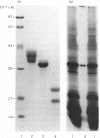
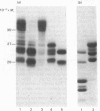
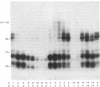
Abstract
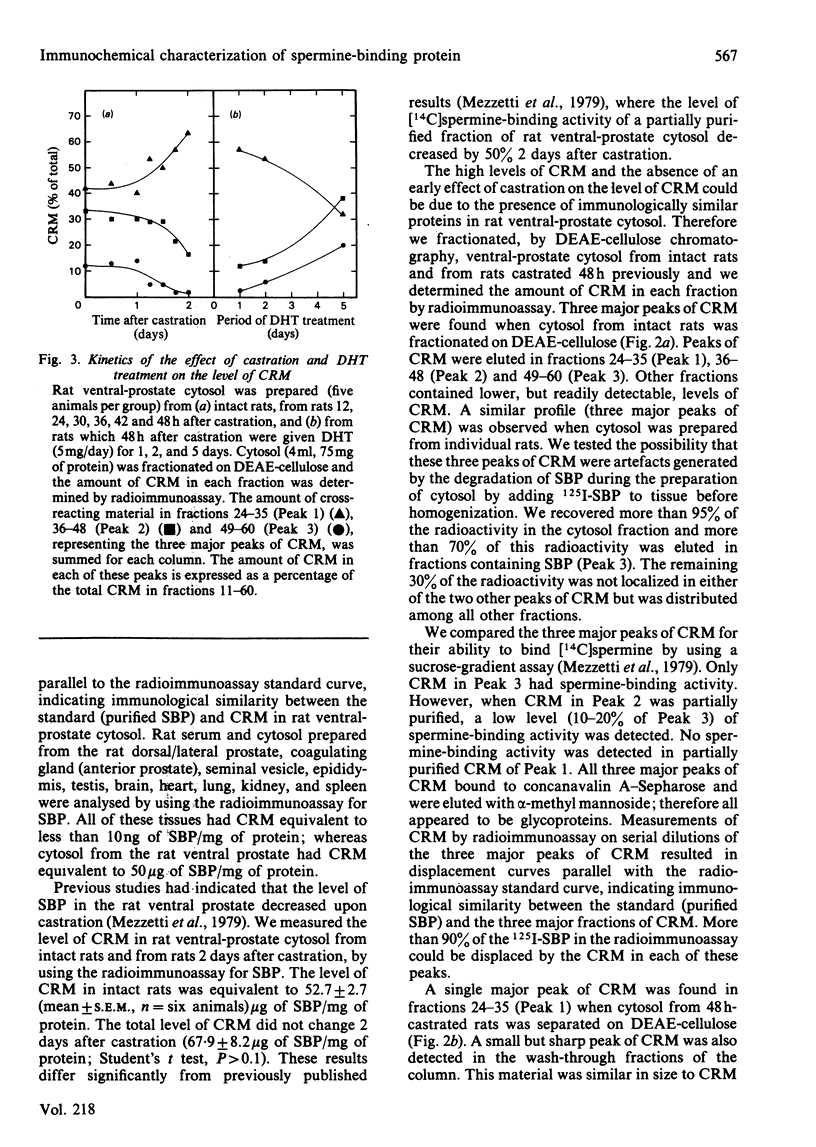
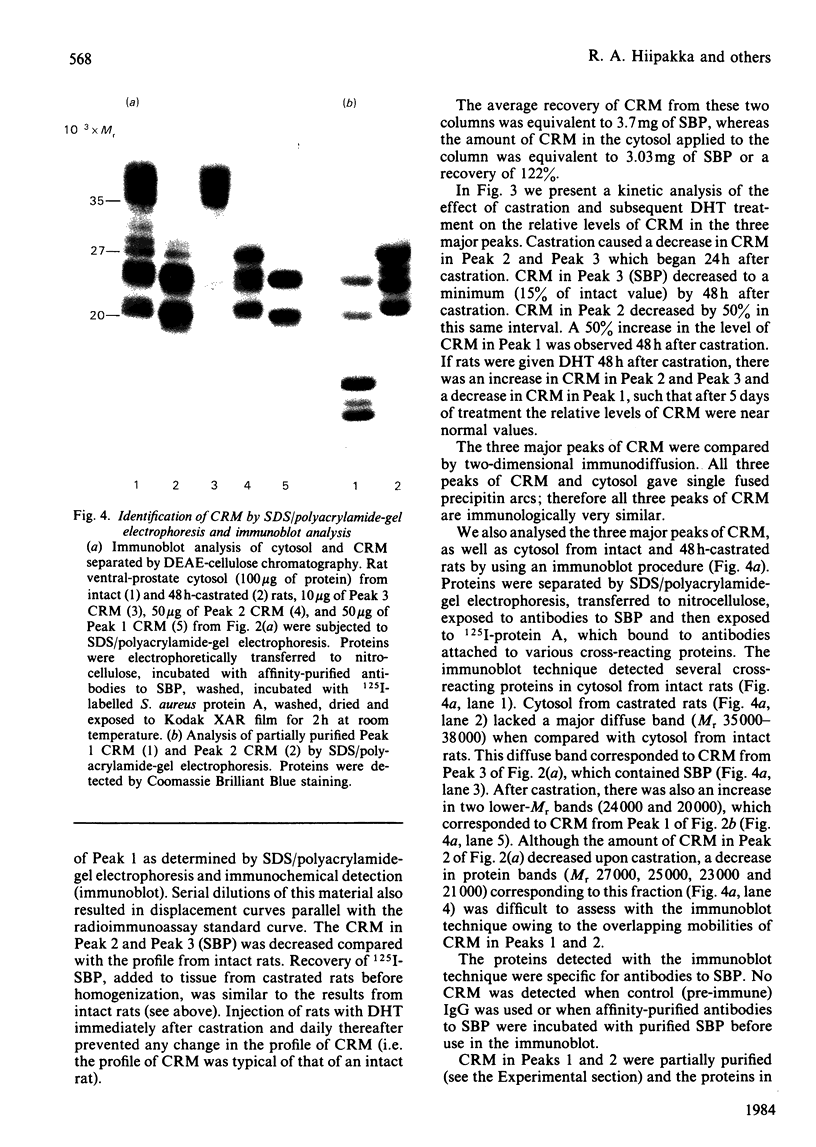
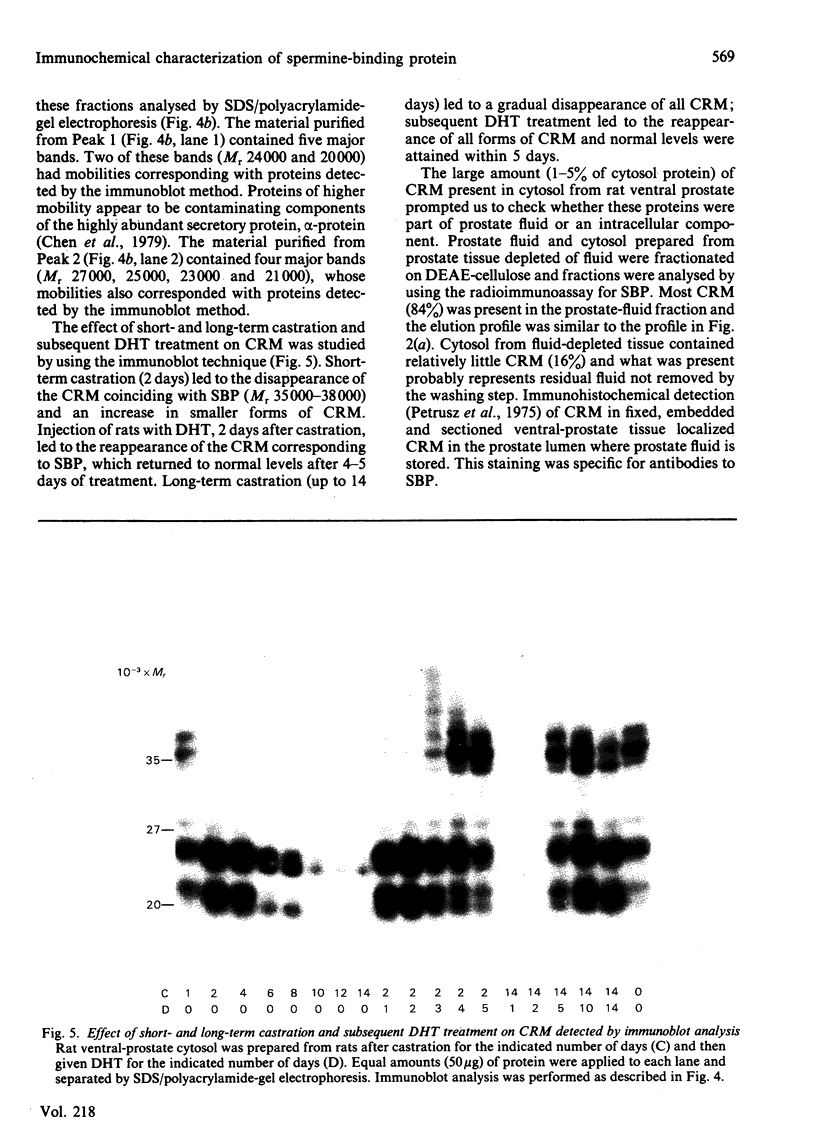
A solid-phase radioimmunoassay was developed to measure the level of the androgen-dependent spermine-binding protein (SBP) in the cytosol fraction of the rat ventral prostate during endocrine manipulation. The concentration of SBP and immunologically cross-reacting material (CRM) in the ventral prostate was at least 5000 times higher than the level of CRM detected in rat serum or cytosol from other rat tissues. Cytosol from the ventral prostate of intact rats was separated by DEAE-cellulose chromatography into three major fractions of CRM. One of these fractions corresponded to the elution position of SBP. Cytosol prepared from rats 48 h after castration lacked SBP and one of the two other fractions of CRM. This loss coincided with an increase in CRM in the remaining fraction. No significant difference was detected in the total level of CRM when intact and 48 h-castrated rats were compared. Injection of rats with 5 alpha-dihydrotestosterone (DHT) immediately after castration prevented these changes in the profile of CRM. Several proteins cross-reacting with antibodies to purified SBP were detected in cytosol by using an immunoblot procedure. The highest-Mr band corresponded to SBP. The effect of short- and long-term castration and subsequent DHT treatment on CRM was studied by using the immunoblot technique. Short-term castration (2 days) led to the disappearance of CRM coinciding with SBP (Mr 35 000-38 000) and an increase in smaller forms of CRM (Mr 24 000 and 22 000). Injection of rats with DHT 2 days after castration led to the reappearance of CRM corresponding to SBP, which returned to normal levels within 4 to 5 days of treatment. Long-term castration (up to 14 days) led to a gradual disappearance of all CRM; subsequent DHT treatment led to the reappearance of all forms of CRM and normal levels were attained within 5 days. We have identified SBP and the various forms of CRM as a secretory product of the rat ventral prostate by immunohistochemical staining and by DEAE-cellulose fractionation of prostatic fluid. Prostatic fluid is rich in proteolytic activity and these proteinases may be responsible for processing SBP to small forms of CRM.
Full text
PDF


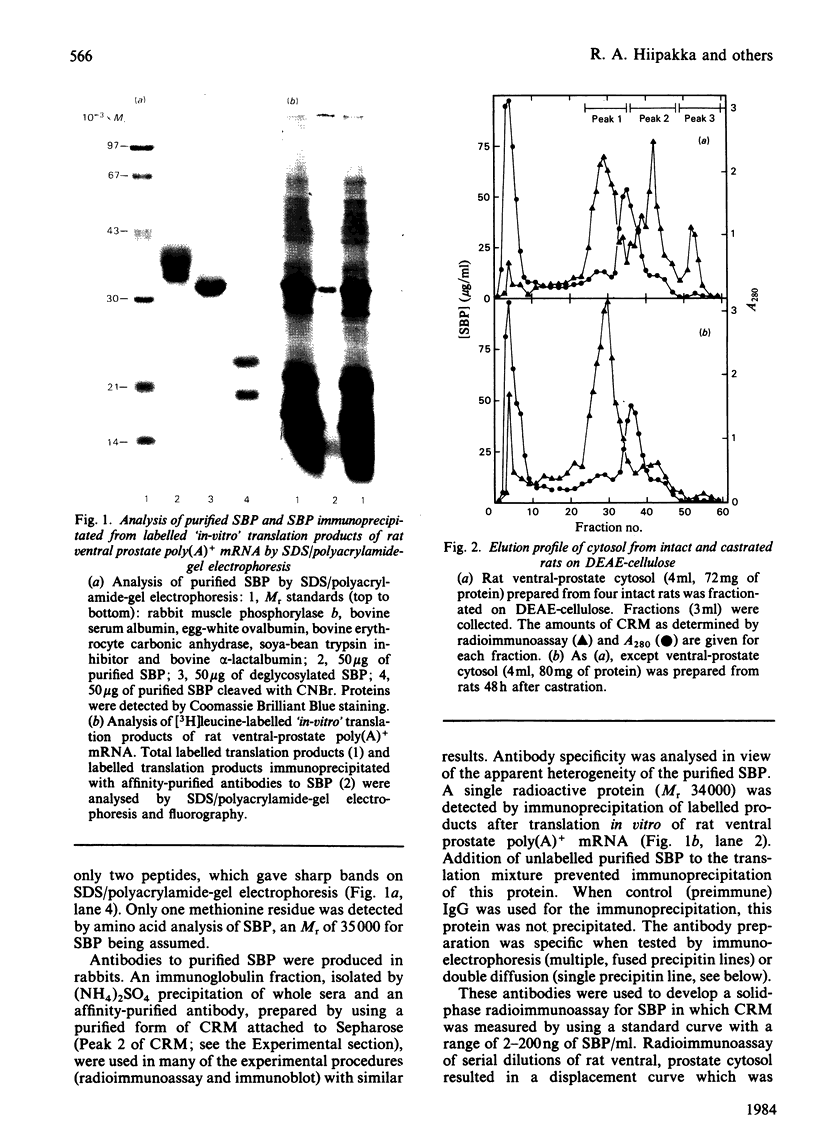


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Wilson M. J., Goueli S. A., Williams-Ashman H. G. Effects of polyamines on prostatic chromatin- and non-histone-protein-associated protein kinase reactions. Biochem J. 1978 Dec 15;176(3):739–750. doi: 10.1042/bj1760739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980 Aug;28(8):836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chamberlin L. L., Mpanias O. D., Wang T. Y. Isolation, properties, and androgen regulation of a 20-kilodalton protein from rat ventral prostate. Biochemistry. 1983 Jun 21;22(13):3072–3077. doi: 10.1021/bi00282a008. [DOI] [PubMed] [Google Scholar]
- Chen C., Hiipakka R. A., Liao S. Prostate alpha-protein: subunit structure, polyamine binding, and inhibition of nuclear chromatin binding of androgen-receptor complex. J Steroid Biochem. 1979 Jul;11(1B):401–405. doi: 10.1016/0022-4731(79)90058-x. [DOI] [PubMed] [Google Scholar]
- Chen C., Schilling K., Hiipakka R. A., Huang I. Y., Liao S. Prostate alpha-protein. Isolation and characterization of the polypeptide components and cholesterol binding. J Biol Chem. 1982 Jan 10;257(1):116–121. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Heyns W., Van Damme B., De Moor P. Secretion of prostatic binding protein by rat ventral prostate: influence of age and androgen. Endocrinology. 1978 Oct;103(4):1090–1095. doi: 10.1210/endo-103-4-1090. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981 Dec 25;256(24):12836–12839. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang T., Mezzetti G., Chen C., Liao S. Selective polyamine-binding proteins. Spermine binding by an androgen-sensitive phosphoprotein. Biochim Biophys Acta. 1978 Sep 6;542(3):430–441. doi: 10.1016/0304-4165(78)90374-4. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Mariotti S., Oger J. J., Fragu P., Antel J. P., Kuo H. H., DeGroot L. J. A new solid-phase radioimmunoassay to measure IgG secreted by cultured human lymphocytes. J Immunol Methods. 1980;35(3-4):189–199. doi: 10.1016/0022-1759(80)90246-x. [DOI] [PubMed] [Google Scholar]
- Mezzetti G., Loor R., Liao S. Androgen-sensitive spermine-binding protein of rat ventral prostate. Purification of the protein and characterization of the hormonal effect. Biochem J. 1979 Nov 15;184(2):431–440. doi: 10.1042/bj1840431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler M. K., Kistler W. S. Purification and cell-free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem. 1979 Jan 25;254(2):383–390. [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Qi D. F., Schatzman R. C., Mazzei G. J., Turner R. S., Raynor R. L., Liao S., Kuo J. F. Polyamines inhibit phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1983 Aug 1;213(2):281–288. doi: 10.1042/bj2130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Ewing L. L., Williams-Ashman H. G. Protein phospholinase reactions in mammalian testis. Stimulatory effects of adenosine 3':5'-cyclic monophosphate on the phosphorylation of basic proteins. Biochem J. 1971 Apr;122(3):333–345. doi: 10.1042/bj1220333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Breckenridge W. C., Vincendon G. Analysis of monosaccharides by gas-liquid chromatography of the O-methyl glycosides as trifluoroacetate derivatives. Application to glycoproteins and glycolipids. J Chromatogr. 1972 Jul 5;69(2):291–304. doi: 10.1016/s0021-9673(00)92897-8. [DOI] [PubMed] [Google Scholar]