Abstract
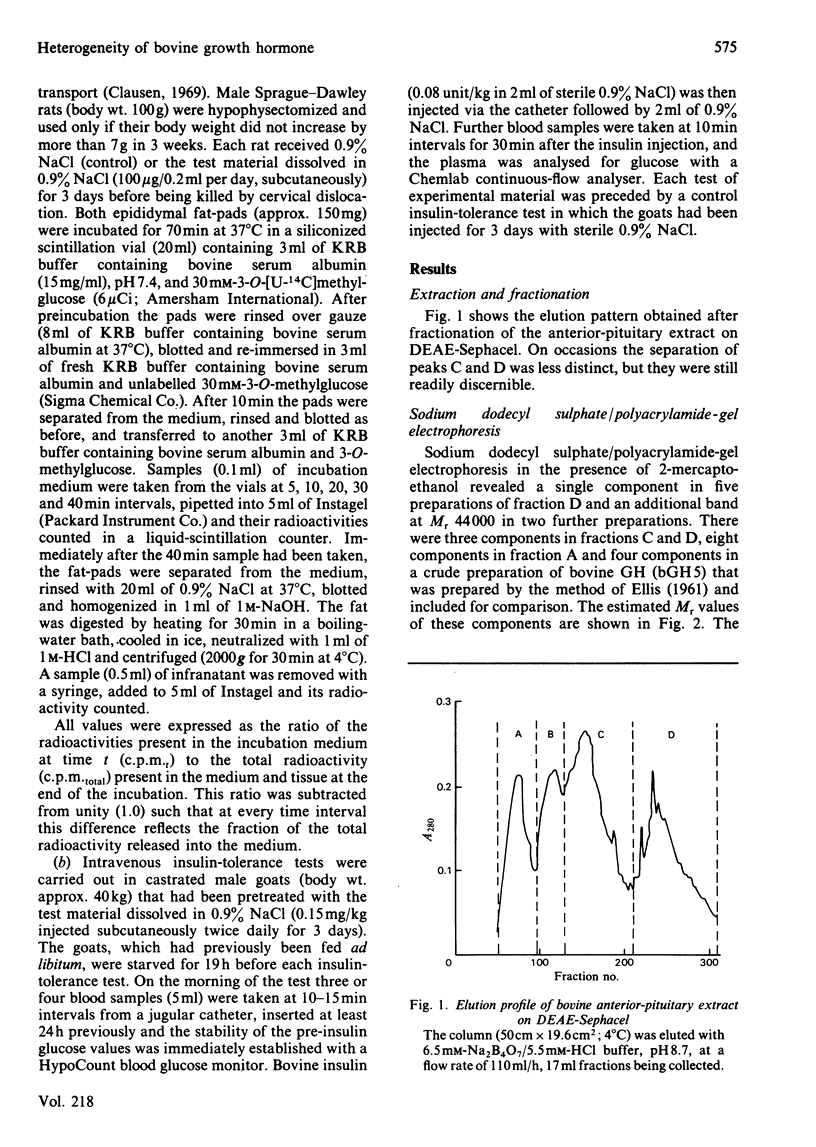
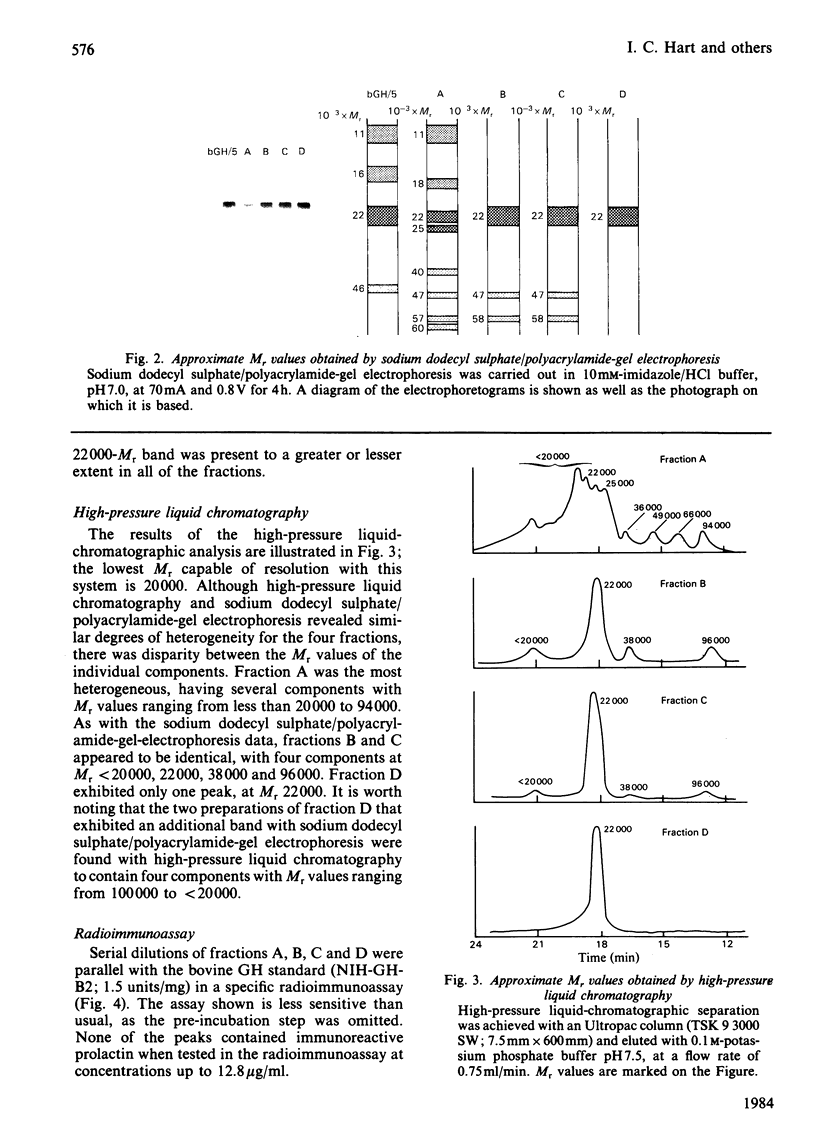
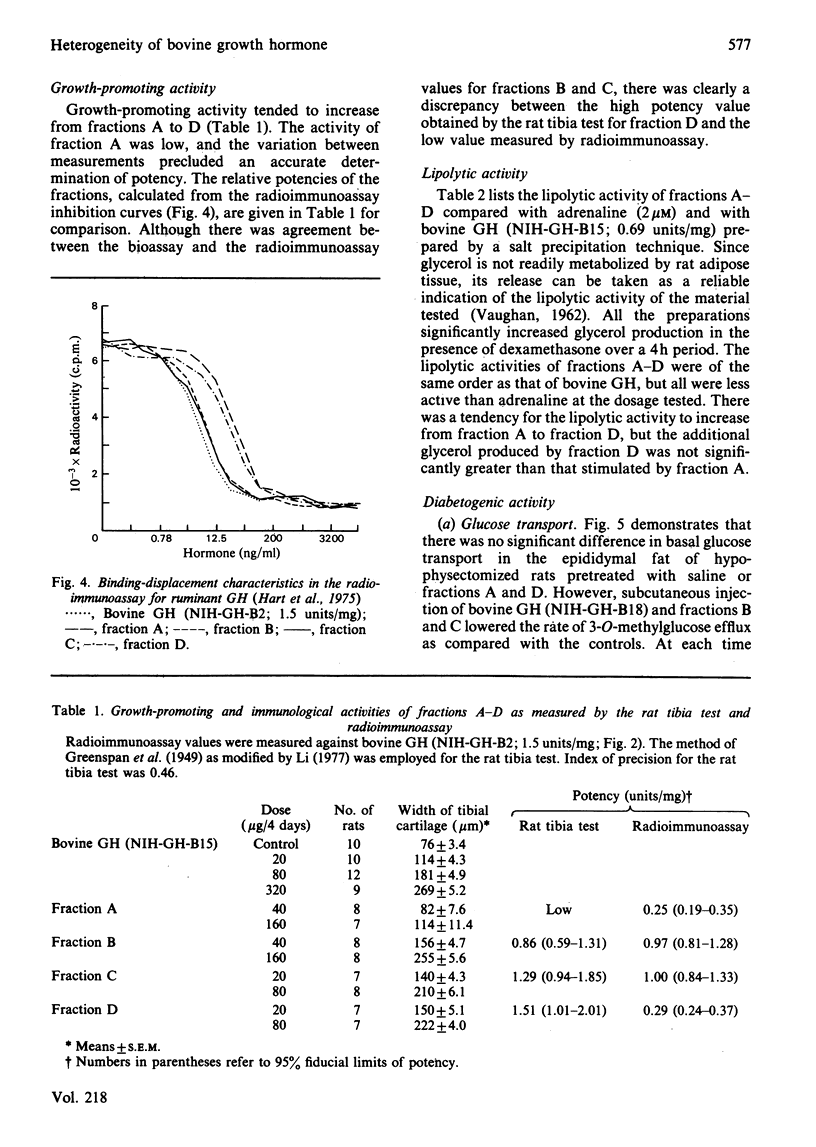
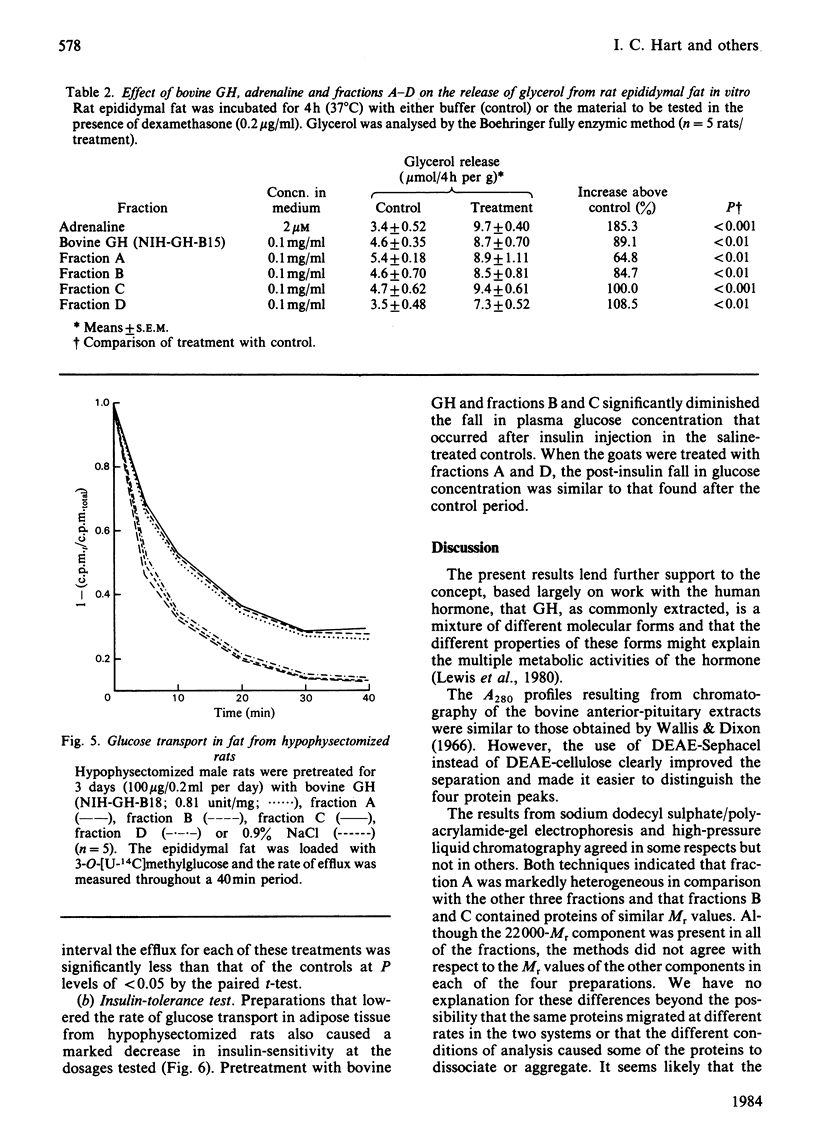
Bovine growth hormone (somatotropin) was extracted from anterior pituitaries and fractionated into four protein peaks (A-D) by chromatography on DEAE-Sephacel. Analysis by high-pressure liquid chromatography and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis indicated that the homogeneity of the material increased from fraction A through to D. The properties of the fractions were examined in the following manner: immunological activity (radioimmunoassays for ruminant growth hormone and prolactin); growth-promoting activity (rat tibia test); lipolytic activity (release of glycerol from rat epididymal fat in the presence of dexamethasone); diabetogenic activity (rate of glucose transport in epididymal fat of hypophysectomized rats and intravenous insulin-tolerance tests in goats). None of the fractions contained immunoreactive prolactin and all were equally lipolytic. Although fraction A contained a small quantity of immunoreactive growth hormone it had no growth-promoting or diabetogenic activities. Both fractions B and C were diabetogenic and contained high concentrations of immunoreactive growth hormone, consistent with their growth-promoting activity. Although the growth-promoting activity of fraction D was higher than that of the other three fractions, it was not diabetogenic and was only weakly immunoreactive. These results for bovine growth hormone support the contention that growth hormone, as commonly extracted, is a mixture of different molecular forms and that these different metabolic properties of the hormone might be explained in terms of this heterogeneity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assa S., Laron Z. The purification and characterization of a lipolytic factor from bovine pituitaries. Horm Metab Res. 1977 Jul;9(4):294–299. doi: 10.1055/s-0028-1093516. [DOI] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. V. Stimulating effect of ouabain, K+-free medium and insulin on efflux of 3-O-methylglucose from epidimal adipose tissue. Biochim Biophys Acta. 1969;183(3):625–634. doi: 10.1016/0005-2736(69)90175-8. [DOI] [PubMed] [Google Scholar]
- DELLACHA J. M., SONENBERG M. PURIFICATION OF BOVINE GROWTH HORMONE. J Biol Chem. 1964 May;239:1515–1520. [PubMed] [Google Scholar]
- ELLIS S. Studies on the serial extraction of pituitary proteins. Endocrinology. 1961 Sep;69:554–570. doi: 10.1210/endo-69-3-554. [DOI] [PubMed] [Google Scholar]
- Ellis S., Vodian M. A., Grindeland R. E. Studies on the bioassayable growth hormone-like activity of plasma. Recent Prog Horm Res. 1978;34:213–238. doi: 10.1016/b978-0-12-571134-0.50009-0. [DOI] [PubMed] [Google Scholar]
- Frigeri L. G. Absence of in vitro dexamethasone-dependent lipolytic activity from highly purified growth hormone. Endocrinology. 1980 Sep;107(3):738–743. doi: 10.1210/endo-107-3-738. [DOI] [PubMed] [Google Scholar]
- Frigeri L. G., Robel G., Stebbing N. Bacteria-derived human growth hormone lacks lipolytic activity in rat adipose tissue. Biochem Biophys Res Commun. 1982 Feb 11;104(3):1041–1046. doi: 10.1016/0006-291x(82)91354-7. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- Gay V. L. A stereotaxic approach to transauricular hypophysectomy in the rat. Endocrinology. 1967 Nov;81(5):1177–1179. doi: 10.1210/endo-81-5-1177. [DOI] [PubMed] [Google Scholar]
- Hart I. C. A solid phase radioimmunoassay for ovine and caprine prolactin using sepharose 6B: its application to the measurement of circulating levels of prolactin before and during parturition in the goat. J Endocrinol. 1972 Oct;55(1):51–62. doi: 10.1677/joe.0.0550051. [DOI] [PubMed] [Google Scholar]
- Hart I. C., Bines J. A., Morant S. V., Ridley J. L. Endocrine control of energy metabolism in the cow: comparison of the levels of hormones (prolactin, growth hormone, insulin and thyroxine) and metabolites in the plasma of high- and low-yielding cattle at various stages of lactation. J Endocrinol. 1978 Jun;77(3):333–345. doi: 10.1677/joe.0.0770333. [DOI] [PubMed] [Google Scholar]
- Hart I. C. Endocrine control of nutrient partition in lactating ruminants. Proc Nutr Soc. 1983 Jun;42(2):181–194. doi: 10.1079/pns19830023. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Benker G., Salacinski P. R., Lloyd T. J., Lowry P. J. Large-scale preparation of highly purified pyrogen-free human growth hormone for clinical use. J Endocrinol. 1979 Jul;82(1):77–86. doi: 10.1677/joe.0.0820077. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Singh R. N., Tutwiler G. F., Sigel M. B., VanderLaan E. F., VanderLaan W. P. Human growth hormone: a complex of proteins. Recent Prog Horm Res. 1980;36:477–508. doi: 10.1016/b978-0-12-571136-4.50019-x. [DOI] [PubMed] [Google Scholar]
- Lewis U. J., Singh R. N., Vanderlaan W. P., Tutwiler G. F. Enhancement of the hyperglycemic activity of human growth hormone by enzymic modification. Endocrinology. 1977 Nov;101(5):1587–1603. doi: 10.1210/endo-101-5-1587. [DOI] [PubMed] [Google Scholar]
- Lostroh A. J., Krahl M. E. Diabetogenic peptide from human growth hormone: partial purification from peptic digest and long-term action in ob/ob mice. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4706–4710. doi: 10.1073/pnas.73.12.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis L. H., Conn J. W. A diabetogenic polypeptide from hog and sheep adenohypophysis similar to that found in lipoatrophic diabetes. Metabolism. 1968 Jun;17(6):475–484. doi: 10.1016/0026-0495(68)90038-3. [DOI] [PubMed] [Google Scholar]
- Louis L. H., Conn J. W., Minick M. C. A diabetogenic polypeptide from bovine adenohypophysis similar to that excreted in lipoatrophic diabetes. Metabolism. 1966 Apr;15(4):309–324. doi: 10.1016/0026-0495(66)90145-4. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., WALLIS M. COMPARISON OF IN VIVO AND IN VITRO ACTIONS OF VARIOUS OX PITUITARY GROWTH HORMONE FRACTIONS. Nature. 1963 Nov 30;200:888–889. doi: 10.1038/200888a0. [DOI] [PubMed] [Google Scholar]
- MacGorman L. R., Rizza R. A., Gerich J. E. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab. 1981 Sep;53(3):556–559. doi: 10.1210/jcem-53-3-556. [DOI] [PubMed] [Google Scholar]
- Muller E. E., Giustina G., Miedico D., Pecile A., Cocchi D., King F. W. Circadian pattern of bioassayable and radiommunoassayable growth hormone in the pituitary of female rats. Proc Soc Exp Biol Med. 1970 Dec;135(3):934–939. doi: 10.3181/00379727-135-35174. [DOI] [PubMed] [Google Scholar]
- RABEN M. S., WESTERMEYER V. W. Differentiation of growth hormone from the pituitary factor which produces diabetes. Proc Soc Exp Biol Med. 1952 May;80(1):83–86. doi: 10.3181/00379727-80-19531. [DOI] [PubMed] [Google Scholar]
- Rogol A. D., Grissom F., Follows R. E., Jr The relationship of the bovine pituitary "diabetogenic peptide" to prolactin. Horm Metab Res. 1978 Nov;10(6):515–520. doi: 10.1055/s-0028-1093382. [DOI] [PubMed] [Google Scholar]
- Salaman M. R. Discrepancies in the fat mobilizing activities of bovine growth hormone fractions determined in vivo and in vitro. J Endocrinol. 1972 Nov;55(2):459–460. doi: 10.1677/joe.0.0550459. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Voigt K. H. Preparation of highly purified human somatotropin (growth hormone). Hoppe Seylers Z Physiol Chem. 1977 Dec;358(12):1557–1564. doi: 10.1515/bchm2.1977.358.2.1557. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Froesch E. R. Glucose transport in adipocytes and its control by growth hormone in vivo. Am J Physiol. 1982 Jun;242(6):E368–E372. doi: 10.1152/ajpendo.1982.242.6.E368. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Froesch E. R. In vivo control of insulin-sensitive phosphodiesterase in rat adipocytes by growth hormone and its parallelism to glucose transport. Endocrinology. 1981 Aug;109(2):561–566. doi: 10.1210/endo-109-2-561. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Singh R. N., Seavey B. K., Rice V. P., Lindsey T. T., Lewis U. J. Modified forms of human growth hormone with increased biological activities. Endocrinology. 1974 Mar;94(3):883–891. doi: 10.1210/endo-94-3-883. [DOI] [PubMed] [Google Scholar]
- Swislocki N. I., Sonenberg M., Kikutani M. Metabolic effects of the major component of bovine growth hormone. Biochem J. 1971 May;122(5):633–640. doi: 10.1042/bj1220633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindal J. S., Blake L. A., Simmonds A. D., Hart I. C., Mizuno H. Control of growth hormone release in goats: effect of vagal cooling, feeding and artificial distension of the rumen. Horm Metab Res. 1982 Aug;14(8):425–429. doi: 10.1055/s-2007-1019035. [DOI] [PubMed] [Google Scholar]
- Tutwiler G. F. Diabetogenic activity of the anterior pituitary (a progress report). Acta Diabetol Lat. 1976 Sep-Dec;13(5-6):177–185. doi: 10.1007/BF02581116. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962 Nov;237:3354–3358. [PubMed] [Google Scholar]
- Vodian M. A., Nicoll C. S. Growth hormone releasing factor and the bioassay-radioimmunoassay paradox revisited. Acta Endocrinol (Copenh) 1977 Sep;86(1):71–80. doi: 10.1530/acta.0.0860071. [DOI] [PubMed] [Google Scholar]
- WALLACE A. L., FERGUSON K. A. The preparation of sheep growth hormone. J Endocrinol. 1963 Apr;26:259–263. doi: 10.1677/joe.0.0260259. [DOI] [PubMed] [Google Scholar]
- Wallis M., Dixon H. B. A chromatographic preparation of ox growth hormone. Biochem J. 1966 Sep;100(3):593–600. doi: 10.1042/bj1000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Schoenle E., Froesch E. R. Effect of insulin on glucose transport and metabolism in adipose tissue and skeletal muscle of hypophysectomized rats. FEBS Lett. 1981 Nov 30;135(1):199–202. doi: 10.1016/0014-5793(81)80976-3. [DOI] [PubMed] [Google Scholar]



