Abstract
Levels of histone acetylation and phosphorylation have been contrasted in two developmental states of Drosophila melanogaster. The 0-2 h nuclei of the syncitial blastula are characterized by rapid mitoses and DNA replication, but there is very little transcription. In the 18 h embryo there is considerable transcription and the mitotic rate is much slower. It has been found that (1) histone H1 from 2h nuclei is not highly phosphorylated. This observation is not in accord with the view that H1 hyperphosphorylation is essential to mitosis, but is compatible with the hypothesis that H1 phosphorylation in Drosophila species is related to heterochromatization. (2) Histone H4 from 2 h embryos shows high levels of the diacetyl form (H4-Ac2), which is principally outside the nucleus. This accords with the hypothesis that H4-Ac2 is the form in which H4 is deposited on to newly replicated DNA and shows that H4 acetylation is linked not only to transcription. (3) Histone H3 acetylation is similar in 2h and in 18h embryos. As with H4, this acetylation probably correlates with chromatin assembly and is not transcription-related. (4) Histone H2B carries no modification in 2h or in 18h embryos, and H2A shows a single modification in 2h embryos and two in 18 h embryos. H2B modification is thus not essential either in mitosis or replication, whereas H2A modification is important in one or both processes. (5) The nucleosomal protein D2 is equally present in 2h and 18 h embryos.
Full text
PDF




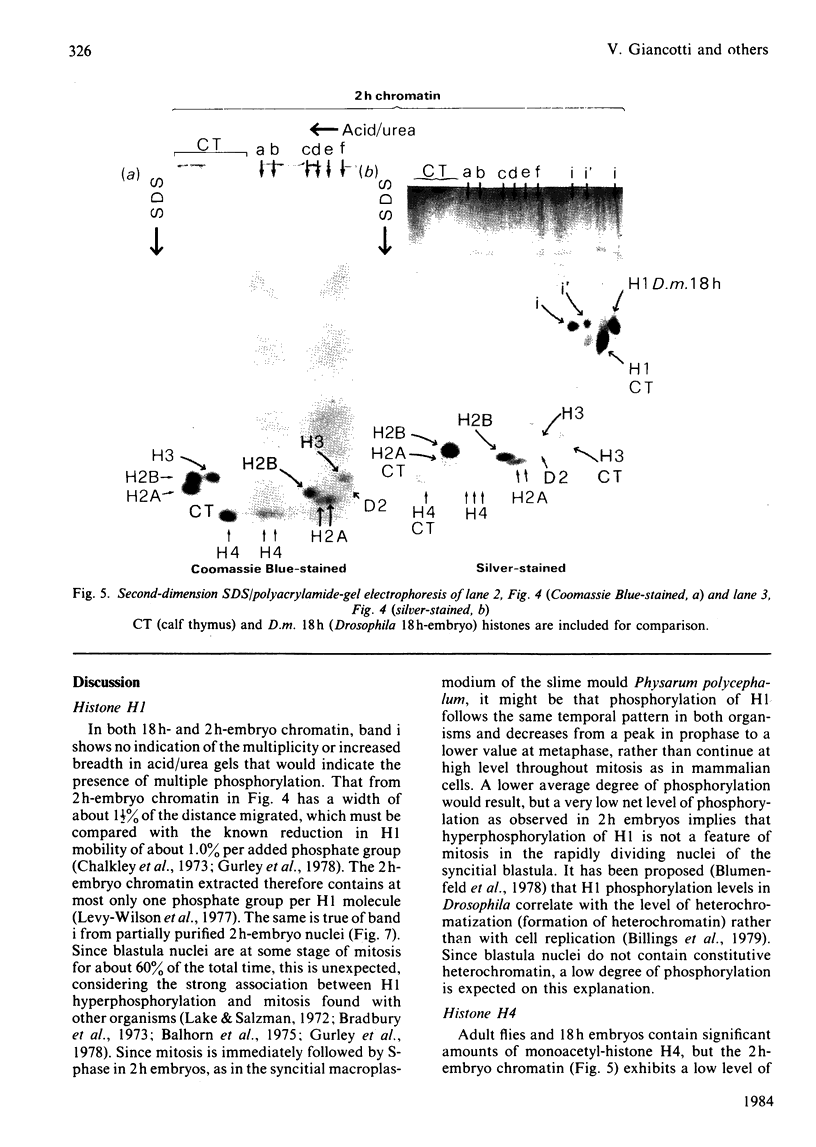

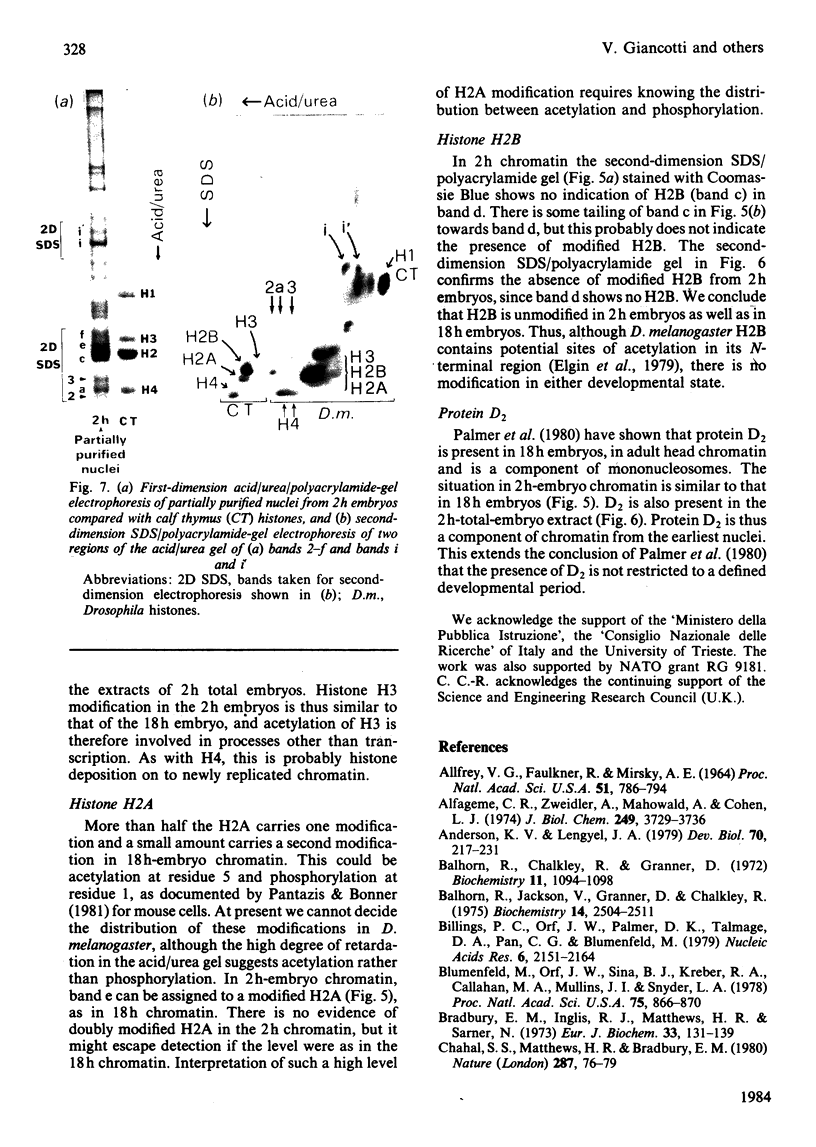

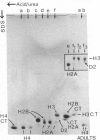
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Anderson K. V., Lengyel J. A. Rates of synthesis of major classes of RNA in Drosophila embryos. Dev Biol. 1979 May;70(1):217–231. doi: 10.1016/0012-1606(79)90018-6. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Chalkley R., Granner D. Lysine-rich histone phosphorylation. A positive correlation with cell replication. Biochemistry. 1972 Mar 14;11(6):1094–1098. doi: 10.1021/bi00756a023. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Jackson V., Granner D., Chalkey R. Phosphorylation of the lysine-rich histones throughout the cell cycle. Biochemistry. 1975 Jun 3;14(11):2504–2511. doi: 10.1021/bi00682a033. [DOI] [PubMed] [Google Scholar]
- Billings P. C., Orf J. W., Palmer D. K., Talmage D. A., Pan C. G., Blumenfeld M. Anomalous electrophoretic mobility of Drosophila phosphorylated H1 histone: is it related to the compaction of satellite DNA into heterochromatin? Nucleic Acids Res. 1979;6(6):2151–2164. doi: 10.1093/nar/6.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld M., Orf J. W., Sina B. J., Kreber R. A., Callahan M. A., Mullins J. I., Snyder L. A. Correlation between phosphorylated H1 histones and satellite DNAs in Drosophila virilis. Proc Natl Acad Sci U S A. 1978 Feb;75(2):866–870. doi: 10.1073/pnas.75.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Chahal S. S., Matthews H. R., Bradbury E. M. Acetylation of histone H4 and its role in chromatin structure and function. Nature. 1980 Sep 4;287(5777):76–79. doi: 10.1038/287076a0. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Hood L. E. Chromosomal proteins of Drosophila embryos. Biochemistry. 1973 Nov 20;12(24):4984–4991. doi: 10.1021/bi00748a026. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Schilling J., Hood L. E. Sequence of histone 2B of Drosophila melanogaster. Biochemistry. 1979 Dec 11;18(25):5679–5685. doi: 10.1021/bi00592a025. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Hohmann P., Tobey R. A., Gurley L. R. Phosphorylation of distinct regions of f1 histone. Relationship to the cell cycle. J Biol Chem. 1976 Jun 25;251(12):3685–3692. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Gjerset R. A., McCarthy B. J. Acetylation and phosphorylation of Drosophila histones. Distribution of acetate and phosphate groups in fractionated chromatin. Biochim Biophys Acta. 1977 Mar 2;475(1):168–175. doi: 10.1016/0005-2787(77)90351-3. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Oliver D., Chalkley R. An electrophoretic analysis of Drosophila histones. I. Isolation and identification. Exp Cell Res. 1972 Aug;73(2):295–302. doi: 10.1016/0014-4827(72)90051-1. [DOI] [PubMed] [Google Scholar]
- Palmer D., Snyder L. A., Blumenfeld M. Drosophila nucleosomes contain an unusual histone-like protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2671–2675. doi: 10.1073/pnas.77.5.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis P., Bonner W. M. Quantitative determination of histone modification. H2A acetylation and phosphorylation. J Biol Chem. 1981 May 10;256(9):4669–4675. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Chromatin structures of main-band and satellite DNAs in Drosophila melanogaster nuclei as probed by photochemical cross-linking of DNA with trioxsalen. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):179–189. doi: 10.1101/sqb.1978.042.01.020. [DOI] [PubMed] [Google Scholar]
- Spiker S. A modification of the acetic acid-urea system for use in microslab polyacrylamide gel electrophoresis. Anal Biochem. 1980 Nov 1;108(2):263–265. doi: 10.1016/0003-2697(80)90579-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Augustine R., Schulman H. Calcium-dependent phosphorylation of histone H3 in butyrate-treated HeLa cells. Nature. 1980 Sep 4;287(5777):74–76. doi: 10.1038/287074a0. [DOI] [PubMed] [Google Scholar]
- Woodland H. R. The modification of stored histones H3 and H4 during the oogenesis and early development of Xenopus laevis. Dev Biol. 1979 Feb;68(2):360–370. doi: 10.1016/0012-1606(79)90210-0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zalokar M. Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev Biol. 1976 Apr;49(2):425–437. doi: 10.1016/0012-1606(76)90185-8. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]