Infections with arboviruses Powassan virus (POWV), Usutu virus (USUV), or Ross River virus (RRV) are rare but can cause severe disease. We report autoantibodies neutralizing type I IFNs in patients with POWV encephalitis, severe USUV infection, and severe RRV infection.
Abstract
Arboviral diseases are a growing global health concern. Pre-existing autoantibodies (auto-Abs) neutralizing type I interferons (IFNs) can underlie encephalitis due to West Nile virus (WNV) (∼40% of patients) and tick-borne encephalitis (TBE, due to TBE virus [TBEV]) (∼10%). We report here that these auto-Abs can also underlie severe forms of rarer arboviral infections. Auto-Abs neutralizing high concentrations of IFN-α2, IFN-β, and/or IFN-ω are present in the single case of severe Powassan virus (POWV) encephalitis studied, two of three cases of severe Usutu virus (USUV) infection studied, and the most severe of 24 cases of Ross River virus (RRV) disease studied. These auto-Abs are not found in any of the 137 individuals with silent or mild infections with these three viruses. Thus, auto-Abs neutralizing type I IFNs underlie an increasing list of severe arboviral diseases due to Flaviviridae (WNV, TBEV, POWV, USUV) or Togaviridae (RRV) viruses transmitted to humans by mosquitos (WNV, USUV, RRV) or ticks (TBEV, POWV).
Introduction
Arboviral diseases are transmitted to humans by mosquitos or, more rarely, by ticks (Davis et al., 2008). There are at least 150 human-tropic arboviruses belonging to the Togaviridae and Flaviviridae families of RNA viruses (Madewell, 2020). The range of clinical presentations of arboviral infections is vast. Most individuals have silent or benign infections, whereas a few suffer from life-threatening diseases (Pierson and Diamond, 2020). Over the last few decades, well-known emerging and re-emerging arboviral diseases have become a growing threat health worldwide (Gould and Solomon, 2008; Wilder-Smith et al., 2017). An estimated 700,000 deaths due to mosquito-borne viral infections alone occur yearly, constituting a major global public health burden (Ketkar et al., 2019). Virulence varies considerably between arboviruses, but interindividual clinical variability is also considerable for each of these viruses and remains unexplained, as in most common infectious diseases (Casanova, 2023; Casanova and Abel, 2024). We recently reported that pre-existing autoantibodies (auto-Abs) neutralizing type I interferons (IFNs) underlie ∼40% of West Nile virus (WNV) encephalitis cases (Gervais et al., 2023) and ∼10% of most severe forms of tick-borne encephalitis (TBE) (Gervais et al., 2024b). Auto-Abs neutralizing type I IFNs have been shown to underlie 5–20% of cases of life-threatening pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Bastard et al., 2020, 2021a, 2021c, 2023, 2024), influenza (Zhang et al., 2022b), or Middle East respiratory syndrome (MERS) (Alotaibi et al., 2023) viruses, and about a third of severe adverse reactions to the attenuated live measles and yellow fever virus (YFV) vaccines (Bastard et al., 2021b). These findings have been replicated worldwide by many studies (Abers et al., 2021; Acosta-Ampudia et al., 2021; Akbari et al., 2023; Akbil et al., 2022; Arrestier et al., 2022; Bastard et al., 2021c; Busnadiego et al., 2022; Carapito et al., 2022; Chang et al., 2021; Chauvineau-Grenier et al., 2022; Credle et al., 2022; Eto et al., 2022; Frasca et al., 2022; Goncalves et al., 2021; Grimm et al., 2023; Hansen et al., 2023; Koning et al., 2021; Lamacchia et al., 2022; Lemarquis et al., 2021; Mathian et al., 2022; Meisel et al., 2021; Petrikov et al., 2022; Philippot et al., 2023; Pons et al., 2023; Raadsen et al., 2022; Savvateeva et al., 2021; Schidlowski et al., 2022; Saheb Sharif-Askari et al., 2023; Simula et al., 2022; Solanich et al., 2021; Soltani-Zangbar et al., 2022; Su et al., 2022; Troya et al., 2021; van der Wijst et al., 2021; Vanker et al., 2023; Vazquez et al., 2021; Wang et al., 2021; Ziegler et al., 2021). These auto-Abs are present in individuals of all ages in the general population, with a prevalence increasing from 0.3% to 1% in individuals under 65 years of age to 4–7% in individuals over 65 years of age (Bastard et al., 2021a).
In this context, we focused on three arboviral infections that are relatively rare in humans: Powassan virus (POWV), Usutu virus (USUV), and Ross River virus (RRV) infections. Most humans infected with these viruses do not develop symptoms or signs of sickness (Ashraf et al., 2015; Dobler, 2010; Harley et al., 2001; Hermance and Thangamani, 2017; Clé et al., 2019; Russell, 2002). POWV is a neurotropic orthoflavivirus transmitted by ticks in North America (Bassam et al., 1999; Hermance and Thangamani, 2017). The seroprevalence of POWV varies significantly between studies, ranging from 0.5% to 3% (Frost et al., 2017; Vahey et al., 2022). An estimated 23% of infections in New Jersey were considered severe, but with a bias toward older people and people reporting tick bites (Vahey et al., 2022). In another study on younger patients with no known history of tick bites, no severe cases were found among the dozen individuals infected (Frost et al., 2017). Fewer than 50 symptomatic cases are reported each year in the United States and almost all these cases are severe, with 60% occurring in people over the age of 60 years (CDC, 2024). Another neurotropic orthoflavivirus, USUV, is transmitted by mosquitoes in Africa and Europe (Ashraf et al., 2015; Clé et al., 2019; Nikolay et al., 2011). In Europe, the estimated seroprevalence for USUV varies considerably, ranging from 0.02% to 3% (Cadar and Simonin, 2022). Just over 100 symptomatic cases were reported in Europe between 2016 and 2021 (European Centre for Disease Prevention and Control, 2023), including about 30 severe neurological forms (meningitis, encephalitis, or meningoencephalitis) (Cadar and Simonin, 2022). The final virus considered here, RRV, is an arthritogenic alphavirus endemic to Oceania, where ∼4,000 symptomatic cases—typically presenting with fever and polyarthralgia or polyarthritis—are reported each year (Yuen and Bielefeldt-Ohmann, 2021). RRV has a median seroprevalence of 19% in endemic regions (Madzokere et al., 2022). No fatal cases of RRV infection have ever been reported and RRV-infected patients usually recover spontaneously or following primary care interventions (Harley et al., 2001; Russell, 2002). We hypothesized that auto-Abs neutralizing type I IFNs (Bastard et al., 2024; Casanova et al., 2024; Hale, 2023) might underlie at least some cases of severe disease due to these three arboviruses.
Results and discussion
Auto-Abs neutralizing IFN-ω in a patient with severe POWV encephalitis
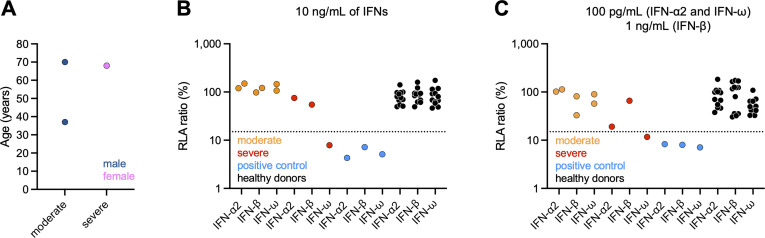
We investigated three patients with POWV disease: two men, aged 37 (P1) and 70 (P2) years, who were hospitalized for a moderate form of the disease with almost complete recovery, and a 68-year-old woman (P3) hospitalized for severe encephalopathy resulting in acute respiratory failure (Fig. 1 A). This patient developed chronic respiratory failure with ventilation dependence and experienced multiple complications, resulting in her death ∼1 year after POWV disease. Plasma samples were obtained from these patients during the first few days after symptom onset (P3) or after the illness (P1 and P2). The diagnosis of viral infection was based on positive results for the detection of anti-POWV IgM in the blood (and CSF for P3) and for a plaque reduction neutralization test (PRNT) against POWV. All three cases originated from and lived in the United States and their ancestry was unknown, as was their medical history, with the exception of POWV infection. Using a previously described luciferase-based neutralization assay (Bastard et al., 2021a), we tested 1:10 dilutions of serum or plasma from all subjects for the neutralization of high (10 ng/ml) or low (100 pg/ml) concentrations of non-glycosylated IFN-α2 and/or IFN-ω, and high (10 ng/ml) or intermediate (1 ng/ml) concentrations of glycosylated IFN-β (Fig. 1, B and C). No neutralization of any of the IFNs tested was observed with plasma samples from P1, P2, or any of the healthy donors. By contrast, plasma from P3-neutralized high and low concentrations of IFN-ω, like plasma from a RAG1-deficient patient known to have neutralizing auto-Abs against IFN-α2, IFN-β, and IFN-ω used as a positive control (Fig. 1, B and C). Unfortunately, the small sample volumes available precluded the testing of auto-Ab levels by another method (e.g., ELISA). Overall, neither of the two cases of moderate POWV disease tested (P1 and P2) displayed any detectable neutralization of type I IFNs, whereas such neutralization was observed for the only case of severe POWV disease tested (P3).
Figure 1.
Auto-Abs neutralizing type I IFNs in individuals infected with POWV. (A) Age and sex distribution of the patients according to POWV disease severity. (B and C) Luciferase-based neutralization assay to detect auto-Abs neutralizing 10 ng/ml IFN-α2, IFN-ω, or IFN-β (B), or 100 pg/ml IFN-α2 and IFN-ω or 1 ng/ml IFN-β (C). The positive control (blue) was plasma from a patient with RAG1 deficiency known to carry auto-Abs neutralizing IFN-α2, IFN-ω, and IFN-β at a concentration of 10 ng/ml. Plasma samples from healthy donors (black) were obtained from individuals from the general population without auto-Abs neutralizing type I IFNs. HEK293T cells were transfected with (1) a plasmid containing the firefly luciferase gene under the control of an ISRE-containing promotor and (2) a plasmid containing the Renilla luciferase gene. The cells were then treated with type I IFNs in the presence of 10% plasma or serum from patients or controls, and RLA was calculated by dividing firefly luciferase activity by Renilla luciferase activity. An RLA <15% of the median RLA for healthy controls was considered to correspond to neutralizing activity (dotted line; Bastard et al., 2021a). The samples of the POWV patients were tested twice and the associated datapoints represent the mean RLA of these independent duplicates.
Auto-Abs neutralizing IFN-α2, -β, and -ω in two patients with severe USUV disease
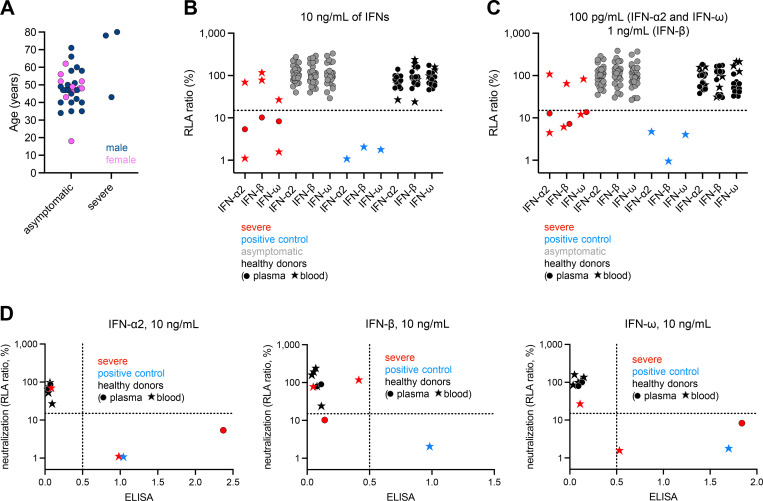
We then investigated a cohort of 34 individuals infected with USUV. In 31 (20 French and 11 Italian) of these individuals, the infection was silent and detected during blood donation. Three patients (1 Hungarian [P4], 1 French [P5], and 1 Italian [P6]) had severe disease (Fig. 2 A). Samples were obtained during the first few days of infection, from the patients with severe disease. P4, P5, and P6 were all male and were 43, 78, and 80 years old, respectively. P4 presented with meningitis and was hospitalized for 7 days. P5 presented with myocarditis and systemic inflammatory response syndrome (SIRS) complicated by acute renal failure. Progression to cardiogenic shock in this patient necessitated intubation and intensive care support. P6 was hospitalized for meningoencephalitis, which progressed, resulting in death within a few days (Gaibani et al., 2023). All three cases tested positive for USUV by RT-qPCR on blood and/or urine. As described above for the patients with POWV disease, we assessed the neutralization of type I IFNs by plasma or serum from the 31 silent cases, cryopreserved whole blood from P4 and P6 (no serum or plasma samples being available for these two cases), and the serum of P5. We also included whole-blood samples from five healthy donors (without neutralizing auto-Abs) and one patient with APS-1 due to AIRE deficiency (with auto-Abs neutralizing high and low concentrations of IFN-α2, and IFN-ω, and intermediate concentrations of IFN-β) (Fig. 2, B and C) to confirm the interpretability of results obtained with whole blood. None of the silently infected individuals had detectable levels of neutralizing auto-Abs against any of the type I IFNs tested. Strikingly, samples from two of the three severe cases (P4 and P5) neutralized IFN-α2 and IFN-ω, respectively, at both high and low concentrations, and IFN-β (at an intermediate concentration for P4 and a high concentration for P5). P6 had no detectable auto-Abs and, notably, had mild COVID-19 6 mo before USUV disease. These results were consistent with the auto-Ab detection results obtained by ELISA (Fig. 2 D). Overall, none of the 31 silently infected individuals displayed detectable neutralization of type I IFNs, whereas such neutralization was observed for two of the three severe cases studied.
Figure 2.
Auto-Abs neutralizing type I IFNs in individuals infected with USUV. (A) Age and sex distribution of the patients according to USUV disease severity. (B and C) Luciferase-based neutralization assay to detect auto-Abs neutralizing 10 ng/ml IFN-α2, IFN-ω, or IFN-β (B), or 100 pg/ml IFN-α2 and IFN-ω or 1 ng/ml IFN-β (C). The positive control (blue) was plasma from a patient with RAG1 deficiency known to carry auto-Abs neutralizing IFN-α2, IFN-ω, and IFN-β at a concentration of 10 ng/ml. Healthy donor plasma (black dots) and whole blood (black stars) samples were tested as negative controls; whole blood was tested because we had only whole-blood samples available for two of the three severe USUV cases. The asymptomatic cases (gray) tested positive for anti-USUV Abs during a blood donation but did not report symptomatic disease. HEK293T cells were transfected with (1) a plasmid containing the firefly luciferase gene under the control of an ISRE-containing promotor and (2) a plasmid containing the Renilla luciferase gene. The cells were then treated with type I IFNs in the presence of 10% plasma or serum from patients or controls, and RLA was calculated by dividing firefly luciferase activity by Renilla luciferase activity. An RLA <15% of the median RLA for healthy controls was considered to correspond to neutralizing activity (dotted line; Bastard et al., 2021a). Each sample was tested once. (D) Correlation between ELISA and neutralization assay results for the detection of auto-Abs neutralizing type I IFNs.
Auto-Abs neutralizing IFN-α2 in the patient with the most severe RRV disease
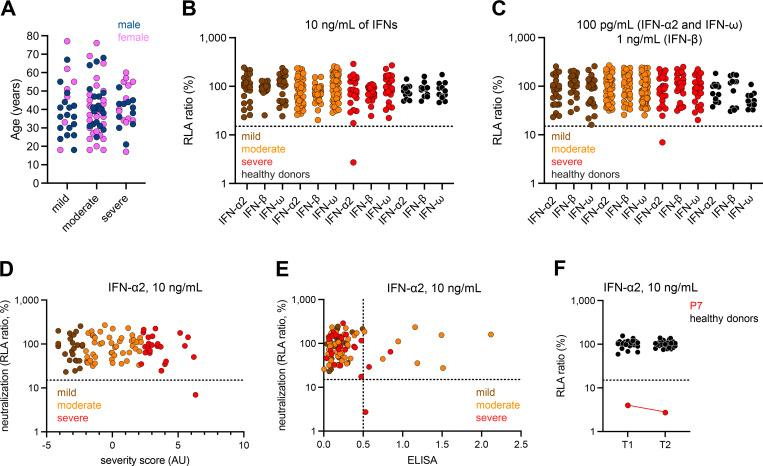
Finally, we investigated a cohort of 96 RRV-infected individuals from Australia. RRV infection was demonstrated by IgG seroconversion or by the detection of anti-RRV IgM and low levels of baseline-avidity anti-RRV IgG. In these patients, clinical severity was determined by calculating a severity score derived from a multidimensional reduction of the severity of prevalent symptoms (e.g., body aches, restless sleep, prolonged tiredness after activity, and febrile manifestations) by principal component analysis (Cvejic et al., 2019). Patients with a score in the top quartile were considered to have severe disease. Those with scores in the bottom quartile were considered to have mild disease, and those in between were considered to have moderately severe disease. Patients with severe disease typically missed work for a mean of 14 days (range: 2–35 days). None of the individuals with severe infection was hospitalized or died. No differences in mean age (standard deviation, SD) were observed between patients with mild (40.7 [5.1] years), moderate (40.8 [14.0] years), and severe (41.0 [10.9]) disease (Fig. 3 A). We assessed the neutralization of type I IFNs by plasma or serum from these patients with the luciferase assay described above (Fig. 3, B and C). No detectable neutralizing auto-Abs against any of the IFNs tested were detected in any of the patients with mild or moderate disease. By contrast, samples from one of the 24 patients with severe disease (P7)—a 55-year-old woman—neutralized high and low concentrations of IFN-α2. P7 had the highest severity score of the entire cohort and therefore had the most severe disease of any of the patients tested (Fig. 3 D). Interestingly, P7 was the only patient to report both headaches and fever most of the time during infection, suggesting an unusual neurotropism of RRV. P7’s auto-Abs against IFN-α2 were also detected by ELISA (Fig. 3 E), and they continued to display neutralizing activity in a follow-up sample obtained 1 year after infection, demonstrating stability over time (Fig. 3 F). Several patients with mild and moderate disease also had detectable titers of auto-Abs against IFN-α2 on ELISA, but these auto-Abs were not neutralizing (Fig. 3 E). None of the 72 individuals with mild or moderate RRV disease displayed detectable neutralization of type I IFNs. Such neutralization was observed for only one of the 24 severe cases (4.2%), the patient with the highest disease severity.
Figure 3.
Auto-Abs neutralizing type I IFNs in individuals infected with RRV. (A) Age and sex distribution of the patients according to RRV disease severity. (B and C) Luciferase-based neutralization assay to detect auto-Abs neutralizing 10 ng/ml IFN-α2, IFN-ω, or IFN-β (B), or 100 pg/ml IFN-α2 and IFN-ω or 1 ng/ml IFN-β (C) in patients with mild (brown), moderate (orange) or severe (red) RRV disease. HEK293T cells were transfected with (1) a plasmid containing the firefly luciferase gene under the control of an ISRE-containing promotor and (2) a plasmid containing the Renilla luciferase gene. The cells were then treated with type I IFNs in the presence of 10% plasma or serum from patients or controls, and RLA was calculated by dividing firefly luciferase activity by Renilla luciferase activity. An RLA <15% of the median RLA for healthy controls was considered to correspond to neutralizing activity (dotted line; Bastard et al., 2021a). Each sample was tested once. (D) Correlation between IFN-α2 neutralization and the severity score of the patients. The severity score was based on the evaluation of symptoms, such as muscle pain after activity, needing to sleep longer, prolonged tiredness after activity, tired muscles after activity, headache, pains in the arms/legs, waking up tired, arms/legs feeling heavy, fevers, back pain, joint pain, and weak muscles. (E) Correlation between ELISA and neutralization assay results for the detection of auto-Abs neutralizing IFN-α2. (F) Neutralization of 10 ng/ml IFN-α2 by the original plasma sample (T1) and a longitudinal plasma sample (T2) from P7 obtained 1 year later. The HDs tested (black) were healthy individuals tested at the time of collection of each of the patient samples, but the HD samples are not longitudinal.
POWV and USUV infections are rare in humans and the corresponding diseases are even rarer. RRV infection is endemic to Australia and many South Pacific islands, but severe cases are also rare. Among the cases we studied, auto-Abs neutralizing type I IFNs were found to underlie the only case of severe POWV encephalitis, two of the three cases of severe USUV disease, and the most severe case of RRV disease. These auto-Abs were absent from cases of moderate POWV disease, individuals with silent USUV infection, and mild and moderate cases of RRV disease. Due to the small number of patients with each arboviral disease, we were unable to calculate the relative risk of developing severe disease conferred by auto-Abs relative to the prevalence of auto-Abs in the corresponding demographic group (Bastard et al., 2021a). However, based on previous estimates of the prevalence of these auto-Abs in the general population (Bastard et al., 2021a) and their pathogenicity in a large proportion of patients with two common arboviral diseases—WNV (40%) (Gervais et al., 2023) and TBEV (10%) encephalitis (Gervais et al., 2024b)—our current findings provide strong evidence that these auto-Abs neutralizing type I IFN may underlie severe POWV, USUV, and RRV diseases (Casanova et al., 2024; Puel et al., 2022). These auto-Abs were probably present before infection with these viruses, as in patients with life-threatening COVID-19, influenza pneumonia (Bastard et al., 2020; Zhang et al., 2022b), or WNV encephalitis (Gervais et al., 2023), and as suggested recently by an elegant longitudinal survey of a large Swiss cohort (Fernbach et al., 2024). However, it was not possible to demonstrate this unequivocally due to the absence of sample collection from the patients before infection.
These findings suggest that people at risk of producing these auto-Abs, such as patients with a history of severe viral disease (Bastard et al., 2024), autoimmunity (Beydon et al., 2022; Mathian et al., 2022), or an inborn error of self-tolerance (Bastard et al., 2021a), and elderly individuals (Bastard et al., 2021a), would benefit from testing for these auto-Abs if they inhabit or plan to travel to areas of endemicity for POWV, USUV, or RRV. Our findings also suggest that treatment with a type I IFN that is not neutralized by these auto-Abs may be beneficial in patients testing positive for these viral infections before or during hospitalization. In principle, patients with auto-Abs neutralizing IFN-α2 could potentially benefit from treatment with IFN-β, whereas those with auto-Abs neutralizing IFN-β would benefit from treatment with IFN-α2 (Bastard et al., 2021e). High doses of the antigenic IFN itself might also be considered as a potentially beneficial means of overcoming these auto-Abs, as reported for the administration of GM-CSF to patients with auto-Abs neutralizing GM-CSF in the context of pulmonary alveolar proteinosis (Tian et al., 2020). We recently developed a rapid diagnostic test that can provide results within a few hours that could be used to screen for these auto-Abs in patients admitted with suspected arboviral disease (Gervais et al., 2024a).
Auto-Abs neutralizing type I IFNs can underlie severe diseases due not only to three respiratory RNA viruses—SARS-CoV-2 (Bastard et al., 2021a, 2023, 2021c, 2020, 2022b; Puel et al., 2022), influenza virus (Zhang et al., 2022b), and MERS (Alotaibi et al., 2023)—but also six flaviviruses: YFV-17D (Bastard et al., 2022a, 2021b; Duncan et al., 2022; Hernandez et al., 2019), WNV (Gervais et al., 2023), TBEV (Gervais et al., 2024b), POWV, and USUV, and a systemic alphavirus, RRV. Unlike the other viruses mentioned, RRV is not usually neurotropic. These auto-Abs against type I IFNs may also underlie severe disease caused by other arboviruses or respiratory viruses, or even non-respiratory viruses. They might also underlie natural viral infections of organs other than the lungs and brain. However, this hypothesis seems unlikely, given the surprisingly narrow range of severe viral diseases seen in patients with autosomal recessive complete genetic deficiencies of IFNAR1 or IFNAR2 (Abolhassani et al., 2022; Bastard et al., 2022a, 2021d; Duncan et al., 2015; Duncan et al., 2022; Hernandez et al., 2019). These patients suffer mostly from adverse reactions to attenuated live viral vaccines (Hernandez et al., 2019), critical viral pneumonia (Meyts and Casanova, 2021; Abolhassani et al., 2022; Bastard et al., 2022a; Duncan et al., 2022; Zhang et al., 2022a), or encephalitis (Bastard et al., 2021d, 2022a; Meyts and Casanova, 2021). The finding of auto-Abs underlying severe WNV encephalitis, TBE, POWV, USUV, or RRV disease in turn suggests that germline genetic deficiencies of type I IFN immunity should be sought in patients with severe arboviral diseases who do not carry auto-Abs against type I IFNs.
Materials and methods
Patients
We enrolled an international cohort of three patients infected with POWV from the US, 40 individuals infected with USUV (20 from France and 20 from Italy), and 96 individuals infected with RRV from Australia. Written informed consent was obtained in the country of residence of each patient, in accordance with local regulations and with institutional review board (IRB) approval. Sampling was performed during acute infection for the severe POWV case and after recovery for the two patients with moderate POWV disease. The USUV patients were sampled within a week of symptom onset and the asymptomatic cases were sampled at undermined times after infection. The RRV cases were sampled a mean of 33 days (range: 6–87 days) after symptom onset, and the severe RRV cases were sampled a mean of 14 days (range: 2–35 days) after symptom onset. In P1, P2, and P3, POWV infection was identified on the basis of the detection of anti-POWV IgM in the blood (and CSF for P3) followed by a PRNT against POWV. For USUV infection, P4 was diagnosed by RT-qPCR on serum samples. P5 was diagnosed by RT-qPCR on serum samples and by the presence of neutralizing IgM anti-USUV antibodies in the serum at day 8 after symptoms onset. Samples from P6 were tested in molecular and serological assays: serum, plasma, and urine specimens were extracted from 500 µl of the sample, eluted in a volume of 55 µl and tested by multiplex real-time PCR, which revealed the presence of USUV in all sample types (Gaibani et al., 2023). The asymptomatic cases were identified on the basis of positive results for RT-qPCR on serum samples. Finally, all RRV cases were diagnosed by serological analysis revealing the presence of anti-RRV IgM antibodies. The experiments for measurement of auto-Abs to type I IFNs were conducted in France and the USA, in accordance with local regulations and guidance from the French National Agency for Medicine and Health Product Safety, the Institut National de la Santé et de la Recherche Médicale in Paris, France, and with the approval of the IRB of the Rockefeller University in New York, NY, USA, respectively.
Luciferase reporter assay
The blocking activity of anti-IFN-α2, anti-IFN-ω, and anti-IFN-β auto-Abs was assessed in a reporter luciferase assay, as previously described (Bastard et al., 2021a). Briefly, HEK293T cells were transfected with a plasmid encoding the firefly luciferase gene under the control of the human IFN-sensitive response element (ISRE) promoter in the pGL4.45 backbone and a plasmid constitutively expressing the Renilla luciferase as a control for transfection (pRL-SV40). Cells were transfected in the presence of the X-tremeGene9 transfection reagent (ref. number 6365779001; Sigma-Aldrich). After 24 h, cells in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific) supplemented with 2% fetal calf serum and 10% control or patient serum/plasma/whole blood (after heat inactivation at 56°C, for 20 min) were either left unstimulated or were stimulated with unglycosylated rhIFN-α2 (ref. number 130-108-984; Miltenyi Biotec), unglycosylated rhIFN-ω (ref. number 300-02J; Peprotech) at a concentration of 10 ng/ml or 100 pg/ml, or glycosylated rhIFN-β (ref. number 300-02BC; Peprotech) at a concentration of 10 or 1 ng/ml for 16 h at 37°C under an atmosphere containing 5% CO2. Finally, the cells were lysed by incubation with a lysis buffer (provided in ref. number E1980; Promega) for 20 min at room temperature and luciferase levels were measured with the Dual-Luciferase Reporter 1000 assay system (ref. number E1980; Promega) according to the manufacturer’s protocol. Luminescence intensity was measured with a VICTOR-X Multilabel Plate Reader (PerkinElmer Life Sciences). Firefly luciferase activity values were normalized against Renilla luciferase activity values. The resulting values (luciferase induction) were then normalized against the median level of induction for non-neutralizing samples and expressed as a percentage (relative luciferase activity [RLA] ratio, %). Samples were considered to be neutralizing if the RLA ratio was below 15% of the median value for controls tested on the same day.
ELISA
ELISA was performed as previously described (Puel et al., 2008). In brief, 96-well ELISA plates (MaxiSorp; Thermo Fisher Scientific) were coated by overnight incubation at 4°C with 1 μg/ml rhIFN-α (ref. number 130-108-984; Miltenyi Biotec), rhIFN-ω (ref. number 300-02J; Peprotech), or rhIFN-β (ref. number 300-02BC; Peprotech). The plates were washed (PBS/0.005% Tween 20), blocked by incubation with the same buffer supplemented with 2% BSA, washed, and incubated with 1:50 dilutions of plasma samples from the patients or positive and negative controls for 2 h at room temperature. Each sample was tested once. Plates were thoroughly washed (PBS/0.005% Tween 20) and horseradish peroxidase–conjugated Fc-specific IgG fractions from polyclonal goat antiserum against human IgG (Nordic Immunological Laboratories) were added to a final concentration of 1 μg/ml. Plates were incubated for 1 h at room temperature and washed. The substrate was added and optical density was measured (450 nm). All the incubation steps were performed with gentle shaking (600 rpm).
Acknowledgments
We thank the patients and their families for participating in our research. We thank all members of both branches of the Laboratory of Human Genetics of Infectious Diseases for discussions and technical and administrative support. We thank Lazaro Lorenzo Diaz, Laboratory of Human Genetics of Infectious Diseases, Necker Branch, Institut National de la Santé et de la Recherche Médicale (INSERM) U1163, Necker Hospital for Sick Children, Paris, France, EU.
The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, The Rockefeller University, the St. Giles Foundation, the Stavros Niarchos Foundation (SNF) as part of its grant to the SNF Institute for Global Infectious Disease Research at The Rockefeller University, the National Institutes of Health (NIH) (R01AI163029), the National Center for Advancing Translational Sciences, the NIH Clinical and Translational Science Award (CTSA) program (UL1TR001866), the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the “Investissement d’Avenir” program launched by the French Government and implemented by the Agence Nationale de la Recherche (ANR) (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), ANR GENVIR (ANR-20-CE93-003), ANR AI2D (ANR-22-CE15-0046), GENFLU (ANR-22-CE92-0004), and ANR AAILC (ANR-21-LIBA-0002) projects, ANRS-COV05, the HORIZON-HLTH-2021-DISEASE-04 program under grant agreement 101057100 (UNDINE), the ANR-RHU COVIFERON Program (ANR-21-RHUS-08), the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, the Battersea & Bowery Advisory Group, William E. Ford, General Atlantic’s Chairman and Chief Executive Officer, Gabriel Caillaux, General Atlantic’s Co-President, Managing Director and Head of Business in EMEA, and the General Atlantic Foundation, the French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), INSERM, REACTing-INSERM, Paris Cité University, and the Imagine Institute. P. Bastard was supported by the MD-PhD program of the Imagine Institute (with support from the Bettencourt-Schueller Foundation), a “Poste CCA-INSERM-Bettencourt” (with support from the Bettencourt-Schueller Foundation), and the FRM (EA20170638020). A. Gervais was supported by a French governmental grant from the French National Agency for Research as part of the Investissement d’Avenir program (ANR-10-LABX-62-01). C.M. Rice and M.R. MacDonald were supported by NIH grants R01 AI124690, R21AI142010, and P01AI138938, and by grant UL1 TR001866 from the National Center for Advancing Translational Sciences NIH CTSA program. The Borghesi group, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, is supported by grants from the Italian Ministry of Health, RC08061819 and RC08061822, and by 5X1000 grant 08061821 from San Matteo Hospital. S.G. Tangye is supported by an Investigator Grant awarded by the National Health and Medical Research Council of Australia (Level 3; 1176665). Y. Simonin is supported by a grant from ANRS-Maladies Infectieuses Emergentes (ANRS-MIE) (reference: ANRS0348) under the “Emergences PRFI 2022” program. Open Access funding provided by Rockefeller University.
Author contributions: A. Gervais: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing, P. Bastard: Investigation, Methodology, Supervision, Writing - review & editing, L. Bizien: Investigation, C. Delifer: Project administration, Resources, P. Tiberghien: Resources, C. Rodrigo: Data curation, Resources, Writing - review & editing, F. Trespidi: Resources, M. Angelini: Data curation, Resources, Writing - review & editing, G. Rossini: Resources, T. Lazzarotto: Resources, Visualization, F. Conti: Resources, Visualization, I. Cassaniti: Methodology, F. Baldanti: Resources, Supervision, F. Rovida: Resources, A. Ferrari: Investigation, Methodology, D. Mileto: Investigation, Resources, A. Mancon: Investigation, Resources, Writing - review & editing, L. Abel: Formal analysis, Writing - review & editing, A. Puel: Funding acquisition, Investigation, Writing - review & editing, A. Cobat: Resources, Writing - review & editing, C.M. Rice: Conceptualization, Writing - review & editing, D. Cadar: Formal analysis, Resources, J. Schmidt-Chanasit: Resources, Writing - review & editing, J.F. Scheid: Investigation, Writing - review & editing, J.E. Lemieux: Investigation, Resources, E.S. Rosenberg: Resources, M. Agudelo: Resources, S.G. Tangye: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing - review & editing, A. Borghesi: Conceptualization, Data curation, Formal analysis, Validation, Writing - review & editing, G.A. Durand: Resources, E. Duburcq-Gury: Resources, B.M. Valencia: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing, A.R. Lloyd: Resources, Supervision, Writing - review & editing, A. Nagy: Resources, M.M. MacDonald: Conceptualization, Funding acquisition, Resources, Writing - review & editing, Y. Simonin: Funding acquisition, Resources, Writing - review & editing, S.-Y. Zhang: Conceptualization, Funding acquisition, Investigation, Supervision, Writing - original draft, Writing - review & editing, J.-L. Casanova: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing.
Data availability
All data supporting the findings of this study are available within the main text and supplemental material and from the corresponding authors upon request.
References
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolhassani, H., Landegren N., Bastard P., Materna M., Modaresi M., Du L., Aranda-Guillén M., Sardh F., Zuo F., Zhang P., et al. 2022. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J. Clin. Immunol. 42:471–483. 10.1007/s10875-022-01215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Ampudia, Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. 2021. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 118:102598. 10.1016/j.jaut.2021.102598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari, A., Hadizadeh A., Amiri M., Najafi N.N., Shahriari Z., Jamialahmadi T., and Sahebkar A.. 2023. Role of autoantibodies targeting interferon type 1 in COVID-19 severity: A systematic review and meta-analysis. J. Transl. Autoimmun. 7:100219. 10.1016/j.jtauto.2023.100219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbil, B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. 2022. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J. Clin. Immunol. 42:1111–1129. 10.1007/s10875-022-01252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi, F., Alharbi N.K., Rosen L.B., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Jeraisy M., Mandourah Y., AlJohani S., Al Harbi S., et al. 2023. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir. Viruses. 17:e13116. 10.1111/irv.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrestier, R., Bastard P., Belmondo T., Voiriot G., Urbina T., Luyt C.E., Gervais A., Bizien L., Segaux L., Ben Ahmed M., et al. 2022. Auto-antibodies against type I IFNs in > 10% of critically ill COVID-19 patients: A prospective multicentre study. Ann. Intensive Care. 12:121. 10.1186/s13613-022-01095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf, U., Ye J., Ruan X., Wan S., Zhu B., and Cao S.. 2015. Usutu virus: An emerging flavivirus in Europe. Viruses. 7:219–238. 10.3390/v7010219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassam, I.A.G., Serge P., and John P.P.. 1999. Powassan encephalitis: A case report with neuropathology and literature review. CMAJ. 161:1419–1422. [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. 2020. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 370:eabd4585. 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. 2021a. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 6:eabl4340. 10.1126/sciimmunol.abl4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. 2021b. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218:e20202486. 10.1084/jem.20202486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. 2021c. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 218:e20210554. 10.1084/jem.20210554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Manry J., Chen J., Rosain J., Seeleuthner Y., AbuZaitun O., Lorenzo L., Khan T., Hasek M., Hernandez N., et al. 2021d. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Invest. 131:e139980. 10.1172/JCI139980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Lévy R., Henriquez S., Bodemer C., Szwebel T.-A., and Casanova J.-L.. 2021e. Interferon-β therapy in a patient with incontinentia pigmenti and autoantibodies against type I IFNs infected with SARS-CoV-2. J. Clin. Immunol. 41:931–933. 10.1007/s10875-021-01023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Hsiao K.-C., Zhang Q., Choin J., Best E., Chen J., Gervais A., Bizien L., Materna M., Harmant C., et al. 2022a. A loss-of-function IFNAR1 allele in Polynesia underlies severe viral diseases in homozygotes. J. Exp. Med. 219:e20220028. 10.1084/jem.20220028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Vazquez S.E., Liu J., Laurie M.T., Wang C.Y., Gervais A., Le Voyer T., Bizien L., Zamecnik C., Philippot Q., et al. 2022b. Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-Abs neutralizing type I IFNs. Sci. Immunol. 8:eabp8966. 10.1126/sciimmunol.abp8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Gervais A., Taniguchi M., Saare L., Särekannu K., Le Voyer T., Philippot Q., Rosain J., Bizien L., Asano T., et al. 2023. Greater risk of COVID-19 pneumonia in children with autoantibodies neutralizing IFN-a than in those with autoantibodies neutralizing IFN-w. J. Exp. Med. 221:e20231353. 10.1084/jem.20231353 [DOI] [Google Scholar]
- Bastard, P., Gervais A., Le Voyer T., Philippot Q., Cobat A., Rosain J., Jouanguy E., Abel L., Zhang S.-Y., Zhang Q., et al. 2024. Human autoantibodies neutralizing type I IFNs: From 1981 to 2023. Immunol. Rev. 322:98–112. 10.1111/imr.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydon, M., Nicaise-Roland P., Mageau A., Farkh C., Daugas E., Descamps V., Dieude P., Dossier A., Goulenok T., Farhi F., et al. 2022. Autoantibodies against IFNα in patients with systemic lupus erythematosus and susceptibility for infection: A retrospective case-control study. Sci. Rep. 12:11244. 10.1038/s41598-022-15508-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnadiego, I., Abela I.A., Frey P.M., Hofmaenner D.A., Scheier T.C., Schuepbach R.A., Buehler P.K., Brugger S.D., and Hale B.G.. 2022. Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease. PLoS Biol. 20:e3001709. 10.1371/journal.pbio.3001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadar, D., and Simonin Y.. 2022. Human Usutu virus infections in Europe: A new risk on horizon? Viruses. 15:77. 10.3390/v15010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapito, R., Li R., Helms J., Carapito C., Gujja S., Rolli V., Guimaraes R., Malagon-Lopez J., Spinnhirny P., Lederle A., et al. 2022. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl. Med. 14:eabj7521. 10.1126/scitranslmed.abj7521 [DOI] [PubMed] [Google Scholar]
- Casanova, J.-L. 2023. From second thoughts on the germ theory to a full-blown host theory. Proc. Natl. Acad. Sci. USA. 120:e2301186120. 10.1073/pnas.2301186120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.-L., and Abel L.. 2024. The microbe, the infection enigma, and the host. Annu. Rev. Microbiol. In press. 10.1146/annurev-micro-092123-022855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.-L., Peel J., Donadieu J., Neehus A.-L., Puel A., and Bastard P.. 2024. The ouroboros of autoimmunity. Nat. Immunol. 25:743–754. 10.1038/s41590-024-01815-y [DOI] [PubMed] [Google Scholar]
- CDC . 2024. Powassan Virus: Historic Data (2004-2023). https://www.cdc.gov/powassan/data-maps/historic-data.html (accessed June 01, 2024).
- Chang, S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. 2021. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 12:5417. 10.1038/s41467-021-25509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvineau-Grenier, A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., Cobat A., Casanova J.L., and Rossi B.. 2022. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J. Clin. Immunol. 42:459–470. 10.1007/s10875-021-01203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clé, M., Beck C., Salinas S., Lecollinet S., Gutierrez S., Van de Perre P., Baldet T., Foulongne V., and Simonin Y.. 2019. Usutu virus: A new threat? Epidemiol. Infect. 147:e232. 10.1017/S0950268819001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle, J.J., Gunn J., Sangkhapreecha P., Monaco D.R., Zheng X.A., Tsai H.J., Wilbon A., Morgenlander W.R., Rastegar A., Dong Y., et al. 2022. Unbiased discovery of autoantibodies associated with severe COVID-19 via genome-scale self-assembled DNA-barcoded protein libraries. Nat. Biomed. Eng. 6:992–1003. 10.1038/s41551-022-00925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic, E., Li H., Hickie I.B., Wakefield D., Lloyd A.R., and Vollmer-Conna U.. 2019. Contribution of individual psychological and psychosocial factors to symptom severity and time-to-recovery after naturally-occurring acute infective illness: The Dubbo Infection Outcomes Study (DIOS). Brain Behav. Immun. 82:76–83. 10.1016/j.bbi.2019.07.034 [DOI] [PubMed] [Google Scholar]
- Davis, L.E., Beckham J.D., and Tyler K.L.. 2008. North American encephalitic arboviruses. Neurol. Clin. 26:727–757. 10.1016/j.ncl.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobler, G. 2010. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 140:221–228. 10.1016/j.vetmic.2009.08.024 [DOI] [PubMed] [Google Scholar]
- Duncan, C.J., Mohamad S.M., Young D.F., Skelton A.J., Leahy T.R., Munday D.C., Butler K.M., Morfopoulou S., Brown J.R., Hubank M., et al. 2015. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci. Transl. Med. 7:307ra154. 10.1126/scitranslmed.aac4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, C.J.A., Skouboe M.K., Howarth S., Hollensen A.K., Chen R., Børresen M.L., Thompson B.J., Stremenova Spegarova J., Hatton C.F., Stæger F.F., et al. 2022. Life-threatening viral disease in a novel form of autosomal recessive IFNAR2 deficiency in the Arctic. J. Exp. Med. 219:e20212427. 10.1084/jem.20212427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto, S., Nukui Y., Tsumura M., Nakagama Y., Kashimada K., Mizoguchi Y., Utsumi T., Taniguchi M., Sakura F., Noma K., et al. 2022. Neutralizing type I interferon autoantibodies in Japanese patients with severe COVID-19. J. Clin. Immunol. 42:1360–1370. 10.1007/s10875-022-01308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . 2023. Surveillance, prevention and control of West Nile virus and Usutu virus infections in the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/surveillance-prevention-and-control-west-nile-virus-and-usutu-virus-infections (accessed June 01, 2024).
- Fernbach, S., Mair N.K., Abela I.A., Groen K., Kuratli R., Lork M., Thorball C.W., Bernasconi E., Filippidis P., Leuzinger K., et al. 2024. Loss of tolerance precedes triggering and lifelong persistence of pathogenic type I interferon autoantibodies. J. Exp. Med. 221:e20240365. 10.1084/jem.20240365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca, F., Scordio M., Santinelli L., Gabriele L., Gandini O., Criniti A., Pierangeli A., Angeloni A., Mastroianni C.M., d’Ettorre G., et al. 2022. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 52:1120–1128. 10.1002/eji.202249824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, H.M., Schotthoefer A.M., Thomm A.M., Dupuis A.P. II, Kehl S.C., Kramer L.D., Fritsche T.R., Harrington Y.A., and Knox K.K.. 2017. Serologic evidence of powassan virus infection in patients with suspected lyme disease. Emerg. Infect. Dis. 23:1384–1388. 10.3201/eid2308.161971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaibani, P., Barp N., Massari M., Negri E.A., Rossini G., Vocale C., Trenti C., Gallerani A., Cantergiani S., Romani F., et al. 2023. Case report of Usutu virus infection in an immunocompromised patient in Italy, 2022. J. Neurovirol. 29:364–366. 10.1007/s13365-023-01148-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais, A., Rovida F., Avanzini M.A., Croce S., Marchal A., Lin S.C., Ferrari A., Thorball C.W., Constant O., Le Voyer T., et al. 2023. Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in approximately 40% of patients. J. Exp. Med. 220:e20230661. 10.1084/jem.20230661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais, A., Le Floc’h C., Le Voyer T., Bizien L., Bohlen J., Celmeli F., Al Qureshah F., Masson C., Rosain J., Chbihi M., et al. 2024a. A sensitive assay for measuring whole-blood responses to type I IFNs. Proc. Natl. Acad. Sci. USA. 121:e2402983121. 10.1073/pnas.2402983121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais, A., Marchal A., Fortova A., Berankova M., Krbkova L., Pychova M., Salat J., Zhao S., Kerrouche N., Le Voyer T., et al. 2024b. Autoantibodies neutralizing type I IFNs underlie severe tick-borne encephalitis in ∼10% of patients. J. Exp. Med. 221:e20240637. 10.1084/jem.20240637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., Pescarmona R., Lombard C., Walzer T., Casanova J.L., et al. 2021. Antibodies against type I interferon: Detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl. Immunol. 10:e1327. 10.1002/cti2.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, E.A., and Solomon T.. 2008. Pathogenic flaviviruses. Lancet. 371:500–509. 10.1016/S0140-6736(08)60238-X [DOI] [PubMed] [Google Scholar]
- Grimm, L., Onyeukwu C., Kenny G., Parent D.M., Fu J., Dhingra S., Yang E., Moy J., Utz P.J., Tracy R., and Landay A.. 2023. Immune dysregulation in acute SARS-CoV-2 infection. Pathog. Immun. 7:143–170. 10.20411/pai.v7i2.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale, B.G. 2023. Autoantibodies targeting type I interferons: Prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur. J. Immunol. 53:e2250164. 10.1002/eji.202250164 [DOI] [PubMed] [Google Scholar]
- Hansen, K.S., Jørgensen S.E., Skouboe M.K., Agergaard J., Schiøttz-Christensen B., Vibholm L.K., Tolstrup M., Østergaard L., Leth S., and Mogensen T.H.. 2023. Examination of autoantibodies to type I interferon in patients suffering from long COVID. J. Med. Virol. 95:e29089. 10.1002/jmv.29089 [DOI] [PubMed] [Google Scholar]
- Harley, D., Sleigh A., and Ritchie S.. 2001. Ross River virus transmission, infection, and disease: A cross-disciplinary review. Clin. Microbiol. Rev. 14:909–932. 10.1128/CMR.14.4.909-932.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermance, M.E., and Thangamani S.. 2017. Powassan virus: An emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis. 17:453–462. 10.1089/vbz.2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, N., Bucciol G., Moens L., Le Pen J., Shahrooei M., Goudouris E., Shirkani A., Changi-Ashtiani M., Rokni-Zadeh H., Sayar E.H., et al. 2019. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J. Exp. Med. 216:2057–2070. 10.1084/jem.20182295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketkar, H., Herman D., and Wang P.. 2019. Genetic determinants of the Re-emergence of arboviral diseases. Viruses. 11:150. 10.3390/v11020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning, R., Bastard P., Casanova J.L., Brouwer M.C., van de Beek D., van Agtmael M., Algera A.G., Appelman B., van Baarle F., Bax D., et al. 2021. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 47:704–706. 10.1007/s00134-021-06392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia, G., Mazzoni A., Spinicci M., Vanni A., Salvati L., Peruzzi B., Bencini S., Capone M., Carnasciali A., Farahvachi P., et al. 2022. Clinical and immunological features of SARS-CoV-2 breakthrough infections in vaccinated individuals requiring hospitalization. J. Clin. Immunol. 42:1379–1391. 10.1007/s10875-022-01325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarquis, A., Campbell T., Aranda-Guillén M., Hennings V., Brodin P., Kämpe O., Blennow K., Zetterberg H., Wennerås C., Eriksson K., et al. 2021. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 148:96–98. 10.1016/j.jaci.2021.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madewell, Z.J. 2020. Arboviruses and their vectors. South. Med. J. 113:520–523. 10.14423/SMJ.0000000000001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madzokere, E.T., Qian W., Webster J.A., Walker D.M.H., Lim E.X.Y., Harley D., and Herrero L.J.. 2022. Human seroprevalence for dengue, Ross River, and Barmah forest viruses in Australia and the Pacific: A systematic review spanning seven decades. PLoS Negl. Trop. Dis. 16:e0010314. 10.1371/journal.pntd.0010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathian, A., Breillat P., Dorgham K., Bastard P., Charre C., Lhote R., Quentric P., Moyon Q., Mariaggi A.A., Mouries-Martin S., et al. 2022. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Ann. Rheum. Dis. 81:1695–1703. 10.1136/ard-2022-222549 [DOI] [PubMed] [Google Scholar]
- Meisel, C., Akbil B., Meyer T., Lankes E., Corman V.M., Staudacher O., Unterwalder N., Kölsch U., Drosten C., Mall M.A., et al. 2021. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 131:e150867. 10.1172/JCI150867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts, I., and Casanova J.L.. 2021. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur. J. Immunol. 51:1039–1061. 10.1002/eji.202048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolay, B., Diallo M., Boye C.S.B., and Sall A.A.. 2011. Usutu virus in Africa. Vector Borne Zoonotic Dis. 11:1417–1423. 10.1089/vbz.2011.0631 [DOI] [PubMed] [Google Scholar]
- Petrikov, S.S., Borovkova N.V., Popugaev K.A., Storozheva M.V., Kvasnikov A.M., and Godkov M.A.. 2022. Anti-interferon alpha autoantibodies and their significance in COVID-19. Infektsiia Immun. 12:279–287. 10.15789/2220-7619-AAA-1789 [DOI] [Google Scholar]
- Philippot, Q., Fekkar A., Gervais A., Le Voyer T., Boers L.S., Conil C., Bizien L., de Brabander J., Duitman J.W., Romano A., et al. 2023. Autoantibodies neutralizing type I IFNs in the bronchoalveolar lavage of at least 10% of patients during life-threatening COVID-19 pneumonia. J. Clin. Immunol. 43:1093–1103. 10.1007/s10875-023-01512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, T.C., and Diamond M.S.. 2020. The continued threat of emerging flaviviruses. Nat. Microbiol. 5:796–812. 10.1038/s41564-020-0714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons, M.J., Mayanga-Herrera A., Palomino-Kobayashi L.A., Quispe A.M., and Ugarte-Gil M.F.. 2023. High anti-interferon-alpha autoantibody levels in severe/critical COVID-19 patients from Peru. J. Interferon Cytokine Res. 43:565–570. 10.1089/jir.2023.0087 [DOI] [PubMed] [Google Scholar]
- Puel, A., Picard C., Lorrot M., Pons C., Chrabieh M., Lorenzo L., Mamani-Matsuda M., Jouanguy E., Gendrel D., and Casanova J.L.. 2008. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180:647–654. 10.4049/jimmunol.180.1.647 [DOI] [PubMed] [Google Scholar]
- Puel, A., Bastard P., Bustamante J., and Casanova J.L.. 2022. Human autoantibodies underlying infectious diseases. J. Exp. Med. 219:e20211387. 10.1084/jem.20211387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsen, M.P., Gharbharan A., Jordans C.C.E., Mykytyn A.Z., Lamers M.M., van den Doel P.B., Endeman H., van den Akker J.P.C., GeurtsvanKessel C.H., Koopmans M.P.G., et al. 2022. Interferon-α2 auto-antibodies in convalescent plasma therapy for COVID-19. J. Clin. Immunol. 42:232–239. 10.1007/s10875-021-01168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, R.C. 2002. Ross River virus: Ecology and distribution. Annu. Rev. Entomol. 47:1–31. 10.1146/annurev.ento.47.091201.145100 [DOI] [PubMed] [Google Scholar]
- Saheb Sharif-Askari, F., Saheb Sharif-Askari N., Hafezi S., Alsayed H.A.H., Selvakumar B., Eladham M.W.A., Mdkhana B., Bayram O.S., Temsah M.-H., and Halwani R.. 2023. Increased blood immune regulatory cells in severe COVID-19 with autoantibodies to type I interferons. Sci. Rep. 13:17344. 10.1038/s41598-023-43675-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvateeva, E., Filippova M., Valuev-Elliston V., Nuralieva N., Yukina M., Troshina E., Baklaushev V., Ivanov A., and Gryadunov D.. 2021. Microarray-based detection of antibodies against SARS-CoV-2 proteins, common respiratory viruses and type I interferons. Viruses. 13:2553. 10.3390/v13122553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schidlowski, L., Iwamura A.P.D., Condino-Neto A., Prando C., and COVID-SUD . 2022. Diagnosis of APS-1 in two siblings following life-threatening COVID-19 pneumonia. J. Clin. Immunol. 42:749–752. 10.1007/s10875-022-01245-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simula, E.R., Manca M.A., Noli M., Jasemi S., Ruberto S., Uzzau S., Rubino S., Manca P., and Sechi L.A.. 2022. Increased presence of antibodies against type I interferons and human endogenous retrovirus W in intensive care unit COVID-19 patients. Microbiol. Spectr. 10:e0128022. 10.1128/spectrum.01280-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanich, X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., Perez-Fernandez X.L., Fuset-Cabanes M.P., Gordillo-Benitez M.A., Suarez-Cuartin G., et al. 2021. Pre-existing autoantibodies neutralizing high concentrations of type I interferons in almost 10% of COVID-19 patients admitted to intensive care in barcelona. J. Clin. Immunol. 41:1733–1744. 10.1007/s10875-021-01136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani-Zangbar, M.S., Parhizkar F., Ghaedi E., Tarbiat A., Motavalli R., Alizadegan A., Aghebati-Maleki L., Rostamzadeh D., Yousefzadeh Y., Jadideslam G., et al. 2022. A comprehensive evaluation of the immune system response and type-I Interferon signaling pathway in hospitalized COVID-19 patients. Cell Commun. Signal. 20:106. 10.1186/s12964-022-00903-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., et al. 2022. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 185:881–895.e20. 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X., Yang Y., Chen L., Sui X., Xu W., Li X., Guo X., Liu L., Situ Y., Wang J., et al. 2020. Inhaled granulocyte-macrophage colony stimulating factor for mild-to-moderate autoimmune pulmonary alveolar proteinosis - a six month phase II randomized study with 24 months of follow-up. Orphanet J. Rare Dis. 15:174. 10.1186/s13023-020-01450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troya, J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martínez A., Abel L., Casanova J.L., and Pujol A.. 2021. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J. Clin. Immunol. 41:914–922. 10.1007/s10875-021-01036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey, G.M., Wilson N., McDonald E., Fitzpatrick K., Lehman J., Clark S., Lindell K., Pastula D.M., Perez S., Rhodes H., et al. 2022. Seroprevalence of powassan virus infection in an area experiencing a cluster of disease cases: Sussex county, New Jersey, 2019. Open Forum Infect. Dis. 9:ofac023. 10.1093/ofid/ofac023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wijst, M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. 2021. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 13:eabh2624. 10.1126/scitranslmed.abh2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanker, M., Särekannu K., Fekkar A., Jørgensen S.E., Haljasmägi L., Kallaste A., Kisand K., Lember M., Peterson P., Menon M., et al. 2023. Autoantibodies neutralizing type III interferons are uncommon in patients with severe coronavirus disease 2019 pneumonia. J. Interferon Cytokine Res. 43:379–393. 10.1089/jir.2023.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez, S.E., Bastard P., Kelly K., Gervais A., Norris P.J., Dumont L.J., Casanova J.L., Anderson M.S., and DeRisi J.L.. 2021. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J. Clin. Immunol. 41:1169–1171. 10.1007/s10875-021-01060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. 2021. Diverse functional autoantibodies in patients with COVID-19. Nature. 595:283–288. 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- Wilder-Smith, A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., and Scott T.W.. 2017. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 17:e101–e106. 10.1016/S1473-3099(16)30518-7 [DOI] [PubMed] [Google Scholar]
- Yuen, K.Y., and Bielefeldt-Ohmann H.. 2021. Ross River virus infection: A cross-disciplinary review with a veterinary perspective. Pathogens. 10:357. 10.3390/pathogens10030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Bastard P., Cobat A., Casanova J.L., and COVID Human Genetic Effort . 2022a. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 603:587–598. 10.1038/s41586-022-04447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Pizzorno A., Miorin L., Bastard P., Gervais A., Le Voyer T., Bizien L., Manry J., Rosain J., Philippot Q., et al. 2022b. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 219:e20220514. 10.1084/jem.20220514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, C.G.K., Miao V.N., Owings A.H., Navia A.W., Tang Y., Bromley J.D., Lotfy P., Sloan M., Laird H., Williams H.B., et al. 2021. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 184:4713–4733.e22. 10.1016/j.cell.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the main text and supplemental material and from the corresponding authors upon request.