Abstract
Cover cropping is a soil restorative strategy that can save degraded soils and offer additional benefits relative to the traditional fallow-based practice in semi-arid cropping systems. This study aimed to (i) quantify the above (shoot)- and belowground (root) biomass production, nutritive value, and tissue carbon and nitrogen concentrations from different annual cool-season cover crop systems, and (ii) determine their effects on soil organic carbon, total nitrogen, soil respiration, and soil microbial population biomass in a semi-arid environment. Treatments used were monocultures of annual ryegrass (Lolium multiflorum Lam.), oat (Avena sativa L.), faba bean (Vicia faba L.), yellow sweetclover [Melilotus officinalis (L.) Lam], winter pea (Pisum sativum L.), two three-species mixtures (Mix 1: annual ryegrass + faba bean + yellow sweetclover and Mix 2: Oat + faba bean + winter pea), and a fallow laid out in a randomized complete block design with three replications. Averaged across years, Mix 2 produced greater shoot biomass (9714 kg DM ha−1; SE = 699) than all other cover crop systems except, the monoculture of oat (7970 kg DM ha−1; SE = 699). The plant tissue C/N ratio of the mixtures and monoculture legumes was mostly similar (range = 19.4–29.1). Overall, legumes produced superior relative feed value (RFV; 112–161) compared to grass monocultures and mixtures (RFV; 80–95). Soil gram-negative bacteria biomass was greatest under the yellow sweetclover monoculture. Based on the results of this study, the mixed cover crop systems (Mix 1 and Mix 2) offered a better chance of fulfilling the dual role of soil health improvement and feed quality for livestock in this semi-arid environment.
Keywords: Cover crop, Biomass, Carbon-to-nitrogen ratio, Forage quality, Microbial biomass, Soil respiration, Soil organic carbon, And soil total nitrogen
1. Introduction
Arid and semi-arid agroecosystems of the United States are characterized by perennial challenges of water deficit, low soil moisture retention, overall poor soil fertility, and vulnerability to both wind and water erosion [1]. The acceleration of soil degradation in this region has been exacerbated by intensive tillage-based cropping systems that pose a threat to sustainable crop production [2]. For example, soil organic carbon and nitrogen are integral to soil fertility, and cropping system approaches are influential in altering these important soil factors to negatively influence soil fertility [3,4]. These constraints impede sustainable agricultural production in these regions and have augmented a drive towards alternative cropping systems that can help mitigate soil degradation and restore soil health. Modern approaches to sustainable agricultural practices rely heavily on three main principles; reduced or no-tillage, maintaining permanent soil cover, and increased diversity through diversification of crop rotations/intercropping [5].
A soil restorative strategy that has been widely studied and practiced on degraded soils or to protect and enhance soil physical, chemical, and biological properties is cover cropping as an approach to sustainable crop cultivation [6]. The integration of cover crops into cropping systems has reduced soil erosion and agrochemical runoff [7], increased water infiltration rate and storage, and provided abundant organic residues to enhance the biological and physical qualities of soils for crop production [8,9]. Legume cover crops can improve soil quality by fixing atmospheric nitrogen whereas non-legume cover crops are effective for improving soils by elevating the organic matter content of the soil due to the increased biomass and high carbon release in the soils [10,11]. Most cover crop studies, however, have focused on monocultures or mixtures of just two species [[12], [13], [14]] and very little research has been done on structured comparisons of polyculture mixtures more so in semi-arid Nevada. In Nebraska, a study of cover crop mixtures using two-, four-, six-, or eight-species showed more productivity by the highly diverse mixtures compared to the lesser entry systems [15]. However, Liebig et al. [16] reported no clear yield advantage among monocultures and mixtures of cover crops with and without functional groups in Mandan, North Dakota. Diverse cover crop species have been proposed as an approach to increasing services from cover crops because of increases in biomass production from the mixtures [17,18].
The selection of species to use in cover crop mixtures is critical for niche complementarity and security more so, in dryland environments where multiple challenges exist [[19], [20], [21]]. Hence, cover crop mixtures that comprise species exhibiting differences in architecture, physiology, and phenology are expected to have reduced production risk and optimum biomass production [16,22]. A selection approach based on multifunctional characteristics may best suit resource-limited agroecosystems. For example, in mixed grass-legume systems, grasses will exploit soil N resources whereas legumes fix atmospheric N that is available for the subsequent crop after residue decomposition and mineralization [[23], [24], [25], [26]]. Several studies have reported that biomass yield from grass-legume mixtures is often the same or greater than yield estimates from monocultures [21,[26], [27], [28]]. Aside from biomass, other cover crop characteristics that facilitate other important ecosystem services from mixtures must be considered. For example, the carbon-to-nitrogen ratio of cover crop biomass is an important functional trait that influences subsequent crop yield [29] and can be manipulated through the species composition of mixtures [17,23,30]. Nutrient recycling is one of the main co-benefits of cover crops to soil productivity. Therefore, the plant tissue characteristics become critical as the carbon-to-nitrogen (C/N) ratio of cover crop residue is the primary driver of nitrogen supply to the following cash crop [31]. Generally, legumes accumulate greater biomass N content than grasses and since they have a lower tissue C/N ratio, the greater release of nitrogen supply from their decomposing residues is common [32]. In contrast, grass cover crops scavenge a greater proportion of the soil organic nitrogen and have a relatively higher C/N ratio, which increases with plant maturity [33].
A unique fit for cover crops in Nevada is between the period after termination and reseeding of alfalfa (Medicago sativa L.) the major cash crop after the traditional 4–5 years of production. The traditional alfalfa cropping system practice in Nevada is rotation with winter cereal small grains, corn, or a fallow period of 1–2 years before reseeding alfalfa in the same field. Incorporating cover crops after the termination of alfalfa serves multiple roles such as minimizing alfalfa autotoxicity [34], enhancing the stability of the agroecosystem with soil cover [35], and can serve as a valuable feed resource for livestock [36]. Despite the increasing number of published studies on different cover cropping systems and their benefits, farmers in the western United States in states such as Nevada are still reluctant to integrate this system into their existing cropping practice. This reluctance by farmers in the western United States to adopt cover cropping practices is due to the prohibitive cost associated with the acquisition of cover crop species seed, scarce and variable irrigation water supply, inadequate knowledge on what cover crop species or mixtures to grow in such environments, and the overall lack of immediate economic benefits from the integration of cover cropping in their existing cropping systems [37,38].
Unlike the majority of referenced studies above that were carried out primarily in rainfed agricultural systems, Nevada is the driest state in the contiguous United States. Nevada is characterized by high desert, low levels of precipitation annually (241 mm annual precipitation), and high water loss through evapotranspiration which means irrigation water is required to optimize crop growth and production [39,40]. Given the benefits and challenges earmarked of cover cropping in water-scarce regions like Nevada, there is an urgent need to find suitable cover cropping systems that will thrive under low agronomic inputs to integrate into such environments that rely solely on irrigation water for crop production. Thus, beyond ecosystem services of soil health, high-quality biomass production and forage nutritive value for animal feeding will incentivize producers to integrate cover cropping during periods of breaks in their cash crop production cycle. Therefore, finding cover crop systems that are adapted and productive on the soils and climatic conditions of this environment will be required for greater adoption of cover crop systems across the western United States and similar environments across the globe. However, there is a paucity of such information on cover crop systems that can serve the dual role of producing high-quality biomass for animal feeding and improved soil health in low-precipitation environments like Nevada. We hypothesized that the two cover crop mixtures would produce greater above (shoot) and belowground (root) biomass, forage nutritive value, lower tissue carbon-to-nitrogen (C/N) ratio, and promote greater soil organic carbon, total nitrogen, and microbial biomass and diversity than monocultures. Thus, the objectives of this study were: (i) to quantify the above- (shoot) and belowground (root) biomass production, forage nutritive value, tissue carbon and nitrogen concentrations, C/N ratio, biomass carbon and nitrogen from different cool-season annual cover crop systems in a semiarid environment, and (ii) to determine the effects of different cool-season annual cover crop systems on soil organic carbon, total nitrogen, soil CO2 respiration, and soil microbial population biomass and diversity.
2. Materials and methods
2.1. Field site description
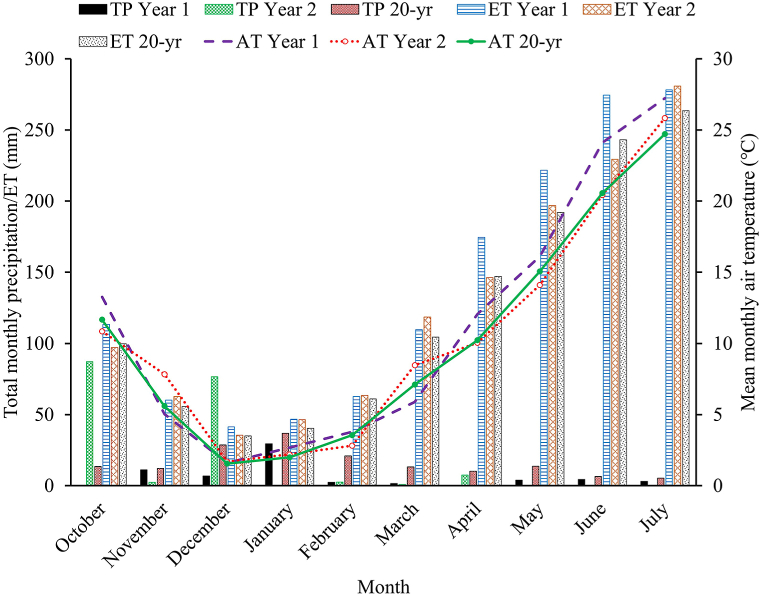
To accomplish the stated objectives, a two-year field experiment was carried out during the fall to early summer of 2020–2021 (Year 1) and 2021–2022 (Year 2) growing seasons at the University of Nevada, Reno Valley Road Field Station, Reno, NV, USA (39°32′19″ N and 119°48′19″ W; elevation 1566 m). The area used for this experiment was under alfalfa for four years. The soil at the experimental site was an Orr gravely sandy loam (Fine-loamy mixed, superactive, mesic Aridic Argixerolls, [41]). Before seeding, soil samples were collected at a depth of 15 cm across the experimental site and composited for the determination of the soil's chemical and biological properties. The initial soil chemical parameters analyzed before seeding were pH (1:1 water), organic matter (loss on ignition method), nitrate-N (KCL extraction), phosphorus (Olsen-P extraction), potassium, calcium, magnesium, and sodium (NH4O acetate extraction), and sulfur (Mehlich 3 extraction) (Table 1). The initial soil biological properties quantified through phospholipid fatty acids analysis (PLFA) were rated very low for microbial biomass, total bacterial, gram-positive and negative bacteria, fungi, actinomycetes, arbuscular mycorrhizal fungi, and saprophytes (Table 5). Soil respiration at the experimental area before seeding was 17.13 μg CO2-C g soil−1 hour−1. The seasonal precipitation and evapotranspiration varied between the two growing seasons and the 20-year average (Fig. 1). Also, there were marginal differences in mean monthly air temperature between the two years of the study relative to the 20-year average (Fig. 1). The cool-season annuals used in this experiment are typically sown in early Fall (Autumn) and Spring in this temperate environment of northwestern Nevada. The active growing periods are from early September to November followed by winter dormancy from mid-November to March and then active growth resumes from mid-March to July followed by physiological maturity (senescence). The summer months from June to August are typically very hot and dry in this region as evapotranspiration exceeds precipitation.
Table 1.
Initial soil chemical characteristics of the experimental site sampled at the 15 cm depth in Reno, Nevada, USA.
| Soil parameter | Value |
|---|---|
| pH | 7.2 |
| Organic matter (g kg−1) | 28.0 |
| Cation Exchange Capacity (CEC) (meq/100 g) | 15.4 |
| Nitrate-Nitrogen (NO3-N) (mg kg−1) | 11.0 |
| Phosphorus (P) (mg kg−1) | 25.0 |
| Potassium (K) (mg kg−1) | 147 |
| Magnesium (Mg) (mg kg−1) | 452 |
| Calcium (Ca) (mg kg−1) | 2729 |
| Sodium (Na) (mg kg−1) | 24.0 |
| Sulfur (S) (mg kg−1) | 7.0 |
Table 5.
Cover crop systems crude protein (CP), acid (ADF) and neutral detergent fiber (NDF) concentrations and relative feed value (RFV) in each year and the two-year average in Reno, Nevada, USA.
| Cover crop system |
CP |
ADF |
NDF |
RFV |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
|
| Monocultures | ---------------------------------------------------------------g kg−1----------------------------------------------------------------- | |||||||||||
| Annual ryegrass | 117.0 | 54.0da | 85.5c | 411.3ab | 465.2b | 438.2a | 617.6a | 676.7a | 647.1a | 86c | 73c | 80c |
| Oat | 131.8 | 69.6d | 100.7bc | 379.1b | 435.6bc | 407.3ab | 585.2a | 623.2ab | 604.2a | 95c | 82c | 89c |
| Faba bean | 146.9 | 168.6a | 157.7a | 287.2d | 371.6d | 329.4c | 354.6c | 409.9d | 382.2c | 175a | 148a | 161a |
| Yellow sweetclover | 149.6 | 121.8bc | 135.7a | 328.4c | 521.3a | 425.0a | 411.7bc | 588.1b | 499.9b | 147b | 77c | 112b |
| Winter pea | 132.3 | 143.2b | 137.8a | 286.1d | 418.7c | 352.3bc | 453.8b | 487.9c | 470.9b | 151b | 111b | 131b |
| Mixtures | ||||||||||||
| Mix 1b | 152.5 | 106.3c | 129.4ab | 425.8a | 457.4b | 441.6a | 626.0a | 604.7b | 615.3a | 83c | 83c | 83c |
| Mix 2b | 90.8 | 101.9c | 96.3bc | 396.8ab | 446.2bc | 421.5a | 616.7a | 607.0b | 611.8a | 88c | 83c | 86c |
| SEc | 32.4 | 7.5 | 18.7 | 17.9 | 13.0 | 19.5 | 32.6 | 20.0 | 21.7 | 9.0 | 9.0 | 8.0 |
| P-value | 0.106 | <0.001 | 0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Means within columns that are followed by the same lowercase letter superscripts are not different among cover crop systems (P > 0.05).
Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea).
SE; standard error.
Fig. 1.
Total monthly precipitation (TP), evapotranspiration (ET), and mean air temperature (AT) during 2020–2022 growing seasons and 20-year average (2001–2020) at the University of Nevada, Reno Valley Road Field Laboratory, Reno, NV.
2.2. Cover crop treatments and experimental design
This study consisted of eight (8) cover crop (CC) treatments (systems) that included monocultures and mixtures (Table 2). The cool-season annual forage species used in this study were annual ryegrass (Lolium multiflorum Lam.), oat (Avena sativa L.), and host-specific Rhizobia spp. pre-inoculated faba bean (Vicia faba L.), yellow sweetclover [Melilotus officinalis (L.) Lam], and winter pea (Pisum sativum subsp. Arvense). The eight cover crop systems consisted of five monocultures, two three-species mixtures, and a control (Fallow) of no cover crop (Table 2). The two cover crop mixtures were formulated based on the recommended monoculture seeding rate for the grass and legume species used (Table 2). The mixture comprised 50 % of the grass seed rate, 25 % of the first legume seed rate, and 25 % of the second legume seed rate. The formulation and use of only two three-species mixtures was considered to be the most practical approach at this initial phase of cover crop evaluation in this region. The cover crop systems were laid out in a randomized complete block design with three replications of each treatment (n = 24).
Table 2.
Cover crop systems, species, cultivars, seed mixture ratio, and seeding rate used in this study.
| Cover crop system | Scientific name | Cultivars | Seed mixture ratio | Seeding rate (kg PLS/ha)a |
|---|---|---|---|---|
| Monocultures | ||||
| Annual ryegrass | Lolium multiflorum Lam. | Hercules | 34 | |
| Oat | Avena sativa L. | Goliath | 112 | |
| Faba bean | Vicia faba L. | Bell | 56 | |
| Yellow sweetclover | Melilotus officinalis (L.) Lam | VNSb | 17 | |
| Winter pea | Pisum sativum subsp. Arvense | Montech 4193 | 56 | |
| Mixtures | ||||
| Mix 1c | 50:25:25d | 36 | ||
| Mix 2c | 50:25:25d | 84 | ||
| Control | ||||
| Fallow | ||||
Pure live seed (PLS).
Variety not stated (VNS).
Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea).
The grass-legume-legume cover crop mixtures were formulated based on the recommended seeding rate of each species planted alone. The mixture comprised 50 % of the grass seed rate, 25 % of the first legume seed rate, and 25 % of the second legume seed rate.
2.3. Cover crop plot establishment and management
Before seeding and land preparation, the experimental site was sprayed with a mixture of 2,4-D [2-(2,4-dichlorophenoxy) acetic acid] at 0.8 kg a.e. ha−1 and glyphosate [isopropylamine salt of N-(phosphonomethyl)glycine] at 1.12 kg a.e. ha−1 glycine herbicides to control both grass and broadleaf weeds. The plot area was minimum tilled (single-pass) using a rototiller, then leveled, and compacted using a preseeding roller to create a uniform seedbed. The plot size was 6.1 m long × 1.5 m wide (9.15 m2) separated by 0.6 m between plots and 1.5 m alleys between blocks. All cover crop systems were seeded based on the seeding rate in Table 2 in early October of each growing season using an XL Plotseed cone seeder (Wintersteiger AG., Salt Lake City, UT) in a 20-cm row spacing to a depth of 1 cm. However, due to extensive damage of seedlings by marmots (Marmota spp.) in the first year (2020), the plots were reseeded in the spring (mid-March) of 2021. In the second year, the cover crop systems were seeded in the same plots after minimum tillage as described above. Fertility management for these cover crop systems in this region is based on a low-input approach. Thus, based on the soil test recommendation from a commercial laboratory, a single application rate of 30 kg P ha−1 was applied using triple superphosphate fertilizer material to all cover crop systems. Even though the previous crop cultivated was alfalfa, fertilizer nitrogen was applied at 60 kg ha−1 using urea to the grass and mixed systems in mid-April of each year. This 60 kg N ha−1 was 20 units below the traditional 80 kg N ha−1 used in low-input non-legume forage production systems in Nevada. Supplemental irrigation was carried out for the first three weeks after sowing (end of October), and thereafter from the end of March to June of each year. Irrigation was applied uniformly through a K-Line irrigation system (St Joseph, MI, USA) set at a pressure of 262 KPa to replace the total grass reference Penman evapotranspiration every seven days during the first month of the active growth period (March–April) and thereafter, every 14 days based on data collected from the UNR Valley Road Weather Station. The 14-day irrigation schedule is commonly utilized by producers in this region. Irrigation was terminated two weeks before the last harvest. Seasonal total irrigation water applied was 718 mm in the first and 661 mm in the second year. Hand weeding was carried out intermittently to remove weeds from plots. For the fallow plots, glyphosate [N -(phosphonomethyl) glycine] was applied to control weeds (chemical fallow).
2.4. Data collection
Botanical composition in the two mixed cover crop systems was quantified by sampling randomly two 0.5 m2 quadrats in undisturbed areas of each plot before biomass sampling at the two sampling dates to represent early (end of June) and late termination (end of July). Samples were thereafter separated by hand into each species component and oven-dried as described below. The dried sample components were weighed, and their proportion was computed as a percentage of the whole sample dry weight. Cover crop shoot biomass was sampled twice at the end of June (representing early termination) and the end of July (representing late termination) as if termination were to be done at these scheduled times from an area of 1.9 m2 in each plot excluding border rows. All plots were harvested with a 36A RCI engineering plot harvester (Mayville, WI, USA) set to 12.7 cm residual stubble height. The fresh weight of harvested shoot biomass from each cover crop system was recorded. Thereafter, a representative subsample of approximately 500 g was randomly collected at different depths of the biomass harvested from each cover crop system for dry matter, tissue carbon, nitrogen, and forage nutritive value determination. The subsamples were oven-dried using a forced-air oven set at 60 °C for 72 h before dry weight was recorded. The shoot biomass for each cover crop system was calculated on a dry matter basis. Dry subsample from each cover crop system plot was ground separately using a Wiley mill (Model 4, Thomas Scientific, Swedesboro, NJ) to pass a 1-mm screen and stored in Whirl-Pak (Nasco, Fort Atkinson, WI) sample bags. After biomass data collection, all biomass from each plot was incorporated into their respective plots. Each sample for the cover crop systems was analyzed by dry combustion to determine the concentration of carbon (C) and nitrogen (N) using a Leco 928 CN analyzer (St. Joseph, MI, USA). Each cover crop system's C/N ratio was calculated by dividing the concentration of C by that of N. The cover crop systems fiber fractions of acid detergent fiber (ADF) and neutral detergent fiber (NDF) were analyzed using the filter bag technique with an Ankom Fiber Analyzer (ANKOM Technology, Macedon, NY, USA), using the modified detergent procedures of Van Soest et al. [42]. The crude protein (CP) concentration for each cover crop system was determined by multiplying the tissue N content by 6.25. The estimated relative feed value (RFV) of each cover crop system was computed based on the proposed equations of Undersander et al. [43].
| Digestible dry matter (DDM) = 88.9 – (0.779 × %ADF), |
| Dry matter intake (DMI) = 120/%NDF, |
| RFV = (%DDM × %DMI)/1.29, |
2.5. Root biomass and soil sampling
Cover crop system root biomass was quantified to a depth of 15.24 cm × 5.08 cm diameter using a soil core bulk density sampler in two randomly sampled areas in the center of each plot following the final harvest (late sampling). The depth used for the root sample represents the typical root depth at which the vast majority of root biomass of the species used in this study are found. The core samples collected for each experimental unit were dried at 60 °C for 72 h using a forced-air oven for bulk density determination. Thereafter, the soil cores were washed separately in a 425-μm sieve to remove soil and other unwanted materials. Root samples were dried in separate bags following the same drying protocol above. Cover crop root biomass was estimated per hectare based on the 0- to 12.7-cm soil depth and soil bulk density [44]. A day after the final harvest, six random soil samples from each experimental unit were collected from the 0- to 15.24-cm soil, depth using a 1.43-cm inner diameter AMS Gator soil probe (AMS, American Falls, ID, USA) and composited for analysis of soil health parameters. Soil samples were analyzed at a commercial soil testing laboratory (Ward Laboratories, Inc., Kearney, NE, USA).
2.6. Soil biological and chemical properties analysis
The microbial community structure and biomass of soil from the different cover crop treatments were determined by phospholipid fatty acid analysis (PLFA) for total lipid extraction based on a modified version of the method of Bligh and Dyer [45] by Buyer et al. [46]. Briefly, from each cover crop system, 2 g (dry equivalent) of freeze-dried soil was placed in test tubes with 10 ml of methanol, 5 ml of chloroform, and 4 ml of phosphate buffer (50 mM; pH 7.4) and rotated for 1 h after a 10-min sonicated water bath at room temperature. Samples were then centrifuged for 10 min at 2500 rpm and the liquid phase was transferred to clean vials. In the new vials, 5 ml of chloroform and 5 ml of water were added, shaken, and left overnight for separation [46]. The organic fraction was evaporated under N2 and stored at −20 °C [46]. After the lipid class separation was carried out in silica gel columns, neutral lipids, glycolipids, and phospholipids were eluted each using 5 ml of chloroform, 10 ml of acetone, and 5 ml of methanol respectively [46]. Thereafter, the phospholipid fraction was dried under N2, dissolved in 1 ml of methanol, and stored at −20 °C. For fatty acid methyl esters detection, samples were analyzed using a Shimadzu Nexis GC-2030 equipped with an AOC-6000 autosampler and flame ionization detector (Shimadzu Scientific Instruments, Columbia, MD, USA). Peak identification and area calculation were carried out using MIDI software (MIDI Corp, Newark, DE).
The PLFA peak areas for the selected terminal-branched fatty acids were summed into biomarker groups. Actinomycetes were classified based on the methyl-branched fatty acids 16:0 10-methyl, 17:0 10-methyl, and 18:0 10-methyl biomarkers [[47], [48], [49]]. Gram-negative bacteria, the specified monounsaturated and cyclopropyl 10:0 2OH, 10:0 3OH, 11:0 2OH, 11:0 3OH, i11:0 3OH, 12:0 2OH, 12:0 3OH, 14:0 2OH, 14:0 3OH, i14:0 3OH, 16:1ω7c, 16:1ω7t 16:1ω9c, 16:0 2OH, 16:0 3OH, 16:1 2OH, cy17:0, 18:1ω5c, 18:1ω7c, 19:0ω9c, and 19:0ω6c were biomarkers used and for gram-positive bacteria, the iso and anteiso branched fatty acids, i14:0; i15:0, a15:0, i16:0, a16:0, i17:0, and a17:0 were used [[47], [48], [49], [50], [51], [52]]. Arbuscular mycorrhizal was classified based on 16:1ω5c, 16:1ω11c, 20:1ω9c, and 22:1ω9c PLFA signatures [49,53,54]. Saprophytes were classified based on 18:1ω9c, 18:2ω6,9c, 18:2ω6c, 18:3ω3c, 18:3ω6c, and 20:5ω3c biomarkers [52,55]. The total microbial biomass equated to the sum of all the extracted PLFAs (including those PLFAs not assigned as biomarkers, the ‘undifferentiated’). Total bacteria biomass was the sum of Gram-negative and Gram-positive bacteria while total fungal biomass was the sum of arbuscular mycorrhizal and saprophytes. All PLFA microbial biomass was reported in ng g−1 of dry soil.
Soil total organic carbon (TOC) and nitrogen (TN) were determined by dry combustion using a LECO CN 928 analyzer (LECO Corporation, St. Joseph, MI USA). For soil TOC, the samples were pre-treated with sulfurous acid to remove inorganic carbon before dry combustion analysis. Soil CO2 respiration (Haney test) was analyzed using 40 g of ground-dried soil along with 12 ml of deionized water sealed in a mason jar and incubated at 24 °C for 24 h. After the rewetting process, a LI-870 CO2 gas analyzer (LI-COR Biosciences Inc., Lincoln, NE, USA) was used to measure the exhaled CO2 concentration.
2.7. Statistical analysis
The data were analyzed using the generalized linear mixed models procedure (PROC GLIMMIX) in SAS (version 9.4 [56], Cary, NC, USA). The response variables were botanical composition, shoot biomass, tissue C and N concentrations, C/N ratio, nutritive value (CP, ADF, and NDF), forage quality estimate RFV, root biomass, and the measured soil parameters. Because of the difference in planting date in Year 1 (2020–2021) relative to Year 2 (2021–2022) of the study, the data were analyzed to present the two-year average and for each year separately. For the two-year average, the fixed effects were cover crop system and termination date while the random effects were replication (block), year, and their interactions. For each year separately, the random effect was replication. The fallow system (no cover crop) was only included for the analysis of the soil response parameters. For the soil data, the cover crop system and year were considered fixed effects in the model. Because samples were collected on the same plot each year, the year was analyzed as a repeated measurement and the covariance model selection was based on the lowest Akaike information criteria value which indicates a better model fit [57]. Cover crop system effects were considered significant when P ≤ 0.05. Cover crop system means separation was done using the PDIFF option of LSMEANS procedure.
3. Results
3.1. Botanical composition
Analyzed across the two years, the proportion of annual ryegrass (68.2 %; SE = 5.6) in Mix 1 and oat (78.3 %; SE = 5.6) in Mix 2 were similar (P = 0.217). Further, neither the termination date (P = 0.766) nor the interaction with the cover crop system (P = 0.606) altered the proportion of grass components in the two mixed cover crop systems. For faba bean in the mixtures, neither the cover crop system (P = 0.175), termination date (P = 0.175), nor the interaction of the two variables (P = 0.966) influence its proportion. The two-year average proportion of faba bean in Mix 1 was 5.3 % (SE = 3.9) and in Mix 2 was 9.3 % (SE = 3.9). There was a trend (P = 0.093) for a more significant proportion of yellow sweetclover (26.3 %; SE = 5.7) in Mix 1 compared to the proportion of winter pea (12.3 %; SE = 5.7) in Mix 2. Again, neither the termination date (P = 0.837) nor the interaction with cover crop system (P = 0.619) alter the proportion of these two components in the mixed systems.
3.2. Cover crop shoot biomass
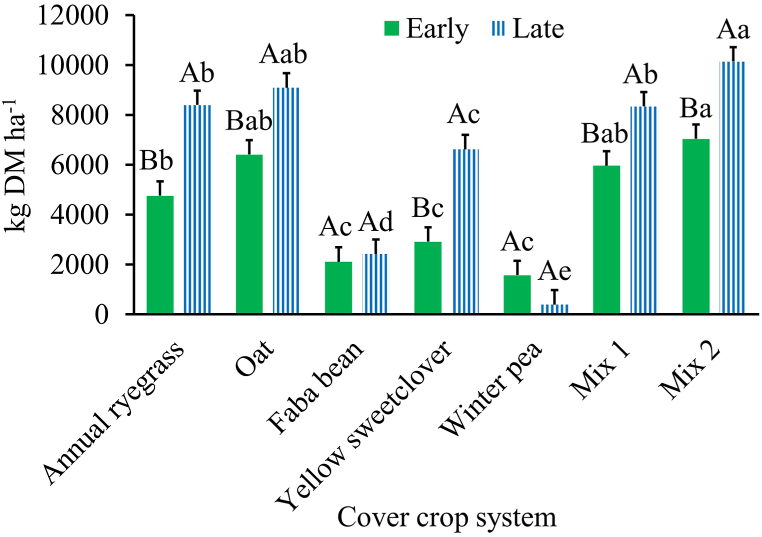
The two-year average shoot biomass was different among cover crop systems (P < 0.001; Table 3), and between the two termination dates (P = 0.004) but it was not affected by the cover crop system × termination date interaction (P = 0.215). The cover crop system Mix 2 produced greater shoot biomass than all other systems except oat whilst the monocultures of faba bean and winter pea produced the least amount of shoot biomass (Table 3). Also, when averaged across years, shoot biomass for the late termination was greater than the early termination date (Early = 5629 kg DM ha− 1; SE = 363 versus Late = 7004 kg DM ha− 1; SE = 363). In Year 1, there was a main effect of cover crop system (Table 3) and a cover crop system × termination date interaction for shoot biomass production (P = 0.0012; Fig. 2). Cover crop system Mix 2 produced greater shoot biomass than all other systems except oat whilst winter pea produced the lowest amount of shoot biomass among the different cover crop systems (Table 3). For the interaction effect at early termination, Mix 2 produced greater shoot biomass than the monocultures of annual ryegrass, faba bean, yellow sweetclover, and winter pea (Fig. 2). Shoot biomass production at late termination differed among cover crop systems and was greater for Mix 2 than annual ryegrass, faba bean, yellow sweetclover, winter pea, and Mix 1 (Fig. 2). For each cover crop system, shoot biomass production was greater at the late than early termination date for annual ryegrass, oat, yellow sweetclover, Mix 1, and Mix 2 (Fig. 2). However, the termination date did not affect shoot biomass production of faba bean and winter pea monocultures (Fig. 2). In Year 2, shoot biomass production was affected by the main effect of cover crop system (P = 0.003) but not by the cover crop system × termination date interaction (P = 0.145). The cover crop system Mix 2 produced the greatest amount of shoot biomass among cover crop systems (Table 3). In Year 2, shoot biomass did not differ (P = 0.250) between the early (6863 kg DM ha−1; SE = 564) and late termination date (7673 kg DM ha−1; SE = 564).
Table 3.
Shoot and root biomass of different cover crop systems in each year and the two-year average in Reno, Nevada, USA.
| Cover Crop System |
Shoot biomass |
Root biomass |
||||
|---|---|---|---|---|---|---|
| Year 1 |
Year 2 |
2-yr avg. |
Year 1 |
Year 2 |
2-yr avg. |
|
| Monocultures | -----------------------------------------------------kg DM ha−1----------------------------------------------- | |||||
| Annual ryegrass | 6576ca | 7022bc | 6799bc | 2988 | 4202 | 3595a |
| Oat | 7753ab | 8187b | 7970ab | 467 | 3175 | 1821bc |
| Faba bean | 2263e | 5052c | 3391d | 467 | 2428 | 1447c |
| Yellow sweetclover | 4768d | 7370bc | 6069c | 3174 | 3081 | 3128ab |
| Winter pea | 982f | 5773bc | 3377d | 1774 | 3454 | 2614abc |
| Mixtures | ||||||
| Mix 1b | 7153bc | 6634bc | 6893bc | 3455 | 4482 | 3968a |
| Mix 2b | 8591a | 10838a | 9714a | 840 | 3081 | 1961bc |
| SEc | 415 | 954 | 656 | 966 | 768 | 677 |
| P-value | <0.001 | 0.004 | <0.001 | 0.109 | 0.296 | 0.011 |
Means within columns that are followed by the same lowercase letter superscripts are not different among cover crop systems (P > 0.05).
Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea).
SE; standard error.
Fig. 2.
Cover crop system × termination date influence on shoot biomass production during Year 1 (2020–2021) growing season. Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea). Across cover crop system for each termination date (early or late), bars with the same lowercase letter are not different. Within each cover crop system, bars with the same uppercase letter are not different. Error bars are standard errors.
3.3. Root biomass
Averaged across years, root biomass was different among cover crop systems (P = 0.011; Table 3). The cover crop systems Mix 1 and annual ryegrass produced greater root biomass than oat and faba bean monocultures, and Mix 2 (Table 3). In Years 1 and 2 separately, cover crop system did not differ in root biomass (Table 3).
3.4. Tissue carbon concentration
The tissue carbon concentration averaged across the two years, was affected by the main effect of cover crop system (P = 0.001). However, carbon concentration was not influenced by the termination date independently (P = 0.985) nor the interaction of cover crop system × termination date (P = 0.564). The carbon concentrations of yellow sweetclover and winter pea were greater than all other cover crop systems (Table 4). In Year 1, tissue carbon concentration differed among cover crop systems, and it was greatest for the monoculture winter pea (Table 4). However, in Year 1, neither the termination date (P = 0.844) nor the interaction of cover crop system × termination date (P = 0.358) was significant for averaged tissue carbon concentration. In Year 2, again tissue carbon concentration was only influenced by the cover crop system (P < 0.001) but contrary to Year 1, it was greatest for yellow sweetclover (Table 4).
Table 4.
Tissue carbon and nitrogen concentrations, carbon-to-nitrogen (C/N) ratio, and biomass carbon and nitrogen of different cover crop systems in each year and the two-year average in Reno, Nevada, USA.
| Cover crop system |
Carbon |
Nitrogen |
C/N ratio |
Biomass carbon |
Biomass nitrogen |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
Year 1 |
Year 2 |
2-yr avg |
|
| Monocultures | -------------------------------------g kg−1---------------------------------- | ----------------------------------kg ha−1----------------------------------- | |||||||||||||
| Annual ryegrass | 417.9ba | 418.6c | 418.3b | 18.7 | 8.6d | 13.7c | 24.6 | 49.9a | 37.2a | 2760b | 2938bc | 2849b | 124ab | 60c | 91.6bc |
| Oat | 420.6b | 423.9b | 422.2b | 21.1 | 11.1d | 16.1bc | 22.6 | 38.9b | 30.8ab | 3257ab | 3472ab | 3365ab | 159ab | 91bc | 124.6abc |
| Faba bean | 417.5b | 425.6b | 421.5b | 23.5 | 27.0a | 25.2a | 22.6 | 16.1e | 19.4d | 948d | 2137c | 1542c | 55.2cd | 128ab | 91.4bc |
| Yellow sweetclover | 422.0b | 437.7a | 429.8a | 23.9 | 19.5bc | 21.7a | 22.8 | 22.8cd | 22.8cd | 2017c | 3232bc | 2624b | 107bc | 141ab | 124.3abc |
| Winter pea | 433.6a | 426.2b | 429.9a | 21.2 | 23.0b | 22.0a | 23.9 | 20.1de | 22.0cd | 423e | 2458bc | 1441c | 21d | 133ab | 76.6c |
| Mixtures | |||||||||||||||
| Mix 1b | 419.7b | 427.5b | 423.6b | 24.4 | 17.0c | 20.7ab | 20.8 | 25.9c | 23.4bcd | 3000b | 2839bc | 2919b | 175a | 107bc | 141ab |
| Mix 2b | 418.4b | 422.8bc | 422.2b | 14.5 | 16.3c | 15.4bc | 31.6 | 26.7c | 29.1bc | 3592a | 4583a | 4087a | 121.3b | 185a | 153.3a |
| SEc | 3.8 | 2 | 26 | 5.1 | 1.2 | 3 | 5.2 | 1.5 | 3.3 | 184 | 407 | 283 | 32.3 | 20.1 | 22 |
| P-value | 0.019 | <0.001 | 0.001 | 0.106 | <0.001 | 0.001 | 0.385 | <0.001 | 0.001 | <0.001 | 0.004 | <0.001 | <0.001 | 0.007 | 0.042 |
Means within columns that are followed by the same lowercase letter superscripts are not different among cover crop systems (P > 0.05).
Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea).
SE; standard error.
3.4.1. Tissue nitrogen concentration
For the average tissue N concentration across two years, there were main effects of cover crop system (P = 0.001) and termination date (P = 0.002) but there was no interaction (P = 0.791) between the two variables. The legumes monocultures of faba bean, yellow sweetclover, and winter pea had greater N concentrations than annual ryegrass, oat, and Mix 2 (Table 4). Terminating late (16.8 g N kg−1 DM; SE = 2.4) resulted in a lower N concentration than early termination (22.0 g N kg−1 DM; SE = 2.4). In Year 1, neither the main effect of cover crop system (Table 4) nor the interaction of cover crop system × termination date (P = 0.499) altered tissue N concentration. However, tissue N concentration was influenced by the termination date (P = 0.007), and it was greater at early termination (23.8 g N kg−1 DM; SE = 4.7) compared to late termination (18.3 g N kg−1 DM; SE = 4.7). In Year 2, tissue N concentration was influenced by the main effects of cover crop system (Table 4) and termination date (P < 0.001) but no interaction between the two variables (P = 0.323) occurred. In Year 2, the faba bean monoculture had the greatest tissue N concentration among cover crop systems while the two grass monocultures ranked lowest (Table 4). Similar to the previous trend, terminating early (19.6 g N kg−1 DM; SE = 0.6) resulted in greater N concentration than late (15.4 g N kg−1 DM; SE = 0.6).
3.4.2. Cover crop systems biomass carbon-to-nitrogen (C/N) ratio
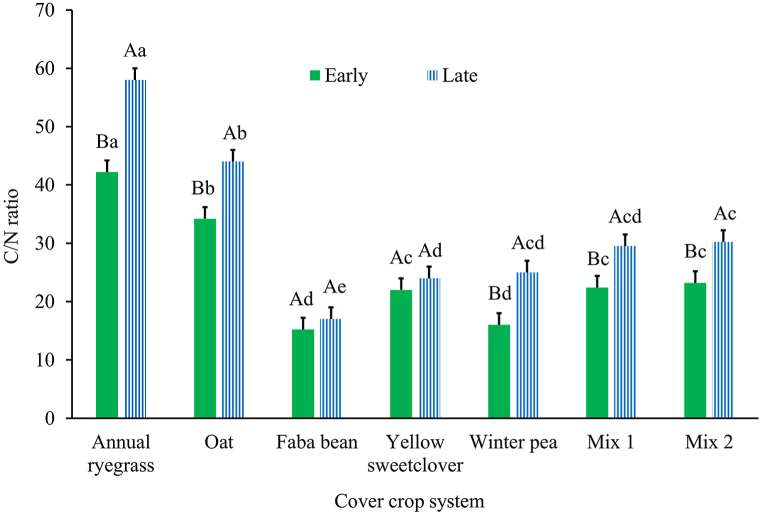
Average across years, cover crop systems (Table 4), and termination dates (P = 0.001) differed in C/N ratio but there was no interaction between the two variables (P = 0.984). Annual ryegrass monoculture produced a greater C/N ratio than all other cover crop systems except oat monoculture (Table 4). Contrary to the previous termination date trends, terminating late (30.0; SE = 2.3) produced a greater C/N ratio compared to early (22.8; SE = 2.3). In Year 1, the C/N ratio was only altered by termination date (P = 0.009) but not by the main effect of cover crop system (Table 4) or their interactions (P = 0.807). The C/N ratio was greater for late (28.0; SE = 4.4) compared to the early termination date (21.0; SE = 4.4). The C/N ratio in Year 2 was altered by the main effect of cover crop system (Table 4) and by the cover crop system × termination date interaction (P = 0.036; Fig. 3). Concerning the main effect of cover crop system, the C/N ratio was lowest for faba bean relative to all other systems except winter pea (Table 4). As it relates to the interaction, at the early termination date, annual ryegrass had the greatest C/N ratio followed by oat whilst faba bean and winter pea were ranked lowest (Fig. 3). At the late termination date, again annual ryegrass had the greatest C/N ratio followed by oat, and again faba bean was ranked lowest (Fig. 3). For each cover crop system, only for faba bean and yellow sweetclover termination date had no impact on the C/N ratio (Fig. 3). All other cover crop systems had greater C/N ratios at late compared to early termination (Fig. 3).
Fig. 3.
Cover crop system × termination date influence on carbon-to-nitrogen ratio during Year 2 (2021–2022) growing season. Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea). Across cover crop system for each termination date (early or late), bars with the same lowercase letter are not different. Within each cover crop system, bars with the same uppercase letter are not different. Error bars are standard errors.
3.4.3. Cover crop systems shoot biomass carbon
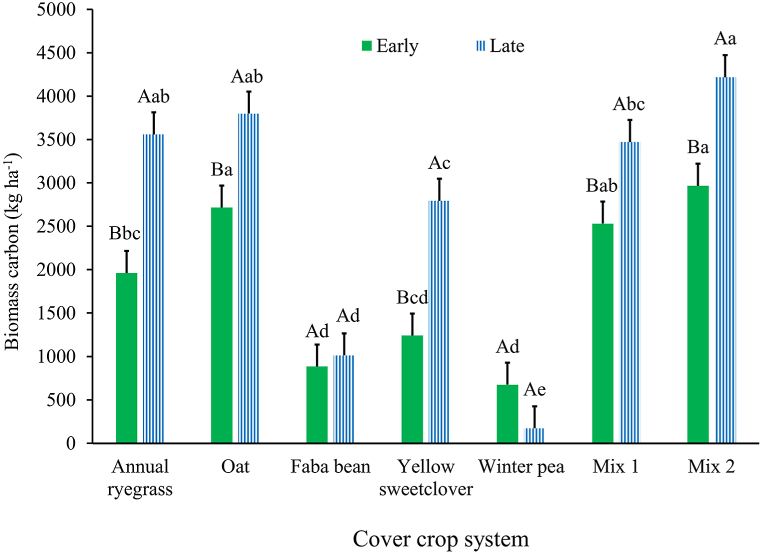
Averaged across years, the shoot biomass carbon was different among cover crop systems (P < 0.001; Table 4), and between termination dates (P = 0.005) but the interaction of the two variables was not significant (P = 0.206). The system Mix 2 produced greater shoot biomass carbon than all other systems except oat (Table 4). Shoot biomass carbon produced was lower for the early (2384 kg C ha−1; SE = 160) relative to the late termination date (2996 kg C ha−1; SE = 160). In Year 1, shoot biomass carbon was affected by the main effect of cover crop system (Table 4) and cover crop system × termination date interaction (P = 0.002; Fig. 4). The lowest shoot biomass carbon was produced by the winter pea monoculture (Table 4). Concerning the interaction, at the early termination date, oat and Mix 2 produced greater shoot biomass carbon than the monocultures of annual ryegrass, faba bean, yellow sweetclover, and winter pea (Fig. 4). At the late termination date, annual ryegrass, oat, and Mix 2 produced greater shoot biomass carbon than the legume monocultures of faba bean, yellow sweetclover, and winter pea (Fig. 4). The systems annual ryegrass, oat, yellow sweetclover, and Mix 2 produced greater shoot biomass carbon at late compared to early termination date (Fig. 4). In Year 2, shoot biomass carbon was only influenced by cover crop system (P = 0.004; Table 4) and it was greater for Mix 2 compared to all other systems except the oat monoculture (Table 4).
Fig. 4.
Cover crop system × termination date influence on biomass carbon during Year 1 (2020–2021) growing season. Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea). Across cover crop system for each termination date (early or late), bars with the same lowercase letter are not different. Within each cover crop system, bars with the same uppercase letter are not different. Error bars are standard errors.
3.4.4. Cover crop systems shoot biomass nitrogen
The two-year average shoot biomass N was only altered by the main effect of cover crop system (P = 0.042; Table 4). Mix 2 produced greater shoot biomass N than annual ryegrass, faba bean, and winter pea monocultures (Table 4). However early (114 kg N/ha; SE = 15) and late termination (115 kg N/ha; SE = 15) produced similar shoot biomass N (P = 0.933). In Year 1, only cover crop system (P < 0.001) influenced shoot biomass N (Table 4). The Mix 1 system produced greater shoot biomass N than faba bean, yellow sweetclover, winter pea, and Mix 2 (Table 4), while early (101 kg N/ha; SE = 28) and late termination (116 kg N/ha; SE = 28) yielded similar shoot biomass N (P = 0.296). In Year 2, shoot biomass N was influenced by cover crop system (P = 0.007) but not the termination date (P = 0.426), nor cover crop system × termination date interaction (P > 0.05). The cover crop system Mix 2 produced greater biomass N than annual ryegrass, oat, and Mix 1 (Table 4). The two termination dates of early (127 kg N/ha; SE = 11) and late (114 kg N/ha; SE = 11) produced similar shoot biomass N.
3.5. Forage quality indices
3.5.1. Crude protein
For crude protein (CP) concentration averaged across the two years, there were main effects of cover crop system (P = 0.001; Table 5) and termination date (P = 0.002) but there was no interaction (P = 0.792) between the two variables. The legumes monocultures of faba bean, yellow sweetclover, and winter pea had greater CP concentrations than annual ryegrass, oat, and Mix 2 (Table 5). Terminating late (105.2 g kg−1 DM; SE = 16.0) resulted in lower CP concentration than early (135.6 g kg−1 DM; SE = 16.0). In Year 1, CP concentration was not influenced by cover crop system (Table 5) but only by the termination date (P = 0.007) and it was greater at early (148.9 g kg−1 DM; SE = 29.6) compared to late termination (114.2 g kg−1 DM; SE = 29.6). In Year 2, CP concentration was influenced by the main effects of cover crop system (P < 0.001; Table 5) and termination date (P < 0.001) but no interaction between the two variables (P = 0.323). The faba bean monoculture had the greatest CP concentration among cover crop systems (Table 5). Terminating cover crop early (122.4 g kg−1 DM; SE = 4.0) resulted in greater CP concentration compared to late (96.3 g kg−1 DM; SE = 4.0).
3.5.2. Acid detergent fiber
Averaged across years, acid detergent fiber (ADF) concentrations varied among cover crop systems (P = 0.002; Table 5) and between termination dates (P = 0.007) but no interaction of the two variables (P = 0.977). The faba bean and winter pea monocultures were ranked among the lowest in ADF concentrations whilst all other systems did not differ in their ADF concentrations (Table 5). The ADF concentration was greater for the late termination date (422.9 g kg−1 DM; SE = 10.4) than for the early (381.5 g kg−1; SE = 9.6). In Year 1, ADF concentration was different among cover crop systems (Table 5) but not between termination dates (early = 350.7 vs late 367.7 g kg−1 DM; SE = 13.4; P = 0.122) nor influenced by the interaction between the two variables (P = 0.264). The monocultures of faba bean and winter pea ranked lowest in ADF concentrations among cover crop systems (Table 5). In Year 2, there were main effects of cover crop system (P = 0.001) and termination date (P < 0.001) but no cover crop system × termination date interaction (P = 0.087). The monoculture of faba bean ranked lowest among cover crop systems in ADF concentration (Table 5). The ADF concentration was lower at early (412.0 g kg−1 DM; SE = 7.0) compared to late termination (478.0 g kg−1 DM; SE = 7.0).
3.5.3. Neutral detergent fiber
Averaged across years, neutral detergent fiber (NDF) concentration was not influenced by the cover crop system × termination date interaction (P = 0.255) but there were main effects of cover crop system (Table 5) and termination date (P = 0.014). Overall, the monoculture of faba bean had the lowest NDF concentration followed by winter pea and yellow sweetclover (Table 5). The NDF concentration at the late termination date (566.7 g kg−1 DM; SE = 13.3) was greater than that at the early (528.0 g kg−1 DM; SE = 13.3). In Year 1, only the main effect of cover crop system affected NDF concentration (Table 5). Apart from being similar to yellow sweetclover, faba bean ranked lowest among all other cover crop systems in NDF concentration (Table 5). The NDF concentration was similar for early (511.0 g kg−1 DM; SE = 23.0) and late termination (536.0 g kg−1 DM; SE = 23.0). In Year 2, NDF concentration was affected by cover crop system (Table 5) and termination date (P = 0.002) but not by the interaction (P = 0.395) of the two variables. The monoculture of faba bean had the lowest NDF concentration followed by winter pea among cover crop systems (Table 5). Terminating early (544.6 g kg−1 DM; SE = 10.7) resulted in less NDF concentration than late (597.5 g kg−1 DM; SE = 10.7).
3.5.4. Relative feed value
Relative feed value (RFV) when averaged across years was not influenced by the cover crop system × termination date interaction (P = 0.774). However, there were main effects of cover crop system (Table 5) and termination date (P = 0.035). Averaged across the two years, faba bean produced the greatest RFV among cover crop systems followed by winter pea and yellow sweetclover (Table 5). The RFV for the early termination date (112; SE = 4.0) was greater than that of late termination (100; SE = 4.0). In Year 1, RFV was only affected by cover crop system, and it was greatest for faba bean, intermediate for yellow sweetclover and winter pea, and lowest for annual ryegrass, oat, Mix 1, and Mix 2 (Table 5). However, RFV did not differ between early (120; SE = 7.0) and late termination (116; SE = 7.0). In Year 2, there were main effects of cover crop system (Table 5) and termination date (P = 0.004) but no interaction (P = 0.310) of the two variables to alter RFV. Among cover crop systems, the faba bean monoculture had the greatest RFV, and terminating early produced greater RFV (104; SE = 5.0) than late (83; SE = 5.0).
3.6. Soil total organic carbon (TOC) and nitrogen (TN), and CO2-C respiration of cover crop systems
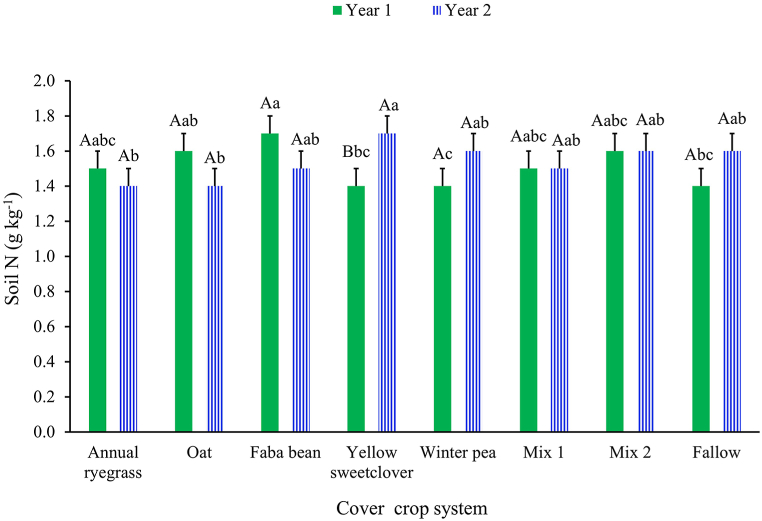
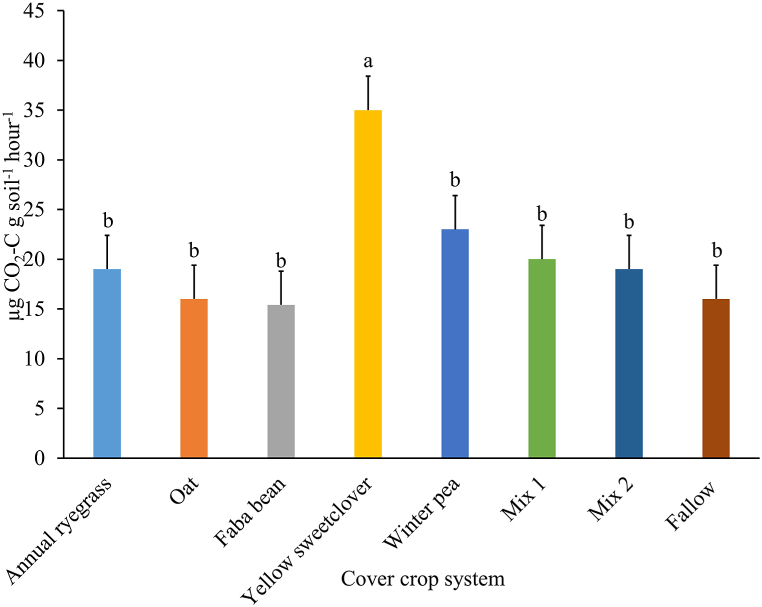
Soil TOC concentration was not influenced by cover crop system (P = 0.442; average = 14.0 g kg−1 soil; SE = 0.8) nor the interaction with year (P = 0.199). However, there was a main effect of year (P = 0.006). Soil TOC concentration was lower in Year 2 (12.2 g kg−1 soil; SE = 0.44) compared to Year 1 (15.8 g kg−1 soil; SE = 0.44) of the study. Soil TN concentration was altered by a cover crop system × year interaction (P = 0.043; Fig. 5). In Year 1, under the faba bean monoculture, soil TN was greater than under yellow sweetclover, winter pea, and the fallow systems (Fig. 5). In Year 2, the soil under the monoculture of yellow sweetclover had greater TN than the grass monocultures of annual ryegrass and oat but did not differ from any other systems (Fig. 5). Only for the yellow sweetclover system did the soil TN differ between years and it was greater in Year 2 compared to Year 1 of the study (Fig. 5). Soil CO2-C respiration differed among cover crop systems (P = 0.007) and between years (P = 0.017), but CO2-C respiration was not affected by the interaction of cover crop system × year (P = 0.447). Under yellow sweetclover monoculture, soil CO2-C respiration was greatest among all cover crop systems (Fig. 6). Between years, soil CO2-C respiration was greater in Year 1 (23.3 μg CO2-C g soil−1 hour−1; SE = 1.7) compared to Year 2 (17.3 μg CO2-C g soil−1 hour−1; SE = 1.7) of the study.
Fig. 5.
Cover crop system × year influence on soil total nitrogen concentration during the two growing seasons in Reno, Nevada, USA. Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea). Across cover crop system for each year, bars with the same lowercase letter are not different. Within each cover crop system, bars with the same uppercase letter are not different. Error bars are standard errors.
Fig. 6.
Soil carbon dioxide respiration across two growing seasons under different cover crop systems in Reno, Nevada, USA. Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea). Bars with the same lowercase letter are not different. Error bars are standard errors.
3.7. Soil microbial community biomass
When averaged across years, apart from the cover crop system effect (P = 0.035) influence on gram-negative bacteria biomass, and the trends for the main effect of cover crop system on total fungi biomass (P = 0.080), and saprophytes biomass (P = 0.064), no other microbial community population biomass was affected by cover crop system or the interaction with year (Table 6). The gram-negative bacteria biomass was greater under the monoculture of yellow sweetclover than the fallow and all other cover crop systems (Table 6). Total fungi biomass tended to be greater under yellow sweetclover monoculture than annual ryegrass, faba bean, Mix 1, and Mix 2 (Table 6). Also, saprophytes biomass tended to be greater under yellow sweetclover than under annual ryegrass, faba bean, Mix 1, and Mix 2 systems (Table 6). For total fungi and saprophytes biomass, no cover crop system generates any advantage over the fallow system (Table 6). However, all soil microbial community populations and the two indices of fungi-bacteria ratio and diversity index differed between the two years (Table 6). For example, total microbial biomass (TMB) increased by 4-fold from Year 1 to Year 2 (Table 7). All other microbial community parameters increased significantly as well from Year 1 to Year 2 (Table 7). In each year separately, there was no effect of cover crop system on soil microbial community biomass and indices (Table 6).
Table 6.
Soil microbial community biomass and indices determined by phospholipid fatty acid analysis (PLFA) for cover crop systems in each year and the two-year average in Reno, Nevada, USA.
| Soil microbial community |
IMBa |
Year |
Annual ryegrass |
Oat |
Faba bean |
Winter pea |
Yellow sweetclover |
Mix 1b |
Mix 2b |
Fallow |
SEc |
P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ----------------------------------------------------------------------ng g soil−1-------------------------------------------------------------- | ||||||||||||
| Total microbial biomass | 1 | 692 | 971 | 785 | 1180 | 874 | 570 | 1136 | 804 | 227 | 0.472 | |
| 2 | 2629 | 4058 | 3297 | 4068 | 5010 | 3959 | 3074 | 3989 | 711 | 0.287 | ||
| 426 | 2-year | 1660 | 2515 | 2041 | 2624 | 2942 | 2265 | 2105 | 2397 | 376 | 0.371 | |
| Total bacteria biomass | 1 | 264 | 378 | 259 | 377 | 304 | 194 | 289 | 329 | 90 | 0.792 | |
| 2 | 1015 | 1593 | 1262 | 1675 | 2170 | 1612 | 1270 | 1668 | 324 | 0.204 | ||
| 214 | 2-year | 640 | 986 | 761 | 1026 | 1237 | 903 | 780 | 998 | 172 | 0.275 | |
| Actinomycetes | 1 | 39 | 62 | 46 | 57 | 42 | 35 | 41 | 68 | 16 | 0.773 | |
| 2 | 288 | 370 | 322 | 418 | 452 | 413 | 341 | 404 | 68 | 0.437 | ||
| 49 | 2-year | 164 | 216 | 184 | 238 | 247 | 224 | 191 | 236 | 37 | 0.577 | |
| Gram-negative bacteria | 1 | 124 | 127 | 80 | 145 | 129 | 73 | 92 | 123 | 42 | 0.881 | |
| 2 | 247 | 462 | 304 | 464 | 865 | 433 | 355 | 462 | 131 | 0.063 | ||
| 102 | 2-year | 185bd | 295b | 192b | 304b | 497a | 253b | 223b | 292b | 69 | 0.035 | |
| Gram-positive bacteria | 1 | 141 | 251 | 180 | 232 | 175 | 121 | 197 | 206 | 56 | 0.687 | |
| 2 | 769 | 1131 | 958 | 1211 | 1306 | 1179 | 916 | 1206 | 201 | 0.359 | ||
| 112 | 2-year | 455 | 691 | 569 | 722 | 741 | 650 | 556 | 706 | 108 | 0.463 | |
| Total fungi biomass | 1 | 65 | 56 | 48 | 80 | 43 | 37 | 28 | 63 | 21 | 0.717 | |
| 2 | 200 | 416 | 265 | 403 | 640 | 313 | 311 | 397 | 100 | 0.102 | ||
| 11 | 2-year | 133b | 236ab | 156b | 241ab | 342a | 175b | 170b | 230ab | 51 | 0.08 | |
| Arbuscular mycorrhizal | 1 | 21 | 22 | 14 | 27 | 11 | 15 | 6 | 23 | 8 | 0.603 | |
| 2 | 41 | 79 | 57 | 80 | 108 | 66 | 66 | 89 | 19 | 0.198 | ||
| 4 | 2-year | 31 | 51 | 36 | 54 | 60 | 41 | 37 | 56 | 11 | 0.342 | |
| Saprophytes biomass | 1 | 44 | 34 | 33 | 53 | 32 | 22 | 22 | 41 | 14 | 0.765 | |
| 2 | 159 | 336 | 208 | 323 | 532 | 247 | 244 | 308 | 82 | 0.091 | ||
| 0 | 2-year | 102b | 185ab | 121b | 188ab | 282a | 134b | 133b | 174ab | 42 | 0.064 | |
| Undifferentiated biomass | 1 | 362 | 537 | 478 | 723 | 527 | 340 | 819 | 412 | 163 | 0.384 | |
| 2 | 1403 | 2038 | 1762 | 1978 | 2185 | 2019 | 1492 | 1908 | 300 | 0.462 | ||
| 208 | 2-year | 883 | 1288 | 1120 | 1350 | 1356 | 1179 | 1155 | 1160 | 171 | 0.589 | |
| Fungal-bacteria ratio | 1 | 0.28 | 0.16 | 0.25 | 0.24 | 0.11 | 0.15 | 0.1 | 0.19 | 0.06 | 0.434 | |
| 2 | 0.2 | 0.26 | 0.2 | 0.24 | 0.3 | 0.19 | 0.25 | 0.23 | 0.02 | 0.059 | ||
| 0.06 | 2-year | 0.24 | 0.21 | 0.23 | 0.24 | 0.21 | 0.17 | 0.17 | 0.21 | 0.03 | 0.737 | |
| Diversity index | 1 | 1.3 | 1.4 | 1.3 | 1.4 | 1.2 | 1.3 | 1.2 | 1.4 | 0.08 | 0.426 | |
| 2 | 1.4 | 1.5 | 1.4 | 1.4 | 1.5 | 1.4 | 1.5 | 1.4 | 0.02 | 0.358 | ||
| 1.09 | 2-year | 1.3 | 1.4 | 1.3 | 1.4 | 1.3 | 1.4 | 1.3 | 1.4 | 0.04 | 0.456 | |
IMB; Initial microbial biomass.
Mix 1 (composition: annual ryegrass + faba bean + yellow sweetclover) and Mix 2 (composition: oat + faba bean + winter pea).
SE; standard error.
Means within rows that are followed by the same lowercase letter superscripts are not different among cover crop systems (P > 0.05).
Table 7.
The main effect of year on soil microbial community biomass and indices determined by phospholipid fatty acid analysis (PLFA) for cover crop systems in Reno, Nevada, USA.
| Year |
TMBa |
TBB |
ACTB |
Gram (−) |
Gram (+) |
TFB |
AMFB |
SB |
UND |
FBR |
DI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| -------------------------------------------------------------------------ng g soil−1------------------------------------------------------------- | |||||||||||
| 1 | 877 | 299 | 49 | 111 | 188 | 53 | 17 | 35 | 525 | 0.19 | 1.3 |
| 2 | 3760 | 1533 | 376 | 449 | 1084 | 368 | 74 | 295 | 1848 | 0.23 | 1.5 |
| SEb | 196 | 99 | 24 | 52 | 63 | 38 | 6 | 29 | 85 | 0.02 | 0.02 |
| P-valuec | <0.001 | <0.001 | <0.001 | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.053 | <0.001 |
TMB; total microbial biomass, TBB; total bacteria biomass, ACTB; actinomycetes biomass, (−) gram-negative bacteria biomass, (+) gram-positive bacteria biomass, TFB; total fungi biomass, AMF; arbuscular mycorrhizal fungi biomass, SB; saprophytes biomass, UND; undifferentiated microbial biomass, FBR; fungi-bacteria ratio, and DI; diversity index.
SE; standard error.
P-value; indicates significance within each column.
4. Discussion
Our study revealed that when differences were observed in biomass production, it generally favored the cover crop mixtures over monocultures which partially supported our hypothesis that cover crop species grown in mixtures provide an overall greater shoot biomass than when planted as monocultures. The generally greater shoot biomass for the cover crop mixtures compared to monocultures in this study was similar to the greater biomass production for mixed cover crop systems relative to the monocultures in several studies ([15,18]; Finey et al., 2016; [14]). Transgressive overyielding (i.e., mixtures produce a higher biomass per unit area compared to the most productive species grown as a monoculture) was also supported by this study, as the average of the mixtures produced 50.4 % greater biomass than the average of the monocultures averaged across years. From a biomass perspective, the monocultures of faba bean and winter pea performed poorly relative to the other systems. The possible reasons for their lower yield were the notably early maturity of both as indicated by the lack of increase in biomass when sampled early compared to late. Also, there was a greater invasion of weeds in the monoculture plots of faba bean and winter pea, which considerably reduced biomass due to suppression particularly, in the first year of the study. The overall lower shoot biomass ranking of faba bean and winter pea than that of the grass monocultures and the mixed systems in both years in this environment possibly reflects that the variety used for these species may not be well suited to this environment [58,59]. This trend may be a result of the differences in the competitiveness of grasses over legumes and more so, under water-limited environments. Generally, grasses tend to scavenge more mineral N and establish faster, producing more biomass than legumes with a slower establishment and are compromised by their unique ability to fix atmospheric nitrogen (Rannels and Wagger 1997; [60,61]). Therefore, grasses have an overall competitive edge over legume establishment in resource-poor environments such as the aridisols that dominate Nevada. The greater shoot biomass at late termination in our study concurred with the common trend of greater biomass at late compared to early cover crop termination date [62,63].
Roots of cover crops serve essential ecosystem services, for example, carbon and nutrient cycling thus enhancing soil organic carbon stock, soil stabilization, and increased microbial activities among numerous other soil properties and processes [[64], [65], [66]]. In addition, the root architecture of cover crops differs among species and thus offers synergistic services. For example, the fibrous root structures of grass species cover crops can enhance soil aggregation and production of mineral exudates [67] whereas, the deep taproot systems of legumes and brassicas are effective at breaking compaction, improving pore space and infiltration [68]. In our study, the superior root biomass of the Mix 1 system across the two years can be attributed to the combination of both fibrous (annual ryegrass) and tap root systems (yellow sweetclover). Our study also indicated that between the grass monocultures (annual ryegrass), and among the legume monocultures, yellow sweetclover can be advantageous to include in mixed cover crop systems because of their root biomass when compared to oat or faba bean respectively. Our results of root biomass were similar to the results reported in studies by Kuo et al. [69] and Pietola and Alakukku [70] of significantly greater root biomass for annual ryegrass over other grass and legume species.
Understanding the tissue C and N characteristics, and C/N ratio (biomass quality) of cover crop systems provides insights into cover crop potential nutrient cycling capabilities and the efficient management of resources in cropping systems [71]. In addition, the quantity of carbon and nitrogen available for recycling provides a guide to nutrient management of the succeeding crop [71]. Our study revealed that the legume monocultures of faba bean, yellow sweetclover, and winter pea produced greater concentrations of C and N relative to the mixtures or grass monocultures. However, their similar C/N ratios but the overall greater shoot biomass C and N of the mixtures are indicative of their balance mineralizable potential compared to the legumes or grass monocultures. The overall greater C/N ratio for the monoculture grasses in our study can be attributed to the lower nitrogen concentrations and this response can favor a slower release of N or even immobilization of N and thus limit N availability for the succeeding crop [31,72]. The termination date of cover crop systems in this study had a marked effect on C and N characteristics and a 31.6 % increase in the C/N ratio of late compared to early termination. This is critical since the higher C/N ratio of late termination can impede mineralization and limit the N supply to the subsequent crop [73]. The C/N ratios among cover crops systems in our study compared favorably to the ranges of 12–53 by Sainju et al. [74,75] and 16–29 by Pantoja et al. [71] for rye and several other cool-season annual legumes and their blends. The shoot biomass C and N quantity in our study followed closely the overall shoot biomass production of each system which was similar to the observation reported by Brennan et al. [76]. The shoot biomass C and N in this study compared favorably for the range of C (360–3700 kg ha−1) and N (31–261 kg ha−1) reported for cool-season cover crop species [74,75,77].
Lately, an attractive aspect of cover crops is their forage quality indices for animal utilization [78,79]. Lower values for ADF, NDF, and a higher RFV indicate greater digestibility, greater available energy, and greater dry matter intake, which corresponds to a higher feed quality [79,80]. The forage quality indices in this study indicated an overall superior feed quality for the legume monocultures than the grass monocultures and the mixed systems. This trend concurred with the findings from several studies that reported greater forage quality of legumes over grasses [[81], [82], [83], [84]]. The lower NDF and ADF concentrations and greater relative feed value of the early termination date samples relative to the late termination is a classical depiction of the loss in forage quality as plants mature over time [85]. The results from this study concurred with findings from other studies, which demonstrate that grasses typically dictate the carbohydrate and fiber fractions concentrations whereas the legume proportion influences the crude protein concentrations within forage crop systems (Sanderson et al., 2010). For example, Zemenchick et al. [86] and Holman et al. [84] reported that variations in crude protein concentrations are highly dependent on the proportion of legumes within a system whereas the total dry matter intake is altered by the proportion of the grass component. The lack of difference in RFV between the grass monocultures and mixtures in this study could be attributed to the relatively low proportion of legumes in the mixtures based on the botanical composition results. Hence, for high-producing ruminant livestock, the grass monocultures and mixed cover crop systems used in this study may need additional supplementation to achieve their required nutritional requirements [78]. However, because of the nature of legumes digestion in ruminant nutrition, the mixed cover crop systems may be more suitable diets because of their better carbohydrate-to-protein balance.
Our study revealed no short-term impact of the cover cropping systems used relative to the fallow (no cover crop) on soil total organic carbon. Like our study, Chu et al. [87] reported no effect of cover cropping on soil organic carbon after three years. While Ghimire et al. [88] reported a cover crop treatment effect on soil organic carbon, none of the cover crop treatments in their study were different than the fallow system which is similar to our results. However, our results differ from those of Strickland et al. [89] who reported a short-term increase in soil organic carbon under cover crops relative to the fallow system. The lack of difference in our study among cover crop systems for soil TOC possibly indicates too short a period for C inputs from the cover crop systems to influence the soil C dynamics [75]. The greater soil TOC in Year 1 compared to Year 2 may have been a result of the possibly slower rate of residue decomposition (decrease in carbon mineralization) in the second compared to the first year of the study. While this trend is a short-term response and potentially shows the dynamic nature of these soil parameters, this decrease in soil TOC in Year 2 may have also been a result of a higher initial level of soil TOC [90] which inadvertently was not measured in this study. In our study, the soil N concentration was impacted by cover crop systems but only during the first year did a cover crop system (faba bean) have greater soil N than the fallow. This trend was similar to the greater soil N under crimson clover-hairy vetch relative to the fallow system by Strickland et al. [89]. However, the results of our study were in contrast to the greater soil N under fallow than cover crops reported in the study of Ghimire et al. [88]. Soil CO2 respiration provides insights into soil microbial activities and is a good biological indicator of soil quality [91]. Soil CO2-C respiration was greater in Year 1 than 2 and was possibly associated with the greater soil organic carbon in Year 1. However, this temporal difference does not correlate with the overall greater microbial biomass in Year 2 compared to Year 1 of this study. Some possible reasons for this reverse trend in soil CO2-C respiration between Years 1 and 2 may have been lower soil temperature and moisture [92,93], and reduced organic matter at the soil surface [94] at the time of sampling in Year 2 compared to Year 1.
This study only showed a partial response of microbial community biomass (gram-negative bacteria, fungi, and saprophytes) to cover crop systems. However, except for yellow sweetclover, no other cover crop systems yielded an advantage over the fallow system for these microbial parameters. The year effect was quite evident in our study, and while there was no interaction with the cover crop system, a close examination of the data substantiated that the greatest increase in microbial population biomass was under the cover crop systems relative to the fallow. While our study did not yield a complete impact of cover crop system on microbial population biomass, it concurred to some extent with some of the results reported in other studies [75,[95], [96], [97], [98], [99]].
As it relates to limitation and future direction, in this study, we have not examined the carryover effect of residual soil N following alfalfa termination on the succeeding cover crop treatments. The reason was that soil test from the experimental area recommended nitrogen application for the grass species used and since these legumes included were all annual, we surmised that N fixation during this short-duration study would have minimal impact on the companion grass species (dominant species) and thus N was applied to the mixtures. However, from a sustainable approach to cover crop integration, future work will focus on the carryover effect of residual soil N after alfalfa termination to enhance agronomic management decisions in the integration of cover crops in semi-arid environments that are typically resource-poor. Also, future research work will expand on these cover crop systems by increasing the number of mixtures across multiple environments and under different irrigation levels over a longer duration (>5 years) in hopes of providing definitive insights into the ecosystem services they offer in this water-scarce environment.
5. Conclusions
Biomass production is an important factor in cover cropping and the results from this two-year study revealed that the mixed cover crop systems on average performed better than the monocultures. Terminating late offered greater shoot biomass yield relative to early termination in this growing environment in Nevada. Our study revealed no difference in root biomass among cover crop systems in each year separately. However, when averaged across the two years, root biomass was greater for the Mix 1 cover crop system over the monocultures of oat and faba bean. Cover crop system tissue carbon and nitrogen were greater for legumes monoculture relative to the mixtures or monoculture grasses. However, the mixtures offered a similar C/N ratio to the monoculture legumes and coupled with their overall greater shoot biomass C and N content (kg ha−1) will be more suitable to utilize instead of the monocultures. Terminating late drastically increases the C/N ratio and may slow the rate of cover crop residue decomposition and release of mineral nutrients for the succeeding crop and thus increase fertilizer input required for nonlegume crops in succession. Based on the relative feed value, the legume monocultures offered superior feed quality compared to the mixtures and grass monocultures. Terminating late decrease cover crop feed quality after two years of evaluation. Based on the soil health parameters evaluated, the monoculture yellow sweetclover had an impactful role in this short-duration study and should be included in mixtures if not desirable as a single species. The significant increase in microbial biomass in the second year compared to the first indicates the potential of cover crops to boost soil health. However, the true impact of the cover crop systems used in this study will require a longer duration of cultivation. Overall, combining cover crop soil health characteristics, feed quality, and maximizing resource use efficiency, both cover crop mixtures (Mix 1 and Mix 2) offered a greater chance of fulfilling the dual role of soil health improvement and feed quality for livestock in this semiarid environment. For producers, a balance between the trade-offs among biomass production, the C/N ratio, and feed quality will be the key determinants in optimizing the dual role (ecosystem services and animal feed) of cover crops in water-scarce environments.
CRediT authorship contribution statement
Akwasi Opoku: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Anuoluwapo M. Ogunleye: Writing – original draft, Formal analysis, Data curation. Juan K.Q. Solomon: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. William A. Payne: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Data and code availability statement
Data will be made available on request.
Declaration of funding
This research was funded by the Office of the Dean, College of Agriculture, Biotechnology & Natural Resources, University of Nevada, Reno, Reno, USA.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express sincere gratitude to Mr. Scott Huber, Research Coordinator at Nevada Agricultural Experiment Station, and his staff for field preparation, planting, irrigation, and all other technical agronomic support throughout the study duration.
References
- 1.Pimentel D. Soil erosion: a food and environmental threat. Environ. Dev. Sustain. 2006;8(1):119–137. doi: 10.1007/s10668-005-1262-8. [DOI] [Google Scholar]
- 2.Badagliacca G., Laudicina V.A., Amato G., Badalucco L., Frenda A.S., Giambalvo D., Ingraffia R., Plaia A., Ruisi P. Long-term effects of contrasting tillage systems on soil C and N pools and on main microbial groups differ by crop sequence. Soil Tillage Res. 2021;211 doi: 10.1016/j.still.2021.104995. [DOI] [Google Scholar]
- 3.Kucharik C.J., Brye K.R., Norman J.M., Foley J.A., Gower S.T., Bundy L.G. Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern Wisconsin: potential for SOC sequestration during the next 50 years. Ecosystems. 2001;4(3):237–258. doi: 10.1007/s10021-001-0007-2. [DOI] [Google Scholar]
- 4.Haddaway N.R., Hedlund K., Jackson L.E., Kätterer T., Lugato E., Thomsen I.K., Isberg P.E. How does tillage intensity affect soil organic carbon? A systematic review. Environ. Evid. 2017;6(1):1–48. doi: 10.1186/s13750-017-0108-9. [DOI] [Google Scholar]
- 5.Eric G M., Yongping Y., Megan H M., Michael A J. SWAT model application to assess the impact of intensive corn-farming on runoff, sediments and phosphorous loss from an agricultural watershed in Wisconsin. J. Water Resour. Protect. 2012;4:423–431. doi: 10.4236/jwarp.2012.47049. [DOI] [Google Scholar]
- 6.Sharma P., Singh A., Kahlon C.S., Brar A.S., Grover K.K., Dia M., Steiner R.L. The role of cover crops towards sustainable soil health and agriculture—a review paper. Am. J. Plant Sci. 2018;9(9):1935–1951. doi: 10.4236/ajps.2018.99140. [DOI] [Google Scholar]
- 7.Chen L., Rejesus R.M., Aglasan S., Hagen S.C., Salas W. The impact of cover crops on soil erosion in the US midwest. J. Environ. Manag. 2022;324 doi: 10.1016/j.jenvman.2022.116168. [DOI] [PubMed] [Google Scholar]
- 8.Adetunji A.T., Ncube B., Mulidzi R., Lewu F.B. Management impact and benefit of cover crops on soil quality: a Review. Soil Tillage Res. 2020;204 doi: 10.1016/j.still.2020.104717. [DOI] [Google Scholar]
- 9.Haruna S.I., Eichas R.C., Peters O.M., Farmer A.C., Lackey D.Q., Nichols J.E., Peterson W.H., Slone N.A. In situ water infiltration: influence of cover crops after growth termination. Soil Sci. Soc. Am. J. 2022;86(3):769–780. doi: 10.1002/saj2.20390. [DOI] [Google Scholar]
- 10.Kuo S., Sainju U.M., Jellum E.J. Winter cover cropping influence on nitrogen in soil. Soil Sci. Soc. Am. J. 1997;61:1392–1399. doi: 10.2136/sssaj1997.03615995006100050016x. [DOI] [Google Scholar]
- 11.Sainju U.M., Singh B.P., Rahman S., Reddy V.R. Tillage, cover cropping, and nitrogen fertilization influence tomato yield and nitrogen uptake. Hortscience. 2000;35(2):217–221. doi: 10.21273/HORTSCI.35.1.78. [DOI] [Google Scholar]
- 12.Akemo M.C., Regnier E.E., Bennett M.A. Weed suppression in spring-sown rye (Secale cereale)–pea (Pisum sativum) cover crop mixes. Weed Technol. 2000;14(3):545–549. doi: 10.1614/0890-037X(2000)014[0545:WSISSR]2.0.CO;2. [DOI] [Google Scholar]
- 13.Villamil M.B., Bollero G.A., Darmody R.G., Simmons F.W., Bullock D.G. No‐till corn/soybean systems including winter cover crops. Soil Sci. Soc. Am. J. 2006;70(6):1936–1944. doi: 10.2136/sssaj2005.0350. [DOI] [Google Scholar]
- 14.Antosh E., Idowu J., Schutte B., Lehnhoff E. Winter cover crops effects on soil properties and sweet corn yield in semi‐arid irrigated systems. Agron. J. 2020;112(1):92–106. doi: 10.1002/agj2.20055. [DOI] [Google Scholar]
- 15.Wortman S.E., Francis C.A., Bernards M.L., Drijber R.A., Lindquist J.L. Optimizing cover crop benefits with diverse mixtures and an alternative termination method. Agron. J. 2012;104:1425–1435. doi: 10.2134/agronj2012.0185. [DOI] [Google Scholar]
- 16.Liebig M.A., Hendrickson J.R., Archer D.W., Schmer M.A., Nichols K.A., Tanaka D.L. Short-term soil responses to late-seeded cover crops in a semi-arid environment. Agron. J. 2015;107:2011–2019. doi: 10.2134/agronj15.0146. [DOI] [Google Scholar]
- 17.Creamer N.G., Bennett M.A., Stinner B.R. Evaluation of cover crop mixtures for use in vegetable production systems. Hortscience. 1997;32(5):866–870. doi: 10.21273/HORTSCI.32.5.866. [DOI] [Google Scholar]
- 18.Smith R.G., Atwood L.W., Warren N.D. Increased productivity of a cover crop mixture is not associated with enhanced agroecosystem services. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper D.U. The role of complementarity and competition in ecosystem responses to variation in plant diversity. Ecology. 1998;79(2):704–719. doi: 10.1890/0012-9658(1998)079[0704:TROCAC]2.0.CO;2. [DOI] [Google Scholar]
- 20.Cardinale B.J., Matulich K.L., Hooper D.U., Byrnes J.E., Duffy E., Gamfeldt L., Balvanera P., O'Connor M.I., Gonzalez A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011;98:572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 21.MacLaren C., Swanepoel P., Bennett J., Wright J., Dehnen-Schmutz K. Cover crop biomass production is more important than diversity for weed suppression. Crop Sci. 2019;59(2):733–748. doi: 10.2135/cropsci2018.05.0329. [DOI] [Google Scholar]
- 22.Hooper D.U., Chapin F.S., III, Ewel J.J., Hector A., Inchausti P., Lavorel S., Lawton J.H., Lodge D.M., Loreau M., Naeem S., Schmid B., Setälä H., Symstad A.J., Vandermeer J., Wardle D.A. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 2005;75:3–35. doi: 10.1890/04-0922. [DOI] [Google Scholar]
- 23.Ranells N.N., Wagger M.G. Nitrogen release from grass and legume cover crop monocultures and bicultures. Agron. J. 1996;88:777–882. doi: 10.2134/agronj1996.00021962008800050015x. [DOI] [Google Scholar]
- 24.Ranells N.N., Wagger M.G. Grass–legume bicultures as winter annual cover crops. Agron. J. 1997;89(4):659–665. doi: 10.2134/agronj1997.00021962008900040019x. [DOI] [Google Scholar]
- 25.Lawson A., Fortuna A.M., Cogger C., Bary A., Stubbs T. Nitrogen contribution of rye–hairy vetch cover crop mixtures to organically grown sweet corn. Renew. Agric. Food Syst. 2013;28(1):59–69. doi: 10.1017/S1742170512000014. [DOI] [Google Scholar]
- 26.Hayden Z.D., Ngouajio M., Brainard D.C. Rye–vetch mixture proportion tradeoffs: cover crop productivity, nitrogen accumulation, and weed suppression. Agron. J. 2014;106(3):904–914. doi: 10.2134/agronj2013.0467. [DOI] [Google Scholar]
- 27.Khan Q.A., McVay K.A. Productivity and stability of multi‐species cover crop mixtures in the northern Great Plains. Agron. J. 2019;111(4):1817–1827. doi: 10.2134/agronj2018.03.0173. [DOI] [Google Scholar]
- 28.Norris R., Chim B.K., Evanylo G., Reiter M., Thomason W. Corn yield and soil nitrogen following winter annual cover crops interseeded into soybean. Crop Sci. 2020;60(5):2667–2682. doi: 10.1002/csc2.20185. [DOI] [Google Scholar]
- 29.Kuo S., Sainju U.M. Nitrogen mineralization and availability of mixed leguminous and non-leguminous cover crop residues in soil. Biol. Fertil. Soils. 1998;26(4):346–353. doi: 10.1007/s003740050387. [DOI] [Google Scholar]
- 30.Teasdale J.R., Abdul-Baki A.A. Comparison of mixtures vs. monocultures of cover crops for fresh-market tomato production with and without herbicide. Hortscience. 1998;33(7):1163–1166. doi: 10.21273/HORTSCI.33.7.1163. [DOI] [Google Scholar]
- 31.Finney D.M., White C.M., Kaye J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016;108(1):39–52. doi: 10.2134/agronj15.0182. [DOI] [Google Scholar]
- 32.Sainju U.M., Singh B.P., Whitehead W.F. Cover crop root distribution and its effects on soil nitrogen cycling. Agron. J. 1998;90(4):511–518. doi: 10.2134/agronj1998.00021962009000040012x. [DOI] [Google Scholar]
- 33.Greenwood D.J., Lemaire G., Gosse G., Cruz P., Draycott A., Neeteson J.J. Decline in percentage N of C3 and C4 crops with increasing plant mass. Ann. Bot. 1990;66(4):425–436. doi: 10.1093/oxfordjournals.aob.a088044. [DOI] [Google Scholar]
- 34.Jennings J.A., Nelson C.J. Influence of soil texture on alfalfa autotoxicity. Agron. J. 1998;90(1):54–58. doi: 10.2134/agronj1998.00021962009000010010x. [DOI] [Google Scholar]
- 35.Van Eerd L.L., Chahal I., Peng Y., Awrey J.C. Influence of cover crops at the four spheres: a review of ecosystem services, potential barriers, and future directions for North America. Sci. Total Environ. 2023;858 doi: 10.1016/j.scitotenv.2022.159990. [DOI] [PubMed] [Google Scholar]
- 36.Bakker C.E., Hite L.M., Wright C.L., Brake D.W., Smart A.J., Blair A.D., Grubbs J.K., Underwood K.R. Impact of feeding cover crop forage containing brassicas to steers during backgrounding on live animal performance, carcass characteristics, and meat color1. Translational Animal Science. 2021;5(3) doi: 10.1093/tas/txab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golden L.A., Hubbard M.L., Utych S., Newman S.M., Hines S., Thomas J., Andrews N., Castellano R.L.S., Collins D.P., Sullivan C. Benefits, barriers, and use of cover crops in the Western United States: regional survey results. J. Soil Water Conserv. 2023;78(3):260–271. doi: 10.2489/jswc.2023.00170. [DOI] [Google Scholar]
- 38.Sullivan C.S., Kleber M. Winter cover cropping to improve soil health in semiarid, irrigated cropping systems of Central Oregon. Agrosystems, Geosciences & Environment. 2024;7(1) doi: 10.1002/agg2.20481. [DOI] [Google Scholar]
- 39.Barnett T.P., Pierce D.W., Hidalgo H.G., Bonfils C., Santer B.D., Das T., Bala G., Wood A.W., Nozawa T., Mirin A.A., Cayan D.R., Dettinger M.D. Human-induced changes in the hydrology of the Western United States. Science. 2008;319(5866):1080–1083. doi: 10.1126/science.1152538. [DOI] [PubMed] [Google Scholar]
- 40.Runkle J., Kunkel K.E., Champion S., Easterling D. NOAA Technical Report NESDIS 149-NV. 2017. Nevada state climate Summary; p. 4. [Google Scholar]
- 41.USDA Soil Survey Staff . USDA-NRCS; 2022. Web Soil Survey.https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx [Google Scholar]
- 42.Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74(10):3583–3597. doi: 10.3168/jds.s0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 43.Undersander D.J., Howard W.T., Shaver R.D. Milk per acre spreadsheet for combining yield and quality into a single term. J. Prod. Agric. 1993;6(2):231–235. doi: 10.2134/jpa1993.0231. [DOI] [Google Scholar]
- 44.Ghimire R., Norton J.B., Pendall E. Alfalfa-grass biomass, soil organic carbon, and total nitrogen under different management approaches in an irrigated agroecosystem. Plant Soil. 2014;374(1):173–184. doi: 10.1007/s11104-013-1854-2. [DOI] [Google Scholar]
- 45.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37(1):911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 46.Buyer J.S., Teasdale J.R., Roberts D.P., Zasada I.A., Maul J.E. Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol. Biochem. 2010;42(5):831–841. doi: 10.1016/j.soilbio.2010.01.020. [DOI] [Google Scholar]
- 47.Frostegård Å., Tunlid A., Bååth E. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 1996;28(1):55–63. doi: 10.1016/0038-0717(95)00100-x. [DOI] [Google Scholar]
- 48.Zelles L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a Review. Biol. Fertil. Soils. 1999;29(2):111–129. doi: 10.1007/s003740050533. [DOI] [Google Scholar]
- 49.Liu M., Sui X., Hu Y., Feng F. Microbial community structure and the relationship with soil carbon and nitrogen in an original Korean pine forest of Changbai Mountain, China. BMC Microbiol. 2019;19(1):1–14. doi: 10.1186/s12866-019-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klamer M., Bååth E. Microbial community dynamics during composting of straw material studied using phospholipid fatty acid analysis. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 1998;27(1):9–20. doi: 10.1111/j.1574-6941.1998.tb00521.x. [DOI] [Google Scholar]
- 51.Buyer J.S., Sasser M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012;61:127–130. doi: 10.1016/j.apsoil.2012.06.005. [DOI] [Google Scholar]
- 52.Willers C., Jansen van Rensburg P.J., Claassens S. Phospholipid fatty acid profiling of microbial communities-a review of interpretations and recent applications. J. Appl. Microbiol. 2015;119(5):1207–1218. doi: 10.1111/jam.12902. [DOI] [PubMed] [Google Scholar]
- 53.Olsson P.A., Bååth E., Jakobsen I., Söderström B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995;99(5):623–629. doi: 10.1016/s0953-7562(09)80723-5. [DOI] [Google Scholar]
- 54.Olsson P. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 1999;29(4):303–310. doi: 10.1016/s0168-6496(99)00021-5. [DOI] [Google Scholar]
- 55.Frostegård A., Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils. 1996;22(1–2):59–65. doi: 10.1007/bf00384433. [DOI] [Google Scholar]
- 56.SAS Institute, Inc. SAS Institute; Cary, NC: 2016. SAS/STAT 9.4 Users' Guide. [Google Scholar]
- 57.Cavanaugh J.E. Unifying the derivations for the Akaike and corrected Akaike information criteria. Stat. Probab. Lett. 1997;33(2):201–208. doi: 10.1016/s0167-7152(96)00128-9. [DOI] [Google Scholar]
- 58.Brasier K., Smither‐Kopperl M., Bullard V., Young‐Matthews A., Bartow A., Friddle M., Bernau C., Humphrey M., Dial H., Wolf M., Hu J., Zakeri H. A multi‐environment analysis of winter faba bean germplasm for cover crop traits. Agron. J. 2021;113(4):3051–3064. doi: 10.1002/agj2.20717. [DOI] [Google Scholar]
- 59.Vann R.A., Reberg‐Horton S.C., Castillo M.S., Murphy J.P., Martins L.B., Mirsky S.B., Saha U., McGee R.J. Differences among eighteen winter pea genotypes for forage and cover crop use in the Southeastern United States. Crop Sci. 2021;61(2):947–965. doi: 10.1002/csc2.20355. [DOI] [Google Scholar]
- 60.Dabney S.M., Delgado J.A., Reeves D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001;32(7–8):1221–1250. doi: 10.1081/CSS-100104110. [DOI] [Google Scholar]
- 61.Blanco‐Canqui H., Shaver T.M., Lindquist J.L., Shapiro C.A., Elmore R.W., Francis C.A., Hergert G.W. Cover crops and ecosystem services: insights from studies in temperate soils. Agron. J. 2015;107(6):2449–2474. doi: 10.2134/agronj15.0086. [DOI] [Google Scholar]
- 62.Keene C.L., Curran W.S., Wallace J.M., Ryan M.R., Mirsky S.B., VanGessel M.J., Barbercheck M.E. Cover crop termination timing is critical in organic rotational no‐till systems. Agron. J. 2017;109(1):272–282. doi: 10.2134/agronj2016.05.0266. [DOI] [Google Scholar]
- 63.Overmyer K., Looker W., Dorrance A.E., Tilmon K.J., Lindsey L.E. Influence of rye/oat cover crop in a two‐year soybean production system. Agron. J. 2023;115(1):303–313. doi: 10.1002/agj2.21244. [DOI] [Google Scholar]
- 64.Six J., Feller C., Denef K., Ogle S., de Moraes Sa J.C., Albrecht A. Soil organic matter, biota and aggregation in temperate and tropical soils-effects of no-tillage. Agronomie. 2002;22(7–8):755–775. doi: 10.1051/agro:2002043. [DOI] [Google Scholar]
- 65.Wilhelm W.W., Johnson J.M.F., Hatfield J.L., Voorhees W.B., Linden D.R. Crop and soil productivity response to corn residue removal: a literature review. Agron. J. 2004;96:1–17. doi: 10.2134/agronj2004.0001. [DOI] [Google Scholar]
- 66.Ruis S.J., Blanco‐Canqui H., Koehler‐Cole K., Jasa P.J., Slater G., Elmore R.W., Ferguson R.B. Winter cover crop root biomass yield in corn and soybean systems. Agrosystems, Geosciences & Environment. 2020;3(1) doi: 10.1002/agg2.20101. [DOI] [Google Scholar]
- 67.Gyssels G., Poesen J., Bochet E., Li Y. Impact of plant roots on the resistance of soils to erosion by water: a review. Prog. Phys. Geogr. 2005;29:189–217. doi: 10.1191/0309133305pp443ra. [DOI] [Google Scholar]
- 68.Ruis S.J., Blanco-Canqui H., Jasa P., Ferguson R., Slater G. Can cover crop use allow increased levels of maize residue removal for biofuel in irrigated and rainfed systems? BioEnergy Research. 2017;10:992–1004. doi: 10.1007/s12155-017-9858-z. [DOI] [Google Scholar]
- 69.Kuo S., Sainju U.M., Jellum E. Winter cover cropping influence on nitrogen mineralization, presidedress soil nitrate test, and maize yields. Biol. Fertil. Soils. 1996;23:310–317. doi: 10.1007/BF00334575. [DOI] [Google Scholar]
- 70.Pietola L., Alakukku L. Root growth dynamics and biomass input by Nordic annual field crops. Agric. Ecosyst. Environ. 2005;108(2):135–144. doi: 10.1016/j.agee.2005.01.009. [DOI] [Google Scholar]
- 71.Pantoja J.L., Woli K.P., Sawyer J.E., Barker D.W. Winter rye cover crop biomass production, degradation, and nitrogen recycling. Agron. J. 2016;108(2):841–853. doi: 10.2134/agronj2015.0336. [DOI] [Google Scholar]
- 72.Jensen E.S. Nitrogen immobilization and mineralization during initial decomposition of 15N-labelled pea and barley residues. Biol. Fertil. Soils. 1997;24(1):39–44. doi: 10.1007/BF01420218. [DOI] [Google Scholar]
- 73.Schomberg H.H., Martini N.L., Diaz‐Perez J.C., Phatak S.C., Balkcom K.S., Bhardwaj H.L. Potential for using sunn hemp as a source of biomass and nitrogen for the Piedmont and Coastal Plain regions of the southeastern USA. Agron. J. 2007;99(6):1448–1457. doi: 10.2134/agronj2006.0294. [DOI] [Google Scholar]
- 74.Sainju U.M., Whitehead W.F., Singh B.P. Biculture legume–cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron. J. 2005;97(5):1403–1412. doi: 10.2134/agronj2004.0274. [DOI] [Google Scholar]
- 75.Sainju U.M., Schomberg H.H., Singh B.P., Whitehead W.F., Tillman P.G., Lachnicht-Weyers S.L. Cover crop effect on soil carbon fractions under conservation tillage cotton. Soil Tillage Res. 2007;96(1–2):205–218. doi: 10.1016/j.still.2007.06.006. [DOI] [Google Scholar]
- 76.Brennan E.B., Boyd N.S., Smith R.F. Winter cover crop seeding rate and variety effects during eight years of organic vegetables: III. Cover crop residue quality and nitrogen mineralization. Agron. J. 2013;105(1):171–182. doi: 10.2134/agronj2012.0258. [DOI] [Google Scholar]
- 77.Yang X.M., Drury C.F., Reynolds W.D., Reeb M.D. Legume cover crops provide nitrogen to corn during a three‐year transition to organic cropping. Agron. J. 2019;111(6):3253–3264. doi: 10.2134/agronj2018.10.0652. [DOI] [Google Scholar]
- 78.Horn K.M., Rocateli A.C., Warren J.G., Turner K.E., Antonangelo J.A. Evaluating cover crops forage nutritive value in Oklahoma Winter Wheat Systems. Agron. J. 2021;113(4):3361–3371. doi: 10.1002/agj2.20708. [DOI] [Google Scholar]
- 79.Obour A.K., Holman D.J., Assefa Y. Single and multispecies dual-purpose cover crop productivity, nutritive value, and profitability. Agrosystems, Geosciences & Environment. 2022;5 doi: 10.1002/agg2.20275. [DOI] [Google Scholar]
- 80.Holman J.D., Arnet K., Dille J., Maxwell S., Obour A., Roberts T., Roozeboom K., Schlegel A. Can cover or forage crops replace fallow in the Semiarid Central Great Plains? Crop Sci. 2018;58(2):932–944. doi: 10.2135/cropsci2017.05.0324. [DOI] [Google Scholar]
- 81.Buxton D.R., Fales S.L. In: Forage Quality, Evaluation, and Utilization. Fahey G.C., editor. 1994. Plant environment and quality. [DOI] [Google Scholar]
- 82.Sanderson M. Nutritive value and herbage accumulation rates of pastures sown to grass, legume, and chicory mixtures. Agron. J. 2010;102(2):728–733. doi: 10.2134/agronj2009.0374. [DOI] [Google Scholar]
- 83.Sanderson M., Johnson H., Hendrickson J. Cover crop mixtures grown for annual forage in a semi‐arid environment. Agron. J. 2018;110(2):525–534. doi: 10.2134/agronj2017.04.0228. [DOI] [Google Scholar]
- 84.Holman J.D., Obour A.K., Assefa Y. Fallow replacement cover crops in a semi‐arid High Plains cropping system. Crop Sci. 2021;61(5):3799–3814. doi: 10.1002/csc2.20543. [DOI] [Google Scholar]
- 85.Ball D.M., Collins M., Lacefield G.D., Martin N.P., Mertens D.A., Olson K.E., Wolf M.W. Understanding forage quality. American Farm Bureau Federation Publication. 2001;1(1) [Google Scholar]
- 86.Zemenchik R.A., Albrecht K.A., Shaver R.D. Improved nutritive value of kura clover– and birdsfoot trefoil–grass mixtures compared with grass monocultures. Agron. J. 2002;94(5):1131–1138. doi: 10.2134/agronj2002.1131. [DOI] [Google Scholar]
- 87.Chu M., Jagadamma S., Walker F.R., Eash N.S., Buschermohle M.J., Duncan L.A. Effect of multispecies cover crop mixture on soil properties and crop yield. Agricultural & Environmental Letters. 2017;2(1) doi: 10.2134/ael2017.09.0030. [DOI] [Google Scholar]
- 88.Ghimire R., Ghimire B., Mesbah A.O., Sainju U.M., Idowu O.J. Soil health response of cover crops in winter wheat-fallow system. Agron. J. 2019;111(4):2108–2115. doi: 10.2134/agronj2018.08.0492. [DOI] [Google Scholar]
- 89.Strickland M.S., Thomason W.E., Avera B., Franklin J., Minick K., Yamada S., Badgley B.D. Short-term effects of cover crops on soil microbial characteristics and biogeochemical processes across actively managed farms. Agrosystems, Geosciences & Environment. 2019;2(1):1–9. doi: 10.2134/age2018.12.0064. [DOI] [Google Scholar]
- 90.Novara A., Catania V., Tolone M., Gristina L., Laudicina V.A., Quatrini P. Cover crop impact on soil organic carbon, nitrogen dynamics and microbial diversity in a Mediterranean semiarid vineyard. Sustainability. 2020;12(8):3256. doi: 10.3390/su12083256. [DOI] [Google Scholar]
- 91.Haney R.L., Haney E.B., Smith D.R., Harmel R.D., White M.J. The soil health tool-theory and initial broad-scale application. Appl. Soil Ecol. 2018;125:162–168. doi: 10.1016/j.apsoil.2017.07.035. [DOI] [Google Scholar]
- 92.Nilahyane A., Ghimire R., Thapa V.R., Sainju U.M. Cover crop effects on soil carbon dioxide emissions in a semiarid cropping system. Agrosystems, Geosciences & Environment. 2020;3(1) doi: 10.1002/agg2.20012. [DOI] [Google Scholar]
- 93.Nguyen L.T.T., Kravchenko A.N. Effects of cover crops on soil CO2 and N2O emissions across topographically diverse agricultural landscapes in corn-soybean-wheat organic transition. Eur. J. Agron. 2021;122 doi: 10.1016/j.eja.2020.126189. [DOI] [Google Scholar]
- 94.Ellman‐Stortz L., Lewis K., Gentry T., DeLaune P., Pierson E., Boogades N. Early impacts of cover crop selection on soil biological parameters during a transition to organic agriculture. Agrosystems, Geosciences & Environment. 2024;7(2) doi: 10.1002/agg2.20532. [DOI] [Google Scholar]
- 95.Sainju U.M., Whitehead W.F., Singh B.P. Cover crops and nitrogen fertilization effects on soil aggregation and carbon and nitrogen pools. Can. J. Soil Sci. 2003;83(2):155–165. doi: 10.4141/S02-056. [DOI] [Google Scholar]
- 96.Wang Q.R., Li Y.C., Klassen W. Changes of soil microbial biomass carbon and nitrogen with cover crops and irrigation in a tomato field. J. Plant Nutr. 2007;30(4):623–639. doi: 10.1080/01904160701209410. [DOI] [Google Scholar]
- 97.Kramer S., Marhan S., Ruess L., Armbruster W., Butenschoen O., Haslwimmer H., Kuzyakov Y., Pausch J., Scheunemann N., Schoene J., Schmalwasser A., Totsche K.U., Walker F., Scheu S., Kandeler E. Carbon flow into microbial and fungal biomass as a basis for the belowground food web of agroecosystems. Pedobiologia. 2012;55(2):111–119. doi: 10.1016/j.pedobi.2011.12.001. [DOI] [Google Scholar]
- 98.Njeru E.M., Avio L., Bocci G., Sbrana C., Turrini A., Bàrberi P., Oehl F. Contrasting effects of cover crops on ‘hot spot’arbuscular mycorrhizal fungal communities in organic tomato. Biol. Fertil. Soils. 2015;51(2):151–166. doi: 10.1007/s00374-014-0958-z. [DOI] [Google Scholar]
- 99.Thapa V.R., Ghimire R., Acosta-Martínez V., Marsalis M.A., Schipanski M.E. Cover crop biomass and species composition affect soil microbial community structure and enzyme activities in semiarid cropping systems. Appl. Soil Ecol. 2021;157 doi: 10.1016/j.apsoil.2020.103735. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.