’s HSD post hoc test)
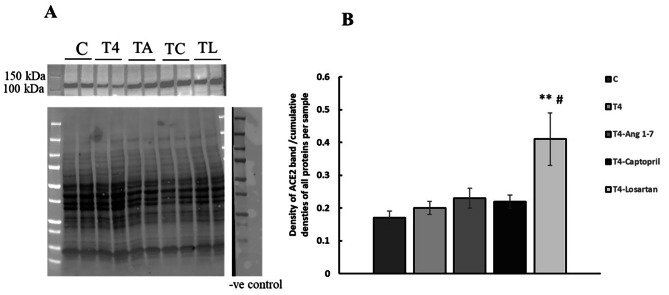
The effect of effects of T4, T4-Ang 1–7 T4-Captopril, and T4- losartan on renal expression of ACE2
ACE2 bands were detected at the expected size (Fig. 5). There was no effect of thyroxine, thyroxine-Ang 1–7, or thyroxine captopril, or on ACE2 expression. However, treatment with losartan caused a significant increase in renal ACE2 expression in T4-treated rats (p < 0.01, Fig. 5).
Fig. 5.
The effect of all treatments on renal expression of ACE2 protein. (A) Representative Western blot of ACE2 protein expression in renal homogenates, total protein, and negative control. Observed band size is 105–110 kDa. (B) Western blot analysis and quantification of ACE2 protein expression. Results are expressed as mean ± SEM, n = 6 for all groups. C = control, T4 = Thyroxine. **p < 0.01 when compared to control, #p < 0.01 when compared to thyroxine treated (One-way ANOVA followed by Tukey’s HSD post hoc test)
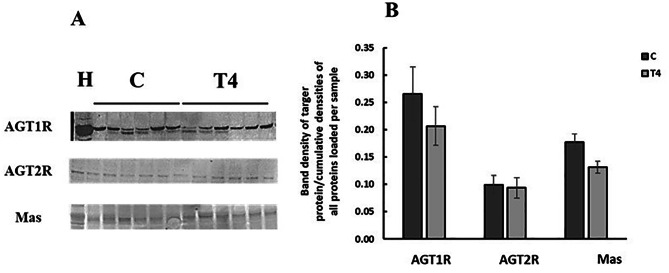
The effect of T4 on renal expression of AT1R, AT2R and MasR in renal membranes
Thyroxine treatment had no effect on the expression of AT1R, AT2R, or MasR expression (Fig. 6) in renal membrane fractions.
Fig. 6.
The effect of thyroxine treatment on the expression of AT1R, AT2R and MasR proteins in renal membranes. (A) Representative Western blot of AT1R protein expression in renal membranes. Observed band sizes is 50kDa. (B) Western blot analysis and quantification of receptors protein expression. Results are expressed as mean ± SEM, n = 6 for all groups. Results are compared using unpaired student t test. C = Control, T4 = Thyroxine-treated, H = Heart sample
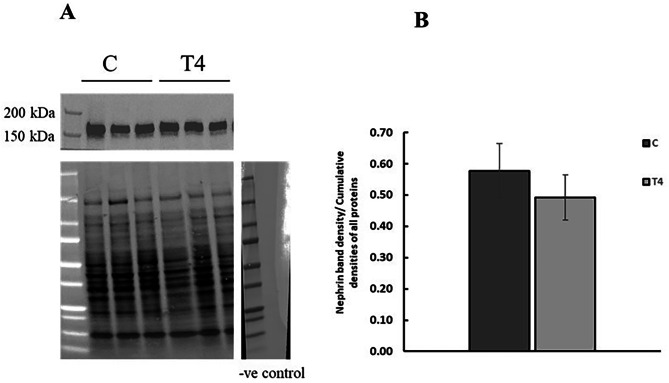
The effect of T4 on the renal expression of nephrin
Thyroxine treatment had no effect on nephrin expression in renal membrane fractions (Fig. 7).
Fig. 7.
The effect of thyroxine on the expression of nephrin protein in renal membranes. (A) Representative Western blot of nephrin protein expression in renal membranes, total protein, and negative control. Observed band size is 170 kDa. (B) Western blot analysis and quantification of nephrin protein expression. Results are expressed as mean ± SEM, n = 6 for all groups. Results are compared using unpaired student t test. C = Control, T4 = Thyroxine-treated
Discussion
Treatment of experimental animals with thyroxine was shown to reproduce the effects of hyperthyroidism [1, 2]. Thyroxine is produced by the thyroid gland and converted to the active form of the hormone triiodothyronine (T3) by iodothyronine deiodinases of which type 1 (D1) is expressed in the liver, kidney, thyroid, and pituitary gland [30]. This study is exploring the possible protective role of Ang 1–7 against thyroxine-induced renal functional and structural changes in comparison to inhibiting the formation or blocking the effect of Ang II.
We studied the effect of thyroxine on creatinine clearance, which is a measurement of glomerular filtration rate (GFR). Human studies show that hyperthyroidism is associated with higher-than-normal eGFR and lower than normal serum creatinine [31]. Animal studies are conflicting with some reporting a decrease in creatinine clearance [32] and others reporting no change in creatinine clearance [33]. Thyroxine did not affect creatinine clearance in our study and nor did the treatments with captopril or losartan [33]. However, Ang 1–7 caused an increase in creatinine clearance in rats treated with thyroxine indicating an increase in GFR. Ang 1–7 was shown to cause afferent arterial vasodilation in rabbits [34] through the production of NO [34]. However, studies on the effect of Ang 1–7 on renal function are contradictory with some showing that Ang 1–7 increases renal blood flow [35] while others show no effect of Ang 1–7 on GFR [36]. A study showed that deletion of MasR caused hyperfiltration [37]. van Twist et al., (2013) [38] suggested that the renal vasodilatory effect of Ang 1–7 seen also in humans depends on the level of RAS activation. The effect seen on creatinine clearance in our study is most likely due to Ang 1–7 since thyroxine alone had no effect on renal function.
Thyroxine also caused an increase in protein excretion rate as previously reported in experimental animals [33, 39] and human studies [40, 41]. Proteinuria in hyperthyroidism suggests either impairment of the glomerular filtration barrier permeability or the re-absorptive mechanisms in the proximal tubule [42]. We studied the effect of thyroxine on the expression of nephrin, an important constituent of the glomerular filtration barrier and is pivotal for the selectivity of the glomerular filtration barrier against plasma proteins [43]. Nephrin expression in renal membranes was not altered after thyroxine treatment, consistent with a study that shows that T3 restores nephrin expression in podocytes after exposure to a high glucose media [44]. However, despite the lack of change in total nephrin expression in renal membranes, abnormal distribution of nephrin [45], structural changes to podocyte cytoskeleton or foot processes effacement cannot be ruled out as a cause of increased protein excretion rate. Proteinuria in this model could also be of tubular origin as thyroid hormone affects many tubular transport proteins such as Na+-H+ exchanger [46] Na+-K+-ATPase [47] and aquaporin 1 [32]. However, protein reabsorption in the proximal tubule is mainly dependent on two brush boarder proteins megalin and cubilin. Megalin is responsible for the binding and uptake of the thyroxine carrier transthyretin [48]. In addition, low levels of thyroxine due to thyroidectomy decreased the expression of cubilin in rat intestine which was restored to normal by thyroxine [49]. These studies suggest the significance of these proteins for the renal uptake of thyroid hormone, and it is unlikely that they would be altered by thyroxine. Hence, the effect of thyroid hormone on renal protein handling should be explored further.
Thyroxine-induced increase in protein excretion rate was prevented by treatment with losartan but not Ang 1–7 suggesting that proteinuria is mediated by Ang II. However, proteinuria was not prevented with the ACE1 inhibitor captopril, which is likely due to the fact that captopril reduces only ACE-dependent production of Ang II, while losartan blocks the action of Ang II regardless of its source [50]. The protective role of Ang 1–7 to the glomerular filtration barrier has been shown mainly in diabetic animals [25], however, it was not protective against the increase in protein excretion rate in this model.
As for morphometric changes, thyroxine caused glomerular hypertrophy, which was prevented by captopril and losartan treatments. However, with Ang 1–7 treatment, glomerular volume was not different from thyroxine-treated rats suggesting that captopril and losartan are more effective than Ang 1–7 in reducing glomerular hypertrophy contrary to what was reported in diabetic rat models using the same dose and treatment method of Ang 1–7 [20, 25].
Thyroxine had no effect on the expression of AT1R, AT2R or MasR in renal membranes. A study showed that thyroidectomy caused a decrease in AT1R and an increase in AT2R [51]. However, they studied whole kidney homogenates and not membrane fractions and since AT1R is internalized after binding to the ligand [52], membrane expression is more informative.
Renal ACE1 expression was not affected by thyroxine, Ang 1–7 or losartan, however, was increased with captopril treatment as expected. We could not measure renal Ang II or Ang 1–7 levels, however, the prevention of thyroxine-induced proteinuria and glomerular hypertrophy with the AT1R blocker losartan strongly suggests that these changes are caused by increased levels of renal Ang II despite unaltered ACE1 expression since increased enzyme activation cannot be ruled out. However, the role of renal Ang 1–7 against injury cannot be ruled out as losartan’s effect could be a combination of blocking AT1 receptors and increasing renal ACE2 expression as we have detected and was reported earlier [53], hence increased renal Ang 1–7 levels as was detected in the heart [17]. In addition, theoretically, blocking AT1 receptors eliminates the negative feedback effect on renin secretion, leading to more angiotensin 1 and subsequently more Ang 1–7 via neprilysin. We conclude that losartan was more protective than captopril against thyroxine-induced renal changes while Ang 1–7 offered no protection.
Acknowledgements
We gratefully acknowledge Ms. Mini Verkey and Ms. Sajida Tappa for their excellent technical assistance.
Author contributions
Slava Malatiali: Conceptualization, project administration, funding acquisition, methodology, data analysis, manuscript writing, reviewing, and editing. Mabayoje Oriowo: Critical review and editing of the manuscript, co-investigator on the project.
Funding
The project was funded by the Research Sector, Kuwait University, grant # MY01/18, and Research Core Facility, Health Sciences Centre, Kuwait University, grant # #SRUL02/13.
Data availability
Data is available upon request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oriowo MA, Oommen E, Khan I. Hyperthyroidism enhances 5-HT-induced contraction of the rat pulmonary artery: role of calcium-activated chloride channel activation. Eur J Pharmacol. 2011;669(1–3):108–14. [DOI] [PubMed] [Google Scholar]
- 2.Hassanpour H, Afzali A, Fatemi Tabatabaie R, Torabi M, Alavi Y. Cardiac renin-angiotensin system (gene expression) and plasma Ang II in chickens with T3-induced pulmonary hypertension. Br Poult Sci. 2016;57(4):444–50. [DOI] [PubMed] [Google Scholar]
- 3.Basset A, Blanc J, Messas E, Hagège A, Elghozi JL. Renin-angiotensin system contribution to cardiac hypertrophy in experimental hyperthyroidism: an echocardiographic study. J Cardiovasc Pharmacol. 2001;37(2):163–72. [DOI] [PubMed] [Google Scholar]
- 4.Asahi T, Shimabukuro M, Oshiro Y, Yoshida H, Takasu N. Cilazapril prevents cardiac hypertrophy and postischemic myocardial dysfunction in hyperthyroid rats. Thyroid. 2001;11(11):1009–15. [DOI] [PubMed] [Google Scholar]
- 5.Kobori H, Ichihara A, Miyashita Y, Hayashi M, Saruta T. Mechanism of hyperthyroidism-induced renal hypertrophy in rats. J Endocrinol. 1998;159(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carneiro-Ramos MS, Silva VB, Santos RA, Barreto-Chaves ML. Tissue-specific modulation of angiotensin-converting enzyme (ACE) in hyperthyroidism. Peptides. 2006;27(11):2942–9. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Gómez I, Manuel Moreno J, Jimenez R, Quesada A, Montoro-Molina S, Vargas-Tendero P, Wangensteen R, Vargas F. Effects of arginase inhibition in hypertensive hyperthyroid rats. Am J Hypertens. 2015;28(12):1464–72. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Gomez I, Banegas I, Wangensteen R, Quesada A, Jiménez R, Gómez-Morales M, O’Valle F, Duarte J, Vargas F. Influence of thyroid state on cardiac and renal capillary density and glomerular morphology in rats. J Endocrinol. 2013;216(1):43–51. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Ichihara A, Miyashita Y, Hayashi M, Saruta T. Local renin–angiotensin system contributes to hyperthyroidism-induced cardiac hypertrophy. J Endocrinol. 1999;160(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–87. [DOI] [PubMed] [Google Scholar]
- 11.Kaltenecker CC, Domenig O, Kopecky C, Antlanger M, Poglitsch M, Berlakovich G, Kain R, Stegbauer J, Rahman M, Hellinger R, Gruber C. Critical role of neprilysin in kidney angiotensin metabolism. Circul Res. 2020;127(5):593–606. [DOI] [PubMed] [Google Scholar]
- 12.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–43. [DOI] [PubMed] [Google Scholar]
- 13.Santos RA, e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences. 2003; 100(14):8258-63. [DOI] [PMC free article] [PubMed]
- 14.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1–7), ACE2 and blood pressure regulation. Kidney Blood Press Regul. 2004;143:77–89. [DOI] [PubMed] [Google Scholar]
- 15.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA. Simões E Silva AC, Ribeiro Vieira MA. ACE2–angiotensin-(1–7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci. 2010;119(9):385–94. [DOI] [PubMed] [Google Scholar]
- 16.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1‐7) and M as receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diniz GP, Senger N, Carneiro-Ramos MS, Santos RA, Barreto-Chaves ML. Cardiac ACE2/angiotensin 1–7/Mas receptor axis is activated in thyroid hormone-induced cardiac hypertrophy. Ther Adv Cardiovasc Dis. 2016;10(4):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senger N, Melo MB, Santos MJ, Santos RA, Barreto-Chaves ML. Angiotensin‐(1–7) prevents cardiovascular changes induced by hyperthyroidism. FASEB J. 2017;31:lb642. [Google Scholar]
- 19.Papinska AM, Mordwinkin NM, Meeks CJ, Jadhav S, Rodgers KE. Angiotensin-(1–7) administration benefits cardiac, renal and progenitor cell function in db/db mice. Br J Pharmacol. 2015;172(18):4443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Lo CS, Padda R, Abdo S, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Angiotensin-(1–7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci. 2015;128(10):649–63. [DOI] [PubMed] [Google Scholar]
- 21.Amato D, Núñez-Ortiz AR, del Benítez-Flores C, Segura-Cobos J, López-Sánchez D, Vázquez-Cruz P. Role of Angiotensin-(1–7) on renal hypertrophy in Streptozotocin-Induced Diabetes Mellitus. Pharmacol Pharm. 2016;7(09):379. [Google Scholar]
- 22.Shao Y, He M, Zhou L, Yao T, Huang Y, LU LM. Chronic angiotensin (1–7) injection accelerates STZ-induced diabetic renal injury 1. Acta Pharmacol Sin. 2008;29(7):829–37. [DOI] [PubMed] [Google Scholar]
- 23.Esteban V, Heringer-Walther S, Sterner-Kock A, de Bruin R, van den Engel S, Wang Y, Mezzano S, Egido J, Schultheiss HP, Ruiz-Ortega M, Walther T. Angiotensin-(1–7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS ONE. 2009;4(4):e5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman DL, Zimpelmann J, Xiao F, Gutsol A, Touyz R, Burns KD. The effect of angiotensin-(1–7) in mouse unilateral ureteral obstruction. Am J Pathol. 2015;185(3):729–40. [DOI] [PubMed] [Google Scholar]
- 25.Papinska AM, Rodgers KE. Long-term administration of angiotensin (1–7) to db/db mice reduces oxidative stress damage in the kidneys and prevents renal dysfunction. Oxidative Med Cell Longev. 2018;2018(1):1841046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devonshire IM, Grandy TH, Dommett EJ, Greenfield SA. Effects of urethane anaesthesia on sensory processing in the rat barrel cortex revealed by combined optical imaging and electrophysiology. Eur J Neurosci. 2010 (5):786–97. [DOI] [PubMed]
- 27.Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not Hypertrophy in Diabetic rats. Experimental Diabetes Res. 2008;2008:305–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojević M, Brzica H, Sebastiani A, Thal SC, Sauvant C. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiology-Cell Physiol. 2012;302(8):C1174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172(2):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruinstroop E, van der Spek AH, Boelen A. Role of hepatic deiodinases in thyroid hormone homeostasis and liver metabolism, inflammation, and fibrosis. Eur Thyroid J. 2023; 12(3). [DOI] [PMC free article] [PubMed]
- 31.Naguib R, Elkemary E. Thyroid dysfunction and renal function: a crucial relationship to recognize. Cureus. 2023;15(2). [DOI] [PMC free article] [PubMed]
- 32.Wang W, Li C, Summer SN, Falk S, Schrier RW. Polyuria of thyrotoxicosis: downregulation of aquaporin water channels and increased solute excretion. Kidney Int. 2007;72(9):1088–94. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Gómez I, Sainz J, Wangensteen R, Moreno JM, Duarte J, Osuna A, Vargas F. Increased pressor sensitivity to chronic nitric oxide deficiency in hyperthyroid rats. Hypertension. 2003;42(2):220–5. [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39(3):799–802. [DOI] [PubMed] [Google Scholar]
- 35.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol. 2003;284(6):H1985–94. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira AJ, Pinheiro SV, Castro CH, Silva GA, e Silva AC, Almeida AP, Bader M, Rentzsch B, Reudelhuber TL, Santos RA. Renal function in transgenic rats expressing an angiotensin-(1–7)-producing fusion protein. Regul Pept. 2006;137(3):128–33. [DOI] [PubMed] [Google Scholar]
- 37.Pinheiro SV, Ferreira AJ, Kitten GT, Da Silveira KD, Da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, Da Mota RK, Botelho-Santos GA. Genetic deletion of the angiotensin-(1–7) receptor mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75(11):1184–93. [DOI] [PubMed] [Google Scholar]
- 38.van Twist DJ, Houben AJ, de Haan MW, Mostard GJ, Kroon AA, de Leeuw PW. Angiotensin-(1–7)–induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and Ang II co-infusion. Hypertension. 2013;62(4):789–93. [DOI] [PubMed] [Google Scholar]
- 39.van Hoek I, Lefebvre HP, Peremans K, Meyer E, Croubels S, Vandermeulen E, Kooistra H, Saunders JH, Binst D, Daminet S. Short-and long-term follow-up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol. 2009;36(1):45–56. [DOI] [PubMed] [Google Scholar]
- 40.Agras PI, Kınık ST, Cengiz N, Baskin E, Saatci U. Autoimmune thyroiditis with associated proteinuria: report of two patients. J Pediatr Endocrinol Metab. 2005;18(3):319–22. [DOI] [PubMed] [Google Scholar]
- 41.Benabdelkamel H, Masood A, Ekhzaimy AA, Alfadda AA. Proteomics profiling of the urine of patients with hyperthyroidism after anti-thyroid treatment. Molecules. 2021;26(7):1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueira MF, Castiglione RC, de Lemos Barbosa CM, Ornellas FM, da Silva Feltran G, Morales MM, da Fonseca RN, de Souza-Menezes J. Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC‐5, and CFTR. Physiological Rep. 2017;5(13):e13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci. 1999;96(14):7962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedetti V, Lavecchia AM, Locatelli M, Brizi V, Corna D, Todeschini M, Novelli R, Benigni A, Zoja C, Remuzzi G, Xinaris C. Alteration of thyroid hormone signaling triggers the diabetes-induced pathological growth, remodeling, and dedifferentiation of podocytes. JCI insight. 2019; 4(18). [DOI] [PMC free article] [PubMed]
- 45.Dumont V, Tolvanen TA, Kuusela S, Wang H, Nyman TA, Lindfors S, Tienari J, Nisen H, Suetsugu S, Plomann M, Kawachi H. PACSIN2 accelerates nephrin trafficking and is upregulated in diabetic kidney disease. FASEB J. 2017;31(9):3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinsella J, Sacktor B. Thyroid hormones increase Na+-H + exchange activity in renal brush border membranes. Proc Natl Acad Sci. 1985;82(11):3606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capasso G, De Tommaso G, Pica A, Anastasio P, Capasso J, Kinne R, De Santo N. Effects of thyroid hormones on heart and kidney functions. Miner Electrolyte Metab. 1999;25(1–2):56–64. [DOI] [PubMed] [Google Scholar]
- 48.Sousa MM, Norden AG, Jacobsen C, Willnow TE, Christensen EI, Thakker RV, Verroust PJ, Moestrup SK, Saraiva MJ. Evidence for the role of megalin in renal uptake of transthyretin. J Biol Chem. 2000;275(49):38176–81. [DOI] [PubMed] [Google Scholar]
- 49.Yammani RR, Seetharam S, Seetharam B. Cubilin and megalin expression and their interaction in the rat intestine: effect of thyroidectomy. Am J Physiology-Endocrinology Metabolism. 2001;281(5):E900–7. [DOI] [PubMed] [Google Scholar]
- 50.Klahr S, Morrissey J. Comparative effects of ACE inhibition and Ang II receptor blockade in the prevention of renal damage. Kidney Int. 2002;62:S23–6. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, Carey LC, Valego NK, Liu J, Rose JC. Thyroid hormone modulates renin and ANG II receptor expression in fetal sheep. Am J Physiology-Regulatory Integr Comp Physiol. 2005;289(4):R1006–14. [DOI] [PubMed] [Google Scholar]
- 52.Guo DF, Sun YL, Hamet P, Inagami T. The Ang II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11(3):165–80. [DOI] [PubMed] [Google Scholar]
- 53.Zaheer J, Kim H, Kim JS. Correlation of ACE2 with RAS components after Losartan treatment in light of COVID-19. Sci Rep. 2021;11(1):24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request from the corresponding author.