Abstract
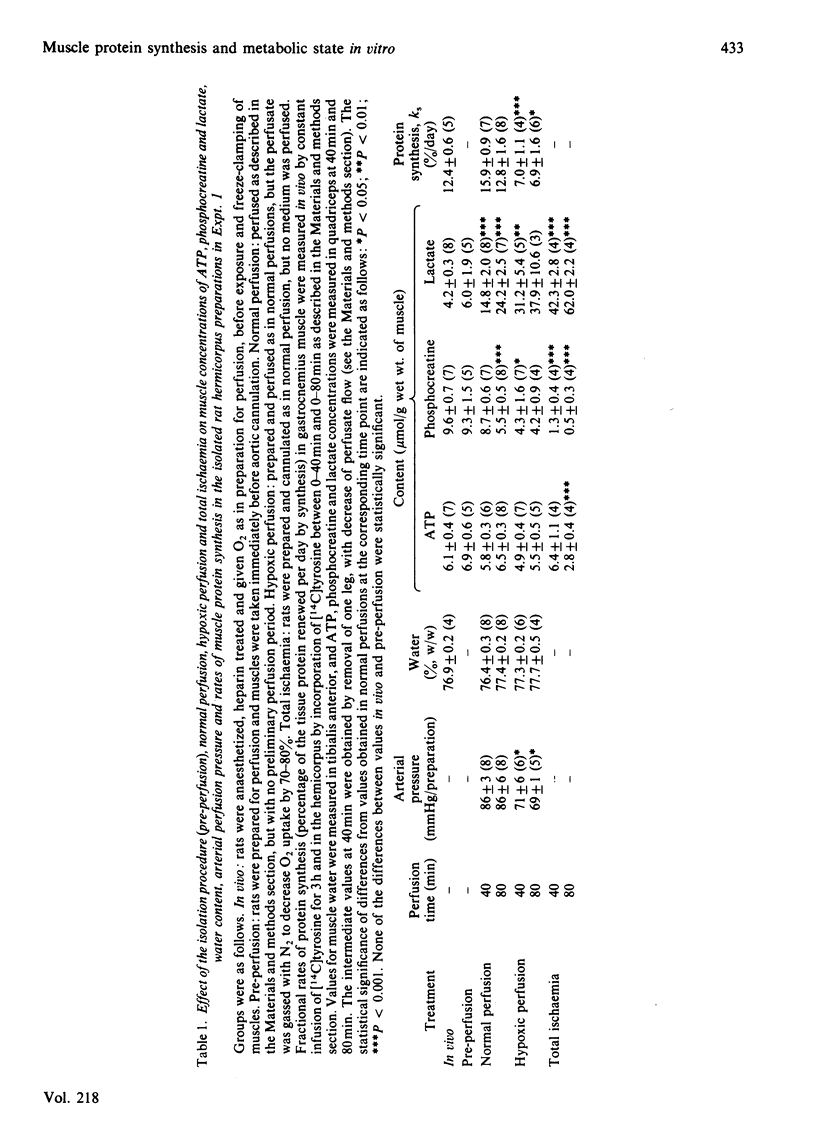
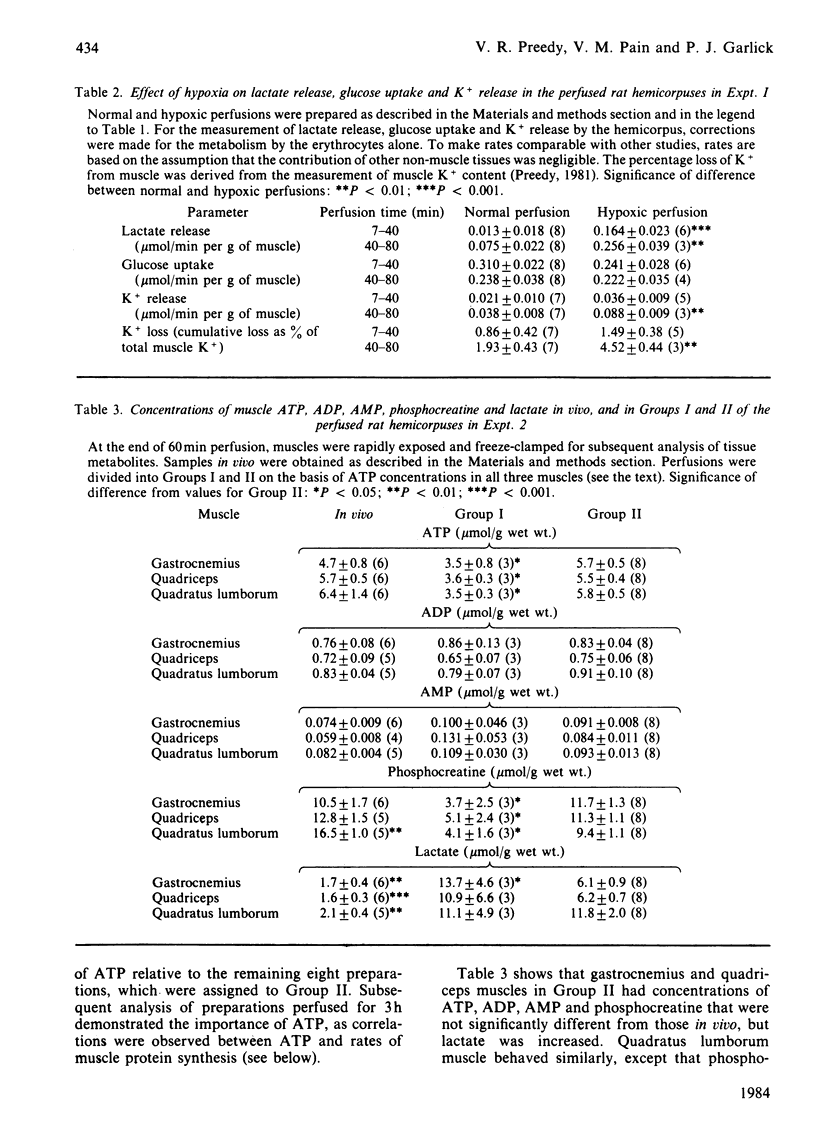
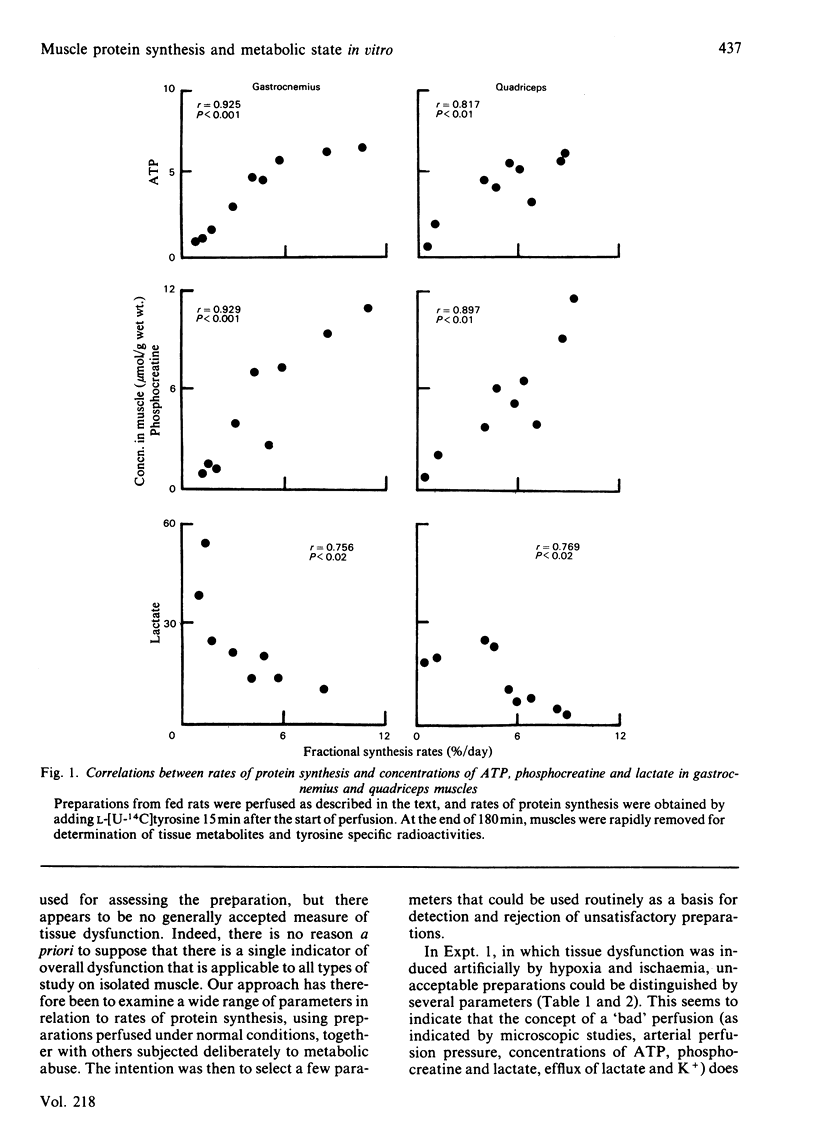
Measures of perfusion adequacy in perfused rat hemicorpus preparations were investigated as potential indices of tissue function during studies of muscle protein metabolism. Perfusion under normal conditions for up to 80 min resulted in rates of protein synthesis and concentrations of ATP in muscle that were similar to those in vivo, but phosphocreatine in muscle gradually decreased and muscle lactate increased. Hypoxic conditions led to lower rates of protein synthesis, lower phospho-creatine and raised lactate contents in muscle compared with normal perfusions, and ATP was slightly decreased. Hypoxic preparations also released more lactate and K+ into the medium and had higher perfusion pressures, but glucose uptake and muscle water content were not altered. In totally ischaemic muscle, concentrations of ATP and phosphocreatine were even lower than in hypoxic muscle, and that of lactate was higher. From 11 preparations perfused for 60 min under normal conditions, three were selected on the basis of lower muscle ATP content than the others. Preparations with low ATP also showed lower muscle phosphocreatine concentrations, O2 uptake and CO2 output, as well as higher perfusion pressure and muscle lactate concentrations than in the remaining preparations, but muscle water, ADP and AMP concentrations and lactate and K+ flux were no different. In perfusions extended to 3 h, deterioration of function was more apparent. There were significant correlations between rates of protein synthesis and the concentrations of ATP, phosphocreatine and lactate in two different muscles (r = 0.756-0.929), but not with any of the other indices investigated. Taken overall, these experiments showed that concentrations of ADP, AMP and water in muscle, rates of lactate and glucose metabolism, K+ output, perfusion pressure and blood gas parameters were unsuitable for distinguishing unsound from sound preparations, because they did not consistently demonstrate differences, or could not be ascribed to only muscle metabolism. It was found that ATP, phosphocreatine and lactate concentrations in muscle were the best indicators of impaired metabolic state in studies of protein synthesis. Measurements of these could be used on a routine basis for rejecting unsatisfactory preparations.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayuso-Parrilla M. S., Parrilla R. Control of hepatic protein synthesis. Differential effects of ATP levels on the initiation and elongation steps. Eur J Biochem. 1975 Jul 15;55(3):593–599. doi: 10.1111/j.1432-1033.1975.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Barak A. J., Beckenhauer H. C., Tuma D. J. A method for studying the uptake of creatine by a perfused hind limb preparation. Can J Physiol Pharmacol. 1971 Jun;49(6):612–614. doi: 10.1139/y71-079. [DOI] [PubMed] [Google Scholar]
- Bloxam D. L. Nutritional aspects of amino acid metabolism. 1. A rat liver perfusion method for the study of amino acid metabolism. Br J Nutr. 1971 Nov;26(3):393–422. doi: 10.1079/bjn19710046. [DOI] [PubMed] [Google Scholar]
- Cain S. M., Chapler C. K. O2 extraction by canine hindlimb during alpha-adrenergic blockade and hypoxic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1980 Apr;48(4):630–635. doi: 10.1152/jappl.1980.48.4.630. [DOI] [PubMed] [Google Scholar]
- Caldwell M. D., Lacy W. W., Exton J. H. Effects of adrenalectomy on the amino acid and glucose metabolism of perfused rat hindlimbs. J Biol Chem. 1978 Oct 10;253(19):6837–6844. [PubMed] [Google Scholar]
- Dobbs B. R., Lee D. The effect of purification of commercial bovine serum albumin on the performance of isolated rat livers perfused at 5 degrees C. Cryobiology. 1979 Oct;16(5):461–467. doi: 10.1016/0011-2240(79)90060-9. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Kasperek G. J., Tapscott E. B., Beecher G. R. Effect of exercise on synthesis and degradation of muscle protein. Biochem J. 1980 Apr 15;188(1):255–262. doi: 10.1042/bj1880255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draznin B., Solomons C. C., Emler C. A., Schalch D. S., Sussman K. E. Decreased insulin binding and degradation associated with depressed intracellular ATP content. Diabetes. 1980 Mar;29(3):221–226. doi: 10.2337/diab.29.3.221. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B., Aoki T. T. Glucose and amino acid metabolism in perfused skeletal muscle. Effect of dichloroacetate. Diabetes. 1978 Nov;27(11):1065–1074. doi: 10.2337/diab.27.11.1065. [DOI] [PubMed] [Google Scholar]
- Haljamäe H., Enger E. Human skeletal muscle energy metabolism during and after complete tourniquet ischemia. Ann Surg. 1975 Jul;182(1):9–14. doi: 10.1097/00000658-197507000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillgartner F. B., Morin D., Hansen R. J. Effect of excessive vitamin A intake on muscle protein turnover in the rat. Biochem J. 1982 Feb 15;202(2):499–508. doi: 10.1042/bj2020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S. A technique for perfusion of an isolated preparation of rat hemicorpus. Methods Enzymol. 1975;39:73–82. doi: 10.1016/s0076-6879(75)39011-3. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Krone W., Huttner W. B., Kampf S. C., Rittich B., Seitz H. J., Tarnowski W. Long-term perfusion of the isolated rat liver: maintenance of its functional state by use of a fluorocarbon emulsion. Biochim Biophys Acta. 1974 Nov 4;372(1):55–71. doi: 10.1016/0304-4165(74)90073-7. [DOI] [PubMed] [Google Scholar]
- Lee D., Holland R. K. Improved performance of the isolated rat liver when perfused with purified bovine serum albumin. Transplantation. 1979 Jun;27(6):384–388. [PubMed] [Google Scholar]
- Li J. B., Jefferson L. S. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta. 1978 Dec 1;544(2):351–359. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- MEHL R. L., PAUL H. A., SHOREY W. D., SCHNEEWIND J. H., BEATTIE E. J., Jr PATENCY OF THE MICROCIRCULATION IN THE TRAUMATICALLY AMPUTATED LIMB--A COMPARISON OF COMMON PERFUSATES. J Trauma. 1964 Jul;4:495–505. doi: 10.1097/00005373-196407000-00005. [DOI] [PubMed] [Google Scholar]
- MILLER L. L., BLY C. G., BALE W. F. Plasma and tissue proteins produced by non-hepatic rat organs as studied with lysine-epsilon-C14; gamma globulins the chief plasma protein fraction produced by non-hepatic tissues. J Exp Med. 1954 Feb;99(2):133–153. doi: 10.1084/jem.99.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian H., Huang S. Effect of sodium azide on the ultrastructural preservation of tissues. J Microsc. 1979 Nov;117(2):243–253. doi: 10.1111/j.1365-2818.1979.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Payne P. R., Stewart R. J. Cubed diets of high and low protein values. Lab Anim. 1972 May;6(2):135–140. doi: 10.1258/002367772781006293. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. Protein synthesis in skeletal muscle of the perfused rat hemicorpus compared with rates in the intact animal. Biochem J. 1983 Aug 15;214(2):433–442. doi: 10.1042/bj2140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. Rates of protein synthesis in skin and bone, and their importance in the assessment of protein degradation in the perfused rat hemicorpus. Biochem J. 1981 Jan 15;194(1):373–376. doi: 10.1042/bj1940373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer F., Löffler G., Hennig G., Wieland O. H. The influence of insulin on glucose and fatty acid metabolism in the isolated perfused rat hind quarter. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):1055–1066. doi: 10.1515/bchm2.1975.356.s1.1055. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Holloszy J. O. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J. 1977 Nov 15;168(2):161–170. doi: 10.1042/bj1680161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rookledge K. A. Comparison of some metabolic parameters in the perfused and the incubated rat diaphragm muscle with diaphragm muscle in vivo. Biochem J. 1971 Nov;125(1):93–96. doi: 10.1042/bj1250093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Kemmer F. W., Goodman M. N., Berger M. Oxygen consumption in perfused skeletal muscle. Effect of perfusion with aged, fresh and aged-rejuvenated erythrocytes on oxygen consumption, tissue metabolites and inhibition of glucose utilization by acetoacetate. Biochem J. 1980 Jul 15;190(1):57–64. doi: 10.1042/bj1900057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourova L. M., Berezov T. T. Puti i vozmozhnosti biokhimichekoi otsenki zhiznesposobnosti organov pri ostroi ishemii. Vestn Akad Med Nauk SSSR. 1975;(7):46–51. [PubMed] [Google Scholar]
- Selman B. J., Tait A. R. Towards blood-gas autoanalysis an evaluation of the radiometer ABL. Br J Anaesth. 1976 May;48(5):487–494. doi: 10.1093/bja/48.5.487. [DOI] [PubMed] [Google Scholar]
- Strohfeldt P., Kettl H., Weinges K. F. Perfusion of the isolated rat hindlimb with a synthetic medium. Horm Metab Res. 1974 Mar;6(2):167–168. doi: 10.1055/s-0028-1095704. [DOI] [PubMed] [Google Scholar]
- Ward L. C., Buttery P. J. The kinetics of myofibrillar protein breakdown in perfused rat skeletal muscle. Biochim Biophys Acta. 1979 Oct 18;587(3):415–423. doi: 10.1016/0304-4165(79)90445-8. [DOI] [PubMed] [Google Scholar]


