Abstract
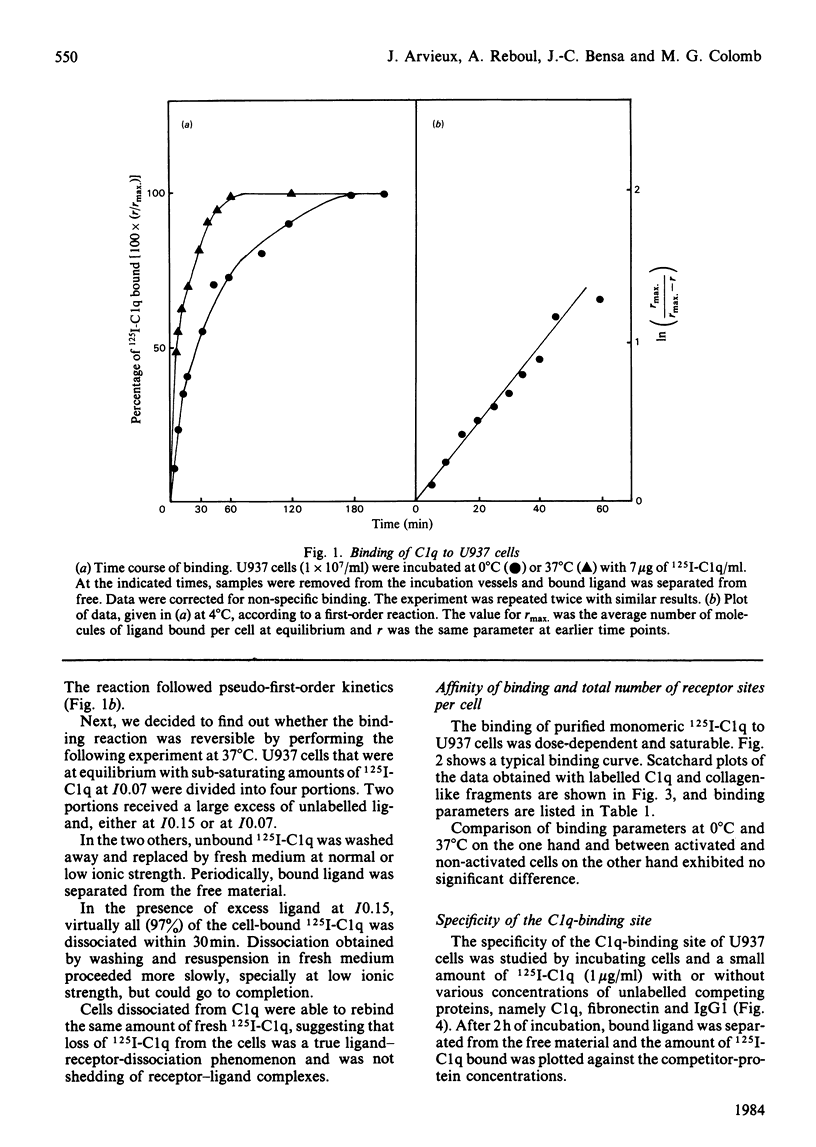
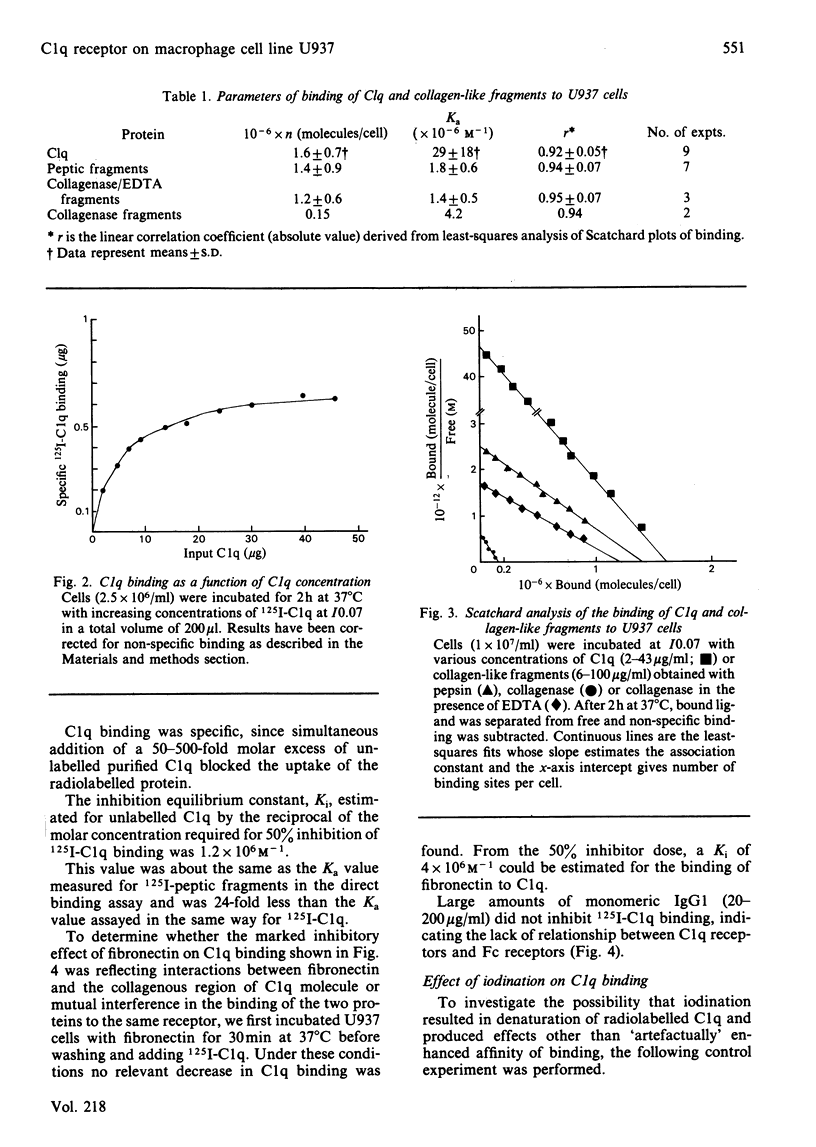
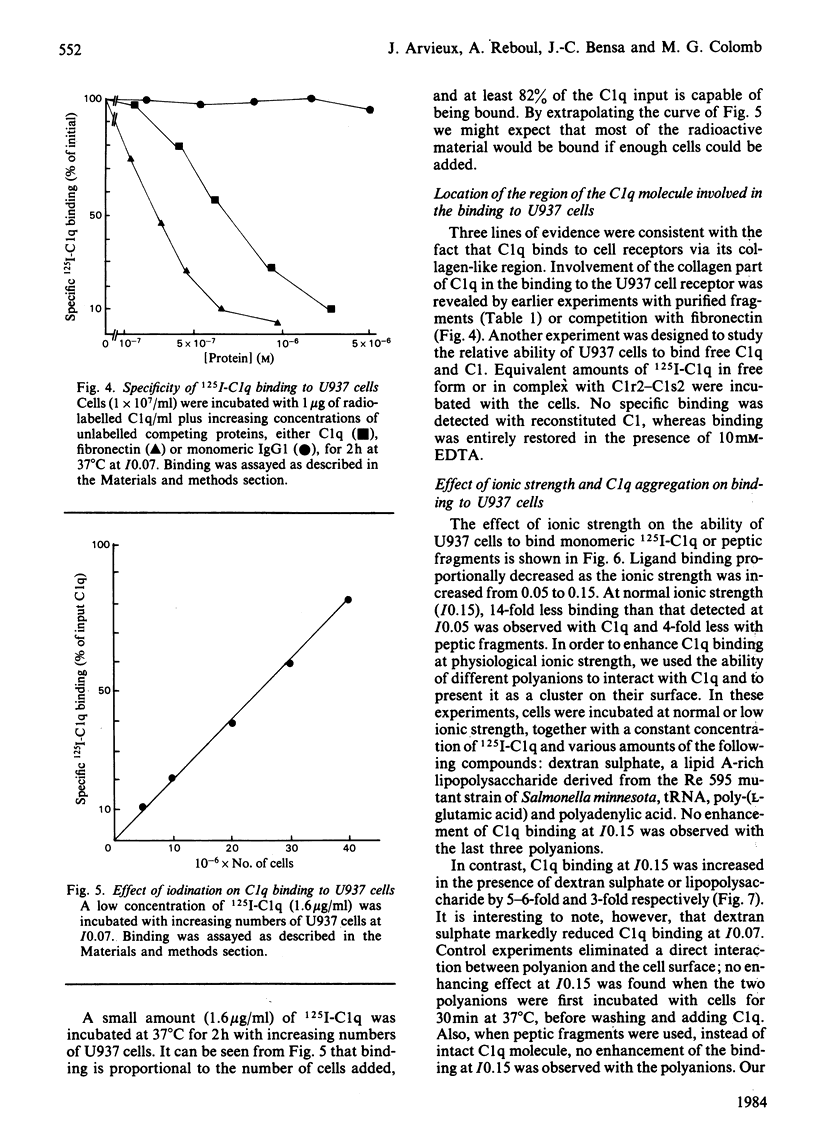
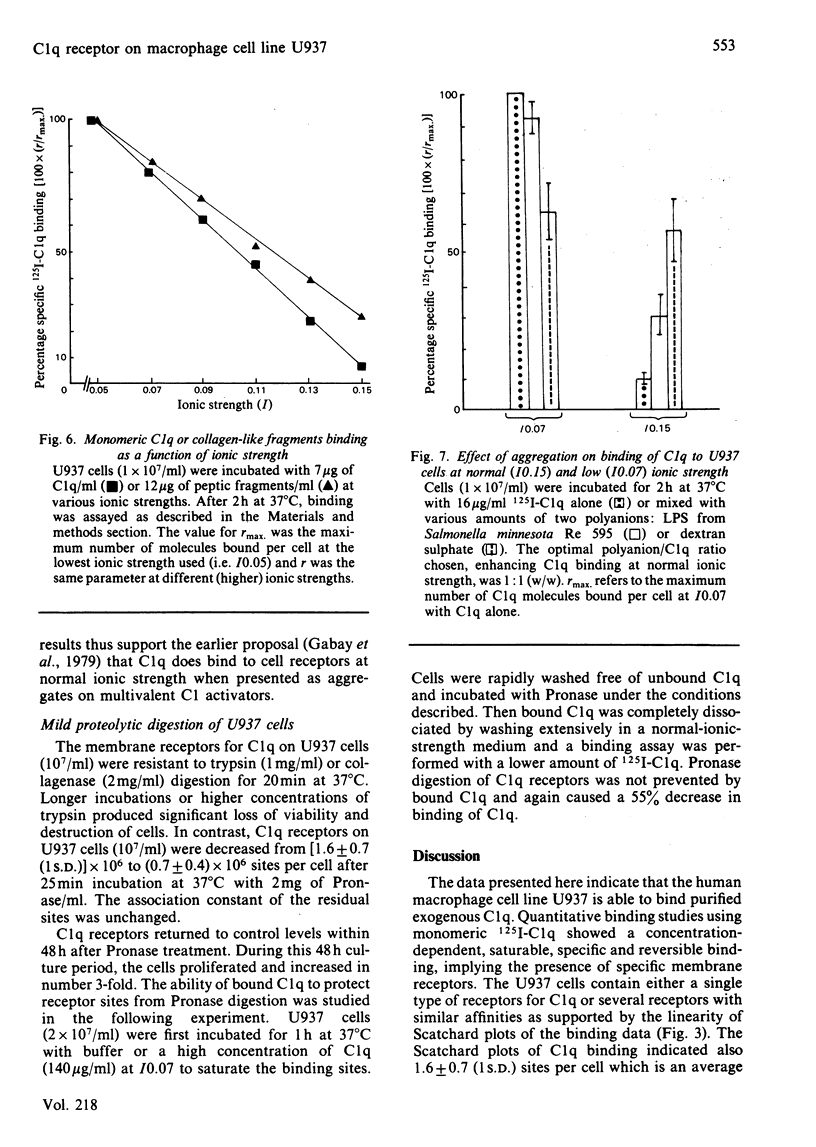
The binding of C1q to the human macrophage cell line U937 has been studied. Fluorescence microscopy with fluorescein-conjugated F(ab')2 anti-C1q antibody showed that 100% of the cell population is able to bind exogenous C1q. Monomeric C1q binding to U937 cells is very weak at normal ionic strength (I0.15) and was therefore investigated at I0.07, conditions which stabilize the binding. However, aggregation of C1q on dextran sulphate or a lipid A-rich lipopolysaccharide allowed a firm, binding at I0.15. Quantitative binding studies with monomeric 125I-C1q showed a concentration-dependent, saturable, specific and reversible binding involving specific membrane receptors. Scatchard plots of C1q binding indicated [1.6 +/- 0.7 (1 S.D.)] X 10(6) sites per cell with an equilibrium constant of (2.9 +/- 1.8) X 10(7) M-1 at I0.07. The location of the molecule region mediating C1q binding was established with collagen-like fragments prepared by partial pepsin digestion, confirming earlier results obtained by inhibition studies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. D., Andrews J. A., Leslie R. G., Wood N. J. The binding of human and guinea-pig IgG subclasses to homologous macrophage and monocyte Fc receptors. Immunology. 1978 Jul;35(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Anderson C. L., Abraham G. N. Characterization of the Fc receptor for IgG on a human macrophage cell line, U937. J Immunol. 1980 Dec;125(6):2735–2741. [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fine J. M., Steinbuch M. A simple technique for the isolation of monoclonal IgG and IgA. Rev Eur Etud Clin Biol. 1970 Dec;15(10):1115–1121. [PubMed] [Google Scholar]
- Gabay Y., Perlmann H., Perlmann P., Sobel A. T. A rosette assay for the determination of C 1 q receptor-bearing cells. Eur J Immunol. 1979 Oct;9(10):797–801. doi: 10.1002/eji.1830091010. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Müller-Eberhard H. J. Lysis of C1Q-coated chicken erythrocytes by human lymphoblastoid cell lines. J Immunol. 1978 Jan;120(1):27–32. [PubMed] [Google Scholar]
- Guyre P. M., Crabtree G. R., Bodwell J. E., Munck A. MLC-conditioned media stimulate an increase in Fc receptors on human macrophages. J Immunol. 1981 Feb;126(2):666–668. [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Isliker H., Bing D. H., Lahan J., Hynes R. O. Fibronectin interacts with Clq, a subcomponent of the first component of complement. Immunol Lett. 1982 Jan;4(1):39–43. doi: 10.1016/0165-2478(82)90075-x. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Loos M., Müller W., Boltz-Nitulescu G., Förster O. Evidence that C1q, a subcomponent of the first component of complement, is an Fc receptor of peritoneal and alveolar macrophages. Immunobiology. 1980 Apr;157(1):54–61. doi: 10.1016/S0171-2985(80)80062-3. [DOI] [PubMed] [Google Scholar]
- Loos M., Storz R., Müller W., Lemmel E. M. Immunofluorescence studies on the subcomponents of the first component of complement (C1): detection of C1q and C1s in different cells of biopsy material and on human as well as on guinea pig peritoneal macrophages. Immunobiology. 1981;158(3):213–224. doi: 10.1016/S0171-2985(81)80071-X. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Reid K. B. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem J. 1976 Apr 1;155(1):5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Proteins involved in the activation and control of the two pathways of human complement. Biochem Soc Trans. 1983 Jan;11(1):1–12. doi: 10.1042/bst0110001. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Yonemasu K. Chemical studies on the isolated collagen-like and globular fragment of complement component C1q. Comparative studies on bovine and human C1q. Biochim Biophys Acta. 1983 Jan 12;742(1):122–128. doi: 10.1016/0167-4838(83)90367-9. [DOI] [PubMed] [Google Scholar]
- Shaala A. Y., Dhaliwal H. S., Bishop S., Ling N. R. Ingestion of dyed-opsonised yeasts as a simple way of detecting phagocytes in lymphocyte preparations. Cytophilic binding of immunoglobulins by ingesting cells. J Immunol Methods. 1979;27(2):175–187. doi: 10.1016/0022-1759(79)90263-1. [DOI] [PubMed] [Google Scholar]
- Siegel R. C., Schumaker V. N. Measurement of the association constants of the complexes formed between intact C1q or pepsin-treated C1q stalks and the unactivated or activated C1r2C1s2 tetramers. Mol Immunol. 1983 Jan;20(1):53–66. doi: 10.1016/0161-5890(83)90105-0. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Reboul A. Preparation and properties of human C1 inhibitor. Methods Enzymol. 1981;80(Pt 100):43–54. doi: 10.1016/s0076-6879(81)80007-9. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Analysis of receptor-mediated C1q binding to human peripheral blood mononuclear cells. J Immunol. 1980 Oct;125(4):1658–1664. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Identification of types of cells in human peripheral blood that bind C1q. J Immunol. 1981 Mar;126(3):1174–1179. [PubMed] [Google Scholar]
- Van Schravendijk M. R., Dwek R. A. Interaction of C1q with DNA. Mol Immunol. 1982 Sep;19(9):1179–1187. doi: 10.1016/0161-5890(82)90328-5. [DOI] [PubMed] [Google Scholar]
- Villiers C. L., Duplaa A. M., Arlaud G. J., Colomb M. G. Fluid phase activation of proenzymic C1r purified by affinity chromatography. Biochim Biophys Acta. 1982 Jan 4;700(1):118–126. doi: 10.1016/0167-4838(82)90299-0. [DOI] [PubMed] [Google Scholar]



