Abstract
Signal transduction enables cells to sense and respond to chemical and mechanical information in the extracellular environment. Recently, phase separation has emerged as a physical mechanism that can influence the spatial organization of signaling molecules and regulate downstream signaling. Although many molecular components of signaling pathways, including receptors, kinases, and transcription factors, have been observed to undergo phase separation, understanding the functional consequences of their phase separation in signal transduction remains an ongoing area of research. In this review, we will discuss recent studies investigating how cells potentially use phase separation to regulate different signaling pathways by initiating signaling, amplifying signaling, or inhibiting signaling. We will also discuss recent observations that suggest a role for phase separation in mechanosensing in the Hippo pathway and at focal adhesions.
Introduction
Signal transduction enables cells to sense and respond to information in the extracellular environment. Cells sense chemical information, such as secreted peptides, hormones, ions, and growth factors, as well as mechanical information, such as tissue stretch, shear force, surface topology, and substrate stiffness1. Recently, phase separation has emerged as a physical mechanism that can influence the spatial organization of signaling molecules and regulate downstream signaling2,3. Phase separation occurs when a homogenous mixture of molecules spontaneously de-mixes to form two or more distinct phases4. Many biological molecules, including proteins and nucleic acids, can undergo phase separation to form liquid-like droplets that concentrate specific collections of molecules3,4. These compartments, termed biomolecular condensates, can be found throughout the kingdoms of life and function to organize cells and potentially regulate diverse cellular processes3. Proteins that undergo phase separation often contain multivalent folded domains and/or intrinsically disordered regions (IDRs) that can mediate multivalent, intermolecular interactions2,3. Phase separation is thermodynamically driven, and whether a solution undergoes spontaneous phase separation depends on the concentration and identities of the macromolecules as well as environmental conditions such as temperature, salt concentration, and pH3,5.
Understanding the functional consequences of phase separation in signal transduction remains an ongoing area of research, and there are challenges to rigorously studying the impact of phase separation on signaling in vivo4,6. For example, phase separation is sensitive to concentration, so experiments caried out with endogenous protein levels are ideal. Recent reviews discuss some challenges and best practices for phase separation experiments in more detail4,6. In this review, we will discuss recent studies investigating how cells use phase separation to regulate signaling pathways (Figure 1). We will also discuss recent observations that suggest a role for phase separation in mechanosensing in the Hippo pathway and at focal adhesions.
Figure 1.
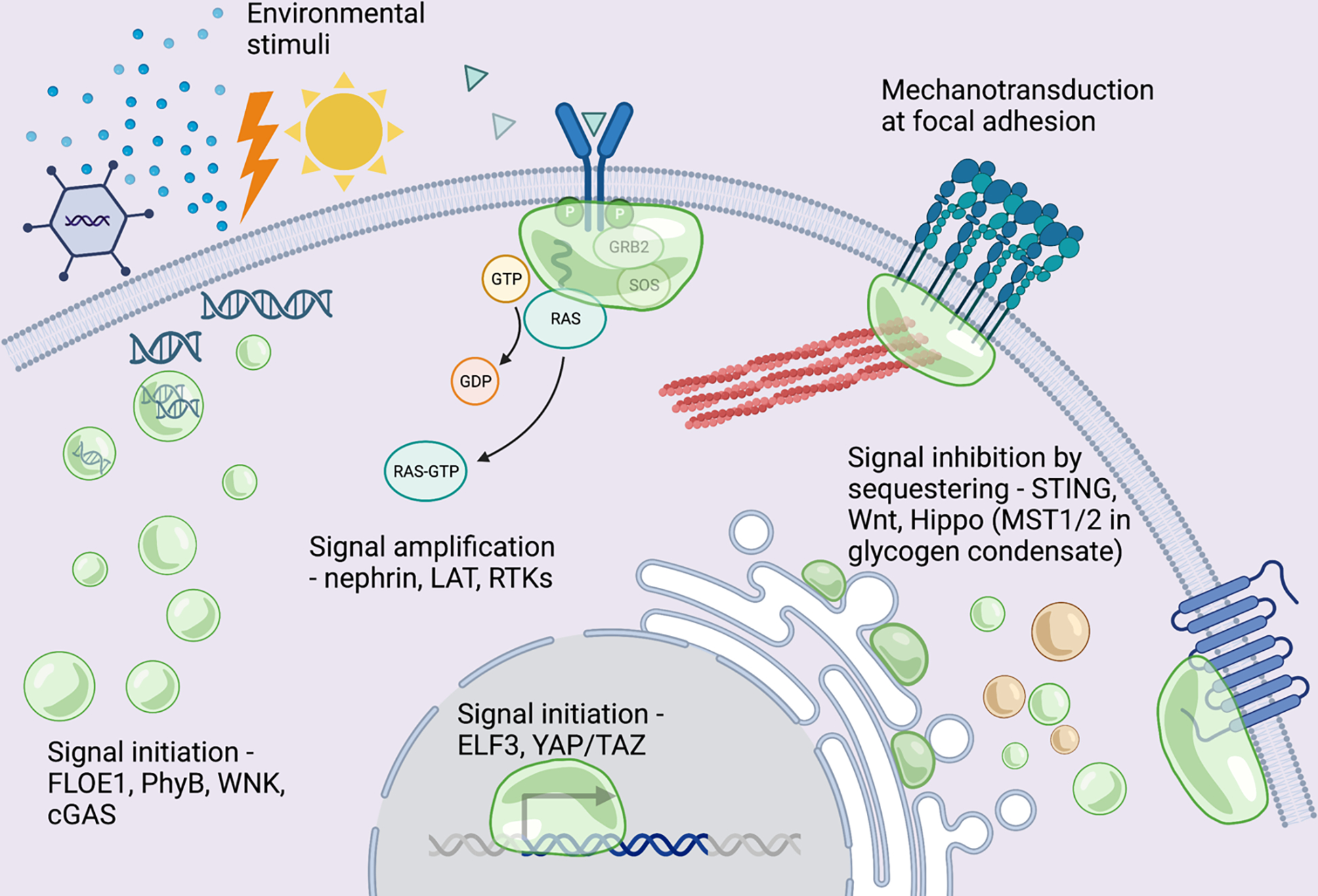
Potential roles for phase separation in chemical and mechanical signaling pathways. Created with BioRender.
Phase separation can initiate signaling.
In many cases, fluctuations in environmental conditions can be directly sensed by the phase separation of intracellular molecules7. Since phase separation is exquisitely sensitive to factors such as pH, temperature, and crowding6, the formation or dissolution of phase separated condensates can be a sensitive switch to initiate signaling in diverse physiological contexts.
Plant growth is sensitive to many environmental variables including hydration, temperature, and light, and plant cells use phase separation to initiate signaling and adapt to environmental fluctuations. A prion-like protein FLOE1 found in Arabidopsis thaliana embryos phase separates upon hydration, resulting in seed germination**8. Mutations that impair FLOE1 phase separation cause inappropriate seed germination in dehydration conditions. The evening complex protein ELF3, a component of the plant circadian clock, undergoes phase separation at high temperatures, leading to a decrease in its activity as a transcriptional repressor9. ELF3 phase separation is mediated by its prion-like domain and this domain is required for thermal responsive growth. Photoreceptor phytochrome B (PhyB), the major red/far-red light receptor in plants, undergoes phase separation to form condensates that selectively incorporate signaling components to activate signaling*10. Since formation of PhyB condensates is sensitive to both light and temperature, PhyB phase separation enables the integration of light and temperature signals.
Mammalian cells can also use phase separation to initiate signaling. Hypertonic stress causes a rapid decrease in cell volume, leading to an increase in intracellular crowding. In response, cells regulate ion transporters to drive net solute influx, leading to a reclamation of water and regulatory volume increase**11. This signaling pathway is initiated by with-no-lysine (WNK) kinases, which form cytoplasmic condensates within seconds of hypertonic stress. WNK kinase phase separation requires the C-terminal IDR and occurs in response to increased crowding, leading to an increase in WNK kinase activity to initiate the regulatory volume increase in response to hyperosmotic stress11.
In the presence of cytosolic double stranded DNA (dsDNA), cyclic GMP-AMP synthase (cGAS) produces the second messenger cGAMP to activate the ER membrane localized STING protein, culminating in the activation of the innate immune response12–14. cGAS-STING signaling is initiated by the presence of double stranded DNA in the cytoplasm. Binding to dsDNA triggers cGAS phase separation, forming condensates that promotes cGAS activation and downstream signaling12,13. Thus, phase separation of cGAS may be used to sense viral infection and initiate signaling.
Phase separation can amplify signaling.
In many signaling pathways, an upstream signal leads to the phosphorylation of the cytosolic domain of a transmembrane receptor on multiple tyrosine residues. These phosphorylated receptors can then interact with collections of cytosolic adaptor proteins containing SH2 domains, SH3 domains, and proline rich motifs (PRMs). Multivalency in these molecules can drive their phase separation, leading to the formation of liquid-like signaling condensates on the plasma membrane3. For example, the cell-cell adhesion receptor nephrin undergoes multivalent interactions with the adaptor protein Nck (which contains one SH2 and three SH3 domains) and the actin regulatory protein N-WASP (which contains multiple PRMs), leading to the formation of phase separated condensates15. Similarly, the linker for activation of T-cells (LAT) receptor undergoes multivalent interactions with the adaptor protein Grb2 (which contains one SH2 and two SH3 domains) and the Ras GEF SOS (which contains multiple PRMs)16. More recently, several receptor tyrosine kinases (RTKs), including EGFR, FGFR, and VEGFR, have been observed to undergo phase separation when combined with cytosolic proteins containing multiple SH2 domains17,18.
In these examples, phase separation requires phosphorylation on multiple tyrosine residues15,19 and is likely regulated by competition between kinase and phosphatase activities. Specific protein interactions and the emergent chemical properties of condensates have been observed to protect receptors from dephosphorylation. Binding of the SH2 domain of phospholipase PLCγ1 to LAT favors phase separation by protecting a specific tyrosine residue from dephosphorylation20. LAT condensates are enriched with negative charge and can exclude negatively charged phosphatases through electrostatic repulsion19. Phosphorylated FGFR forms liquid-like condensates in cells and in vitro that concentrate both the phosphatase SHP2 and PLCγ1. However, the phosphatase activity of SHP2 is significantly reduced by the formation of condensates in vitro. Additionally, FGFR condensates are protected from the activity of nonspecific phosphatases, such as CIP**18. Reducing phosphatase activity through these mechanisms may help to stabilize multivalent phosphotyrosine condensates and sustain downstream signaling.
For Nephrin, LAT, EGFR, and FGFR2, phase separation has been observed to upregulate downstream signaling. Phase separation of nephrin promotes downstream actin polymerization by increasing the membrane dwell time of N-WASP and Arp2/3 complex21. Phase separation of the LAT receptor and EGFR can promote downstream Ras activation by increasing the membrane dwell time of SOS16,17,19. Phase separation of FGFR2 increases FGFR2 kinase activity and the lipolytic activity of PLCγ118. When both kinases and their substrates are localized within engineered synthetic condensates, substrate phosphorylation significantly increases both in vitro and in yeast cells22. Additionally, localization to condensates increased phosphorylation of unfavorable substrates that lacked docking motifs or contained non-consensus phospho-acceptor sequences. These results suggest that concentration of kinases and substrates within condensates can promote phosphorylation of sub-optimal substrates and thus expand kinase specificity22. In these examples, phase separation of signaling molecules propagates and amplifies the initial signal. In cancer, aberrant phase separation may lead to activation or amplification of signaling pathways to promote tumor growth or metastasis23–28.
Phase separation can inhibit signaling.
In some pathways, phase separation has been observed to attenuate or inhibit signaling. During c-Gas-STING signaling, excessively produced cGAMP can trigger the formation of STING condensates on the ER that downregulate innate immunity by sequestering STING away from downstream signaling components*29. Thus, phase separation of STING dampens signaling, providing a mechanism to prevent overactivation of the innate immune response when pathogenic stimulus is too high. The Wnt/β-catenin signaling pathway regulates tissue homeostasis and cell fate decisions during animal development. In the absence of Wnt ligand, signaling is kept off through the formation of the destruction complex, which targets the transcription factor β-catenin for proteasomal degradation. The destruction complex is a condensate that contains the proteins Axin, APC, GSK3β and CK1α, and formation of the destruction complex requires Axin to undergo phase separation via its IDR. β-catenin is recruited to the destruction complex, where it is phosphorylated30. Phosphorylated β-catenin is then recognized by an E3 ligase and rapidly degraded by the proteosome. Ultimately, phase separation of Axin inhibits Wnt signaling by promoting β-catenin degradation in the cytoplasm. Upon Wnt ligand binding to the transmembrane receptor Frizzled, signaling is turned on by the formation of the signalosome at the plasma membrane. This requires recruitment of the cytosolic protein Dishevelled 2 (Dvl2) to the membrane. Additionally, Dvl2 has been observed to undergo phase separation, and mutations in the Dvl2 IDR that impair phase separation also reduce signalosome formation and Wnt signaling31. Axin is slowly recruited to the signalosome condensate at the plasma membrane, which disrupts the phase separation of the destruction complex, leading to an increase in cytosolic β-catenin concentrations31. The activation of Wnt signaling enables β-catenin to enter the nucleus and regulate transcription.
Phase separation can regulate mechanosensitive signaling.
In addition to sensing chemical signals, cells also sense and respond to mechanical signals in their environment. Recent studies suggest that phase separation may contribute to the regulation of certain cellular mechanosensitive signaling pathways. The Hippo pathway is a kinase cascade that negatively regulates YAP/TAZ, a homologous pair of transcriptional coactivators that promote cell proliferation, survival, and maintenance of stem cell fate. The Hippo pathway enables cells to sense and respond to diverse mechanical stimuli, including cell density, cell area, tissue stretch, shear forces, and substrate stiffness32,33. Activation of the Hippo pathway leads to phosphorylation and activation of MST1/2 kinases, which then phosphorylate and activate LATS1/2 kinases, which then phosphorylate YAP/TAZ. Phosphorylated YAP/TAZ is inactive and sequestered in the cytoplasm. Recently, many components of the Hippo pathway have been observed to undergo phase separation34,35. For example, the positive upstream regulators AMOT and KIBRA form condensates that activate Hippo signaling, while the negative upstream regulator SLMAP forms condensates that inhibit Hippo signaling by recruiting MST and its phosphatase. However, these compositionally distinct condensates can coalesce to activate signaling by enriching the kinase cascade and excluding the phosphatase**34. In cancer, several non-protein molecules can dysregulate Hippo pathway phase separation and signaling. Excess glycogen in tumors can undergo phase separation, forming condensates that sequester and inhibit MST1/2**24. The tumor promoting long non-coding (lnc) RNA SNHG9 can bind to LATS1, which promotes LATS1 phase separation and reduces YAP phosphorylation*36. YAP/TAZ can also form liquid-like condensates in the nucleus in direct response to osmotic shock-induced crowding37. Together, these recent studies suggest that phase separation may provide a mechanism for cells to sense and integrate numerous signals that converge on Hippo signaling and YAP/TAZ regulated transcription.
Integrin-dependent signaling is another important mechanosensitive pathway in animal cells. Integrins are heterodimeric receptors that mediate adhesion to the extracellular matrix. Integrins cluster and assemble with numerous cytosolic adaptor proteins, signaling molecules, and actin regulatory proteins to form multiprotein adhesion complexes38. Mechanical forces are transmitted across integrin receptors, and integrin adhesion complexes play a central role in integrating biochemical and mechanical information within cells39,40. Several recent studies provide evidence that phase separation may contribute to the formation, maturation, and turnover of integrin adhesion complexes.
Integrins initially cluster with a subset of cytosolic proteins to form small, diffraction-limited puncta termed nascent adhesions. Several of the cytosolic proteins that localize within nascent adhesions, including phosphorylated p130Cas and focal adhesion kinase (FAK) undergo phase separation at physiological concentrations in vitro**41. Moreover, the p130Cas- and FAK-dependent pathways act synergistically to promote phase separation, integrin clustering, nascent adhesion formation and partitioning of key components in vitro and in cells. Thus, phase separation may provide an intracellular trigger for integrin clustering and nascent adhesion formation. After initial formation, a subset of nascent adhesions are stabilized and undergo a process of force-dependent growth and compositional maturation to form mature focal adhesions that attach to actin stress fibers38,42. The adaptor protein LIMD1 is recruited to maturing focal adhesions in a force-dependent manner, likely by a direct interaction with the protein vinculin. Additionally, LIMD1 also undergoes phase separation both in vitro and in cells to form droplets that enrich additional adaptor proteins such as Zyxin**43. Mutations that disrupt LIMD1 phase separation, such as phosphomimetic point mutations in the IDR, lead to impaired FA dynamics, reduced force transduction, and impaired mechanosensative cell migration. Thus, the force-dependent recruitment of LIMD1 to focal adhesions could trigger its subsequent phase separation to enable the enrichment of specific adaptor proteins, promote focal adhesion maturation, and regulate cellular mechanotransduction. The turnover and disassembly of focal adhesions is regulated by phosphorylation, protease activity, and endocytosis40. Recently, the focal adhesion protein tensin was observed to undergo phase separation as focal adhesions disassemble44. However, how tensin phase separation is regulated and the functional consequences of tensin condensates remain unclear.
Perspectives
Progress in understanding biological phase separation has been rapid, but many open questions remain. Although we have cited many examples of signaling molecules that can undergo phase separation, in some cases the functional consequences of this phase separation remain unclear, and more rigorous studies would help provide deeper insight into whether phase separation specifically impacts signaling6. One exciting approach to assess the functional relevance of phase separation is to explore the selection of phase separation in evolution8,9. While the Arabidopsis protein ELF3 undergoes temperature sensitive phase separation to regulate growth, plants from hotter climates contain an ELF3 protein that does not exhibit temperature sensitive phase separation and these plants lack thermal responsive growth9. Another question is the extent to which the emergent chemical and material properties of condensates have been selected for in evolution6,8,45,46. The material properties of condensates could be particularly important in mechanosensitive signaling pathways where mechanical forces are potentially sensed or transmitted at condensates. At membranes, lipids often play an important role in regulating molecular organization and activity. Recent studies have shown that membrane surfaces can promote protein and RNA driven phase separation47 and that lipid phase separation can be coupled to multivalent protein driven phase separation**48,49. Lipid membranes are likely to regulate protein phase separation in diverse signaling pathways, although the feedback between lipid membranes and protein condensates remains under studied50. In conclusion, growing evidence suggests that diverse signaling molecules undergo phase separation, and future studies should focus on investigating the functional consequences of phase separation in signal transduction.
Acknowledgements
This work was supported by the Air Force Office of Scientific Research (award FA9550–22-1–0207) and the National Institutes of Health (NIGMS Award 1DP2GM149549).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Bibliography
- 1.Ullo MF, Case LB. How cells sense and integrate information from different sources. WIREs Mechanisms of Disease. 2023. [DOI] [PubMed] [Google Scholar]
- 2.Mayer BJ, Yu J. Protein Clusters in Phosphotyrosine Signal Transduction. Journal of Molecular Biology. 2018;430(22):4547–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Case LB, Ditlev JA, Rosen MK. Regulation of Transmembrane Signaling by Phase Separation. Annual Review of Biophysics. 2019;48(1):465–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357(6357):eaaf4382. [DOI] [PubMed] [Google Scholar]
- 5.Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nature Reviews Molecular Cell Biology. 2017;18(5):285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti S, Gladfelter A, Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell. 2019;176(3):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, Drummond DA. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell. 2017;168(6):1028–1040.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.**. Dorone Y, Boeynaems S, Flores E, Jin B, Hateley S, Bossi F, Lazarus E, Pennington JG, Michiels E, De Decker M, Vints K, Baatsen P, Bassel GW, Otegui MS, Holehouse AS, et al. A prion-like protein regulator of seed germination undergoes hydration-dependent phase separation. Cell. 2021;184(16):4284–4298.e27. The authors found that FLOE1 regulates seed germination in response to environmental water status by undergoing hydration-dependent phase separation. By inhibiting germination in unfavorable environments, FLOE1 promotes overall survival of the plant. Additionally, this study links FLOE1 condensate material properties to plant development.
- 9.Jung J-H, Barbosa AD, Hutin S, Kumita JR, Gao M, Derwort D, Silva CS, Lai X, Pierre E, Geng F, Kim S-B, Baek S, Zubieta C, Jaeger KE, Wigge PA. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature. 2020;585(7824):256–260. [DOI] [PubMed] [Google Scholar]
- 10.*. Chen D, Lyu M, Kou X, Li J, Yang Z, Gao L, Li Y, Fan L, Shi H, Zhong S. Integration of light and temperature sensing by liquid-liquid phase separation of phytochrome B. Molecular Cell. 2022;82(16):3015–3029.e6. The authors found that the major red/far-red light receptor Photoreceptor phytochrome B (PhyB) undergoes spontaneous phase separation, forming droplets that selectively incorporate signaling components. PhyB senses both light and temperature, and its intrinsically disordered N terminal extension (NTE) modulates both phase separation and temperature sensing.
- 11.**. Boyd-Shiwarski CR, Shiwarski DJ, Griffiths SE, Beacham RT, Norrell L, Morrison DE, Wang J, Mann J, Tennant W, Anderson EN, Franks J, Calderon M, Connolly KA, Cheema MU, Weaver CJ, et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell. 2022:S0092867422012612. Phase separation provided a robust mechanism for cells to rapidly sense and coordinate responses to osmotic stress. The activation of the signaling pathway for mammalian cells to combat hypertonic stress starts with with-no-lysine (WNK) kinase, which forms cytoplasmic condensates within seconds of hypertonic stress. The formation of WNK condensates promotes WNK kinase activity, leading to phosphorylation of ion channels to restore cell volume via net solute influx.
- 12.Su Z, Dhusia K, Wu Y. Coarse-grained simulations of phase separation driven by DNA and its sensor protein cGAS. Archives of Biochemistry and Biophysics. 2021;710:109001. [DOI] [PubMed] [Google Scholar]
- 13.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361(6403):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F, Yu Z, Zhang D, Chen S, Guan H, Zhou R, Wu Q, Zhang Q, Liu S, Venkat Ramani MK, Yang B, Ba X-Q, Zhang J, Huang J, Bai X, et al. Induced phase separation of mutant NF2 imprisons the cGAS-STING machinery to abrogate antitumor immunity. Molecular Cell. 2021;81(20):4147–4164.e7. [DOI] [PubMed] [Google Scholar]
- 15.Banjade S, Rosen MK. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife. 2014;3:e04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, Groves JT. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science. 2019;363(6431):1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C-W, Nocka LM, Stinger BL, DeGrandchamp JB, Lew LJN, Alvarez S, Phan HT, Kondo Y, Kuriyan J, Groves JT. A two-component protein condensate of the EGFR cytoplasmic tail and Grb2 regulates Ras activation by SOS at the membrane. Proceedings of the National Academy of Sciences. 2022;119(19):e2122531119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.**. Lin C-C, Suen KM, Jeffrey P-A, Wieteska L, Lidster JA, Bao P, Curd AP, Stainthorp A, Seiler C, Koss H, Miska E, Ahmed Z, Evans SD, Molina-París C, Ladbury JE. Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Molecular Cell. 2022;82(6):1089–1106.e12. The authors found that eight different RTKs from the EGFR, FGFR, and VEGFR families undergo phase separation when combined with downstream effector proteins. Condensate formation amplified RTK signaling, increased FGFR2 kinase activity as well as the lipolytic activity of PLCγ1. The formation of FGFR2 condensates also provides a potential mechanism for retaining PLCγ1 at the plasma membrane where it can access its substrate PI(4,5)P2.
- 19.Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, Vale RD. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 2016;352(6285):595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng L, Palaia I, Šarić A, Su X. PLCγ1 promotes phase separation of T cell signaling components. Journal of Cell Biology. 2021;220(6):e202009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case LB, Zhang X, Ditlev JA, Rosen MK. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science. 2019;363(6431):1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang D, Shu T, Pantoja CF, Ibáñez de Opakua A, Zweckstetter M, Holt LJ. Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding. Molecular Cell. 2022;82(19):3693–3711.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulpule A, Guan J, Neel DS, Allegakoen HR, Lin YP, Brown D, Chou Y-T, Heslin A, Chatterjee N, Perati S, Menon S, Nguyen TA, Debnath J, Ramirez AD, Shi X, et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell. 2021;184(10):2649–2664.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.**. Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C, Li J, Su D, Chen L, Zhao Q, Shao H, Zhao H, Chen Q, Li Y, Geng J, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. 2021;184(22):5559–5576.e19. The authors showed that glycogen phase separates to form liquid-like condensates. Partitioning of the Hippo-pathway component MST1/2 kinases into glycogen condensates sequester them away from their substrates. This leads to YAP/TAZ nuclear translocation and subsequent transcriptional activation that drives liver tumor initiation. This study showed that phase separation of accumulated glycogen, a polysaccharide, can regulate a signaling pathway involved in tumorigenesis.
- 25.Mehta S, Zhang J. Liquid–liquid phase separation drives cellular function and dysfunction in cancer. Nature Reviews Cancer. 2022;22(4):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Ye Y, Zhu L, Xiao X, Zhou B, Gu Y, Si H, Liang H, Liu M, Li J, Jiang Q, Li J, Yu S, Ma R, Su S, et al. Niche stiffness sustains cancer stemness via TAZ and NANOG phase separation. Nature Communications. 2023;14(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y, Luo H, Yee PP, Zhang L, Liu Z, Zheng H, Zhang L, Anderson B, Tang M, Huang S, Li W. Paraspeckle Protein NONO Promotes TAZ Phase Separation in the Nucleus to Drive the Oncogenic Transcriptional Program. Advanced Science. 2021;8(24):2102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, Lin J, Dong T, Wang L, Li S, Yang Y, Xu S, Guo W, Zhang X, Shi M, et al. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Molecular Cell. 2021;81(6):1216–1230.e9. [DOI] [PubMed] [Google Scholar]
- 29.*. Yu X, Zhang L, Shen J, Zhai Y, Jiang Q, Yi M, Deng X, Ruan Z, Fang R, Chen Z, Ning X, Jiang Z. The STING phase-separator suppresses innate immune signalling. Nature Cell Biology. 2021;23(4):330–340. The authors found that when pathogenic stimulus is too high in virus infected cells, the excessively produced cGAMP triggers formation of STING condensates on the endoplasmic reticulum. These STING condensates down-regulate innate immunity by sequestering STING from downstream signaling component IRF3. Because STING is potently activated by cGAMP, phase separation spatially blocked signaling, providing a potential mechanism to prevent overactivation of innate immunity.
- 30.Nong J, Kang K, Shi Q, Zhu X, Tao Q, Chen Y-G. Phase separation of Axin organizes the β-catenin destruction complex. Journal of Cell Biology. 2021;220(4):e202012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang K, Shi Q, Wang X, Chen Y-G. Dishevelled phase separation promotes Wnt signalosome assembly and destruction complex disassembly. Journal of Cell Biology. 2022;221(12):e202205069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasgupta I, McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. Journal of Biological Chemistry. 2019;294(46):17693–17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon H, Kim J, Jho E. Role of the Hippo pathway and mechanisms for controlling cellular localization of YAP/TAZ. The FEBS Journal. 2022;289(19):5798–5818. [DOI] [PubMed] [Google Scholar]
- 34.**. Wang L, Choi K, Su T, Li B, Wu X, Zhang R, Driskill JH, Li H, Lei H, Guo P, Chen EH, Zheng Y, Pan D. Multiphase coalescence mediates Hippo pathway activation. Cell. 2022;185(23):4376–4393.e18. Authors showed that multicomponent condensate formation can control several upstream regulators of the Hippo pathway. In response to cell-cell contact or osmotic stress, the positive upstream regulators AMOT and KIBRA form Hippo-activating condensates, while the negative upstream regulator SLMAP forms Hippo inactivating condensates. However, SLMAP condensates can coalesce into a common phase with AMOT/KIBRA condensates to form a Hippo-activating condensates that excludes the phosphatase SIKE. These findings provide a paradigm for restricting the activity of biomolecular condensates without condensate dissolution. Instead, activity is regulated by the emergent properties of the condensate that selectively enrich or exclude certain components.
- 35.Bonello TT, Cai D, Fletcher GC, Wiengartner K, Pengilly V, Lange KS, Liu Z, Lippincott-Schwartz J, Kavran JM, Thompson BJ. Phase separation of Hippo signalling complexes. The EMBO Journal. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*. Li R-H, Tian T, Ge Q-W, He X-Y, Shi C-Y, Li J-H, Zhang Z, Liu F-Z, Sang L-J, Yang Z-Z, Liu Y-Z, Xiong Y, Yan Q, Li X, Ju H-Q, et al. A phosphatidic acid-binding lncRNA SNHG9 facilitates LATS1 liquid–liquid phase separation to promote oncogenic YAP signaling. Cell Research. 2021;31(10):1088–1105. Authors show that LATS1, core component of Hippo signaling kinase cascade, forms condensates. Phosphatidic acids (PA) in the plasma membrane and the lipid-associated long noncoding RNA (lncRNA) SNHG9 enhances LATS1 condensate formation. Activity of LATS1 is inhibited when in condensates, thus lnRNA induced phase separation provides a mechanism for inhibiting the Hippo pathway.
- 37.Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, Lippincott-Schwartz J. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nature Cell Biology. 2019;21(12):1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nature Cell Biology. 2015;17(8):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chastney MR, Conway JRW, Ivaska J. Integrin adhesion complexes. Current Biology. 2021;31(10):R536–R542. [DOI] [PubMed] [Google Scholar]
- 40.Kanchanawong P, Calderwood DA. Organization, dynamics and mechanoregulation of integrin-mediated cell–ECM adhesions. Nature Reviews Molecular Cell Biology. 2023;24(2):142–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.**. Case LB, De Pasquale M, Henry L, Rosen MK. Synergistic phase separation of two pathways promotes integrin clustering and nascent adhesion formation. eLife. 2022;11:e72588. The authors screened numerous focal adhesion proteins and found that phosphorylated pCas130 and focal adhesion kinase (FAK) synergistically promotes phase separation of a group of proteins representing the nascent adhesion components. Using in vitro reconstitution, authors showed that these condensates made of FAK, paxillin, p130Cas, Nck, NWASP, and kindlin can cluster β integrin cytoplasmic tail. This study showed that phase separation driven by these FA proteins provide a potential mechanism for integrin clustering during nascent adhesion formation in cells.
- 42.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature Reviews Molecular Cell Biology. 2010;11(9):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.**. Wang Y, Zhang C, Yang W, Shao S, Xu X, Sun Y, Li P, Liang L, Wu C. LIMD1 phase separation contributes to cellular mechanics and durotaxis by regulating focal adhesion dynamics in response to force. Developmental Cell. 2021;56(9):1313–1325.e7. The authors found that the protein LIMD1 can form condensates both in vitro and in the cytoplasm. The N-terminal IDR promotes phase separation, and phosphorylation of the IDR inhibits phase separation. This study correlated LIMD1 phase separation with cell traction force, focal adhesion longevity, and durotaxis.
- 44.Lee Y-RJ, Yamada S, Lo SH. Phase transition of tensin-1 during the focal adhesion disassembly and cell division. The Proceedings of the National Academy of Sciences. 2022;120(15):e2303037120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Chen D, Guan D, Liang X, Xue J, Zhao H, Song G, Lou J, He Y, Zhang H. Material properties of phase-separated TFEB condensates regulate the autophagy-lysosome pathway. Journal of Cell Biology. 2022;221(5):e202112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Najafi S, Lin Y, Longhini AP, Zhang X, Delaney KT, Kosik KS, Fredrickson GH, Shea J, Han S. Liquid–liquid phase separation of Tau by self and complex coacervation. Protein Science. 2021;30(7):1393–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snead WT, Jalihal AP, Gerbich TM, Seim I, Hu Z, Gladfelter AS. Membrane surfaces regulate assembly of ribonucleoprotein condensates. Nature Cell Biology. 2022;24(4):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.**. Wang H-Y, Chan SH, Dey S, Castello-Serrano I, Rosen MK, Ditlev JA, Levental KR, Levental I. Coupling of protein condensates to ordered lipid domains determines functional membrane organization. SCIENCE ADVANCES. 2023. This study showed that the coupling between lipid- and protein- based phase separation is important for T cell signaling. The authors showed that LAT/Grb2/Sos1 protein condensates are coupled to ordered lipid domains, and the protein condensates and lipid domains reciprocally stabilize each other in cells. Mutations in LAT transmembrane domain that dissociate it from ordered membrane domains and mutations that disrupt protein driven phase separation both abrogate T cell activation.
- 49.Chung JK, Huang WYC, Carbone CB, Nocka LM, Parikh AN, Vale RD, Groves JT. Coupled membrane lipid miscibility and phosphotyrosine-driven protein condensation phase transitions. Biophysical Journal. 2021;120(7):1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Case LB. Membranes regulate biomolecular condensates. Nature Cell Biology. 2022;24(4):404–405. [DOI] [PubMed] [Google Scholar]


