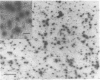
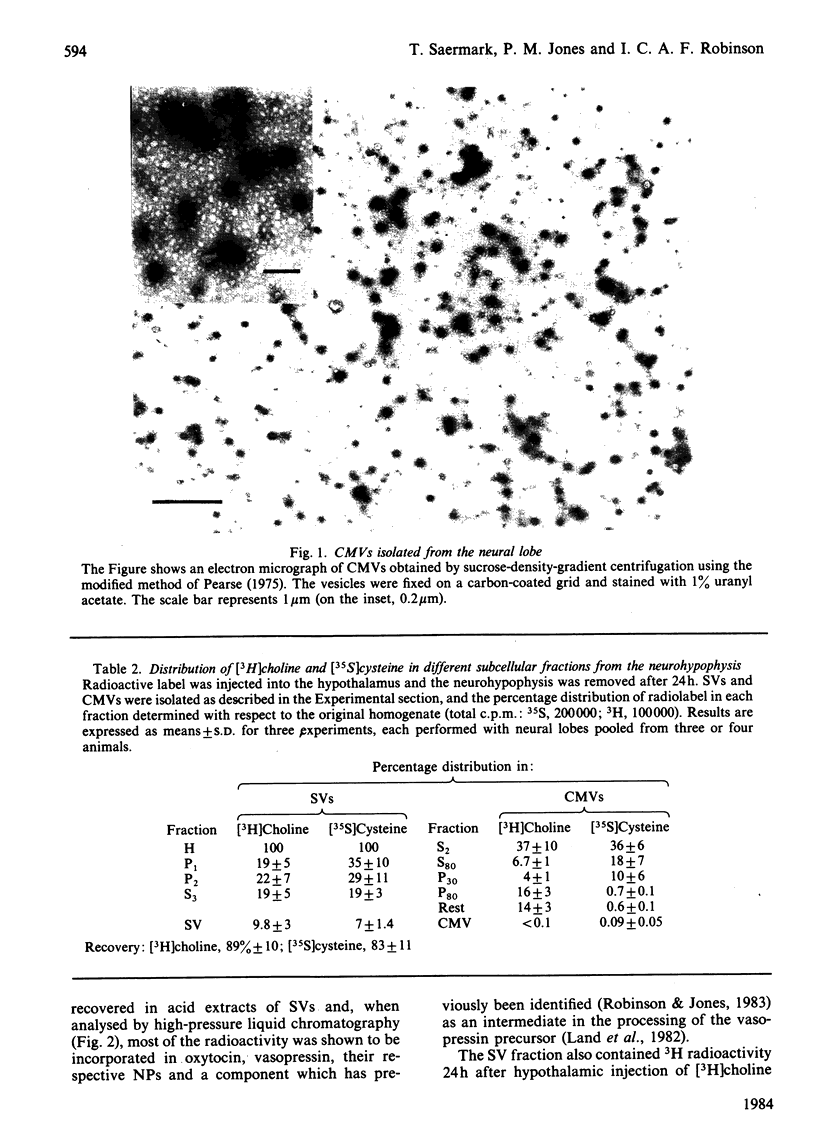
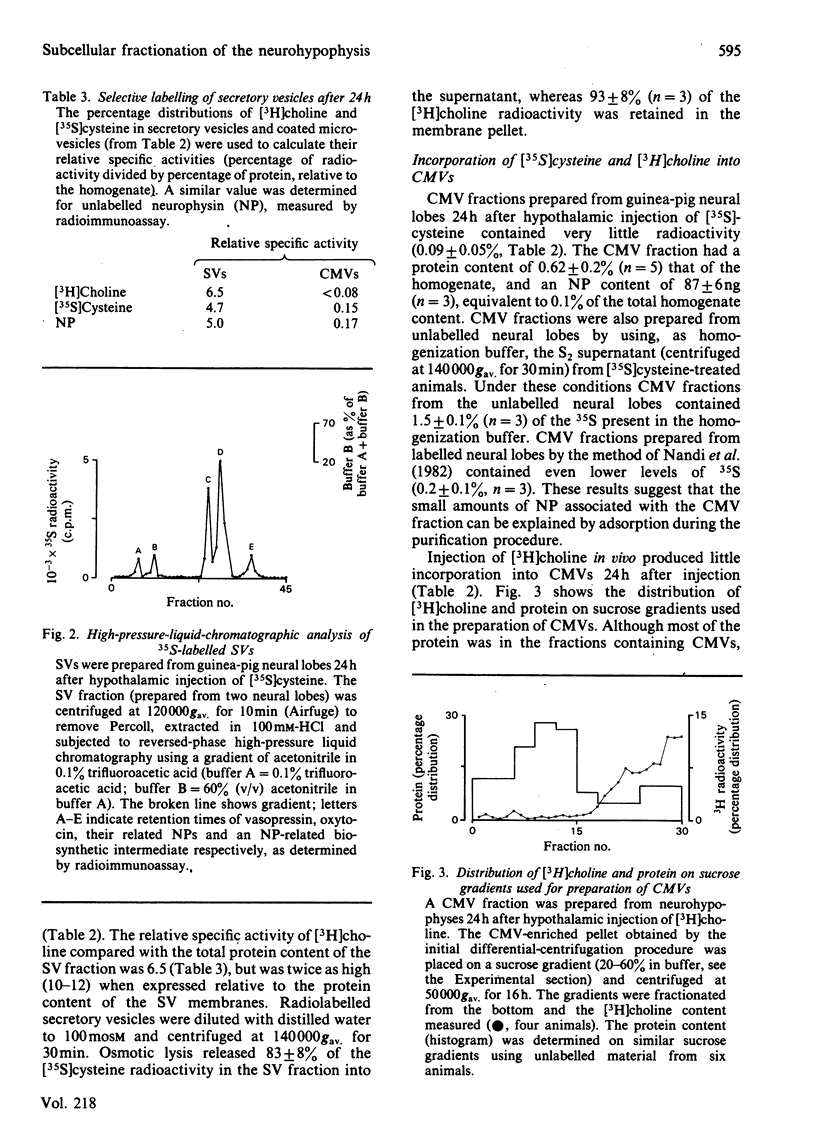
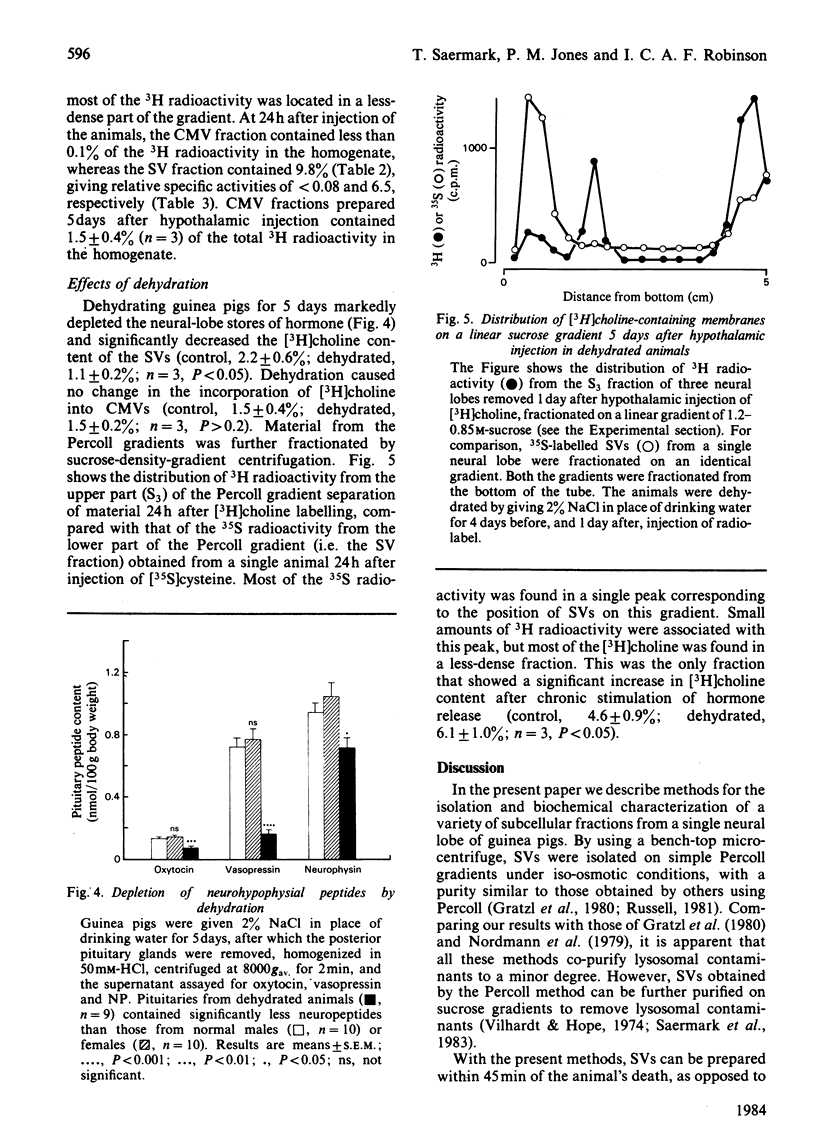
Abstract
We have developed small-scale methods for the isolation and biochemical characterization of subcellular fractions from single guinea-pig posterior-pituitary glands. Secretory vesicles and coated microvesicles produced in this way were of similar purity to those isolated from large amounts of tissue by conventional ultracentrifugation. [35S]Cysteine injected into the hypothalamus was found in the soluble contents of secretory vesicles isolated from the neural lobes 24 h later. High-pressure liquid-chromatographic analysis revealed that the radiolabel was incorporated into the expected neurosecretory products (oxytocin, vasopressin and neurophysin) and also into a biosynthetic intermediate in the vasopressin system. The membranes of secretory vesicles were labelled with [3H]choline 24 h after its hypothalamic injection. Little or no [3H]choline could be demonstrated in coated microvesicles at this time, although these structures were labelled 5 days after injection. Stimulating hormone secretion by chronic dehydration produced a significant fall in [3H]choline content of the secretory-vesicle membranes without any transfer of label into coated microvesicles, suggesting that coated microvesicles are not involved in membrane retrieval in the neurohypophysis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso G., Assenmacher I. The smooth endoplasmic reticulum in neurohypophysial axons of the rat: possible involvement in transport, storage and release of neurosecretory material. Cell Tissue Res. 1979 Jul 17;199(3):415–429. doi: 10.1007/BF00236080. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Ravazzola M., Malaisse-Lagae F. Secretion-dependent uptake of extracellular fluid by the rat neurohypophysis. Nature. 1974 Jul 12;250(462):155–157. doi: 10.1038/250155a0. [DOI] [PubMed] [Google Scholar]
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Nagasawa J., Schulz R. A. Coated microvesicles in neuro-secretory terminals of posterior pituitary glands shed their coats to become smooth "synaptic" vesicles. Nature. 1971 Jul 30;232(5309):340–341. doi: 10.1038/232340a0. [DOI] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Neurophysin biosynthesis: conversion of a putative precursor during axonal transport. Science. 1977 Mar 25;195(4284):1354–1356. doi: 10.1126/science.65791. [DOI] [PubMed] [Google Scholar]
- Gratzl M., Torp-Pedersen C., Dartt D., Treiman M., Thorn N. A. Isolation and characterization of secretory vesicles from bovine neurohypophyses. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1615–1628. doi: 10.1515/bchm2.1980.361.2.1615. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Pickering B. T. Intra-axonal transport and turnover of neurohypophysial hormones in the rat. J Physiol. 1972 Dec;227(2):553–564. doi: 10.1113/jphysiol.1972.sp010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J. H., Pickering B. T. Fate of neurohypophysial granule membranes after secretion in the rat: generation of a new apparent organelle by freezing and thawing secretory granules. J Endocrinol. 1981 Jan;88(1):115–123. doi: 10.1677/joe.0.0880115. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Lescure H., Nordmann J. J. Neurosecretory granule release and endocytosis during prolonged stimulation of the rat neurohypophysis in vitro. Neuroscience. 1980;5(3):651–659. doi: 10.1016/0306-4522(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Nordmann J. J. Membrane recapture after hormone release from nerve endings in the neural lobe of the rat pituitary gland. Neuroscience. 1980;5(3):639–659. doi: 10.1016/0306-4522(80)90061-5. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Nordmann J. J. Membrane retrieval by vacuoles after exocytosis in the neural lobe of Brattleboro rats. Neuroscience. 1982 Jul;7(7):1631–1639. doi: 10.1016/0306-4522(82)90021-5. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W., Schulz R. A. Micropinocytotic origin of coated and smooth microvesicles ("synaptic vesicles") in neurosecretory terminals of posterior pituitary glands demonstrated by incorporation of horseradish peroxidase. Nature. 1971 Jul 30;232(5309):341–342. doi: 10.1038/232341a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W., Schulz R. A. Ultrastructural evidence of secretion by exocytosis and of "synaptic vesicle" formation in posterior pituitary glands. Nature. 1970 Jul 25;227(5256):407–409. doi: 10.1038/227407a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W. Thorium dioxide uptake into adrenal medullary cells and the problem of recapture of granule membrane following exocytosis. Brain Res. 1972 Feb 11;37(1):141–145. doi: 10.1016/0006-8993(72)90356-3. [DOI] [PubMed] [Google Scholar]
- Nandi P. K., Irace G., Van Jaarsveld P. P., Lippoldt R. E., Edelhoch H. Instability of coated vesicles in concentrated sucrose solutions. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5881–5885. doi: 10.1073/pnas.79.19.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Labouesse J. Neurosecretory granules: evidence from an aging process within the neurohypophysis. Science. 1981 Feb 6;211(4482):595–597. doi: 10.1126/science.7455700. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Louis F., Morris S. J. Purification of two structurally and morphologically distinct populations of rat neurohypophysial secretory granules. Neuroscience. 1979;4(9):1367–1379. doi: 10.1016/0306-4522(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Norström A. Axonal transport and turnovers of neurohhypophyseal proteins in the rat. Ann N Y Acad Sci. 1975 Feb 21;248:46–63. doi: 10.1111/j.1749-6632.1975.tb34176.x. [DOI] [PubMed] [Google Scholar]
- North W. G., LaRochelle F. T., Jr, Hardy G. R. Radioimmunoassays for individual rat neurophysins. J Endocrinol. 1983 Mar;96(3):373–386. doi: 10.1677/joe.0.0960373. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Pickering B. T., Jones C. W., Burford G. D., McPherson M., Swann R. W., Heap P. F., Morris J. F. The role of neurophysin proteins: suggestions from the study of their transport and turnover. Ann N Y Acad Sci. 1975 Feb 21;248:15–35. doi: 10.1111/j.1749-6632.1975.tb34174.x. [DOI] [PubMed] [Google Scholar]
- Robinson I. C., Jones P. M. An intermediate in the biosynthesis of vasopressin and neurophysin in the guinea pig posterior pituitary. Neurosci Lett. 1983 Sep 9;39(3):273–278. doi: 10.1016/0304-3940(83)90312-9. [DOI] [PubMed] [Google Scholar]
- Robinson I. C., Jones P. M. Oxytocin and neurophysin in plasma and CSF during suckling in the guinea-pig. Neuroendocrinology. 1982 Jan;34(1):59–63. doi: 10.1159/000123278. [DOI] [PubMed] [Google Scholar]
- Robinson I. C. The development and evaluation of a sensitive and specific radioimmunoassay for oxytocin in unextracted plasma. J Immunoassay. 1980;1(3):323–347. doi: 10.1080/01971528008058475. [DOI] [PubMed] [Google Scholar]
- Robinson I. C., Woolf C. N., Parsons J. A. Suckling in the guinea-pig: the simultaneous release of oxytocin and neurophysin. J Endocrinol. 1981 Aug;90(2):227–236. doi: 10.1677/joe.0.0900227. [DOI] [PubMed] [Google Scholar]
- Russell J. T. The isolation of purified neurosecretory vesicles from bovine neurohypophysis using isoosmolar density gradients. Anal Biochem. 1981 May 15;113(2):229–238. doi: 10.1016/0003-2697(81)90071-3. [DOI] [PubMed] [Google Scholar]
- Sachs H., Fawcett P., Takabatake Y., Portanova R. Biosynthesis and release of vasopressin and neurophysin. Recent Prog Horm Res. 1969;25:447–491. doi: 10.1016/b978-0-12-571125-8.50013-2. [DOI] [PubMed] [Google Scholar]
- Saermark T., Thorn N. A. Ca2+-Mg2+-ATPase activity in brain coated microvesicles purified on immunosorbents. Cell Calcium. 1982 Dec;3(6):561–581. doi: 10.1016/0143-4160(82)90045-8. [DOI] [PubMed] [Google Scholar]
- Saermark T., Thorn N. A., Gratzl M. Calcium/sodium exchange in purified secretory vesicles from bovine neurohypophyses. Cell Calcium. 1983 Jul;4(3):151–170. doi: 10.1016/0143-4160(83)90031-3. [DOI] [PubMed] [Google Scholar]
- Saermark T., Vilhardt H. Isolation and partial characterization of magnesium ion- and calcium ion-dependent adenosine triphosphatase activity from bovine brain microsomal fraction. Biochem J. 1979 Aug 1;181(2):321–330. doi: 10.1042/bj1810321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D., Nordmann J., Henry J. P. Existence of an adenosine 5'-triphosphate dependent proton translocase in bovine neurosecretory granule membrane. Biochemistry. 1982 Feb 16;21(4):687–694. doi: 10.1021/bi00533a016. [DOI] [PubMed] [Google Scholar]
- Swann R. W., Pickering B. T. Incorporation of radioactive precursors into the membrane and contents of the neurosecretory granules of the rat neurohypophysis as a method of studying their fate. J Endocrinol. 1976 Jan;68(1):95–108. doi: 10.1677/joe.0.0680095. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Dreifuss J. J., Orci L. Two classes of microvesicles in the neurohypophysis. Brain Res. 1977 Mar 4;123(1):159–163. doi: 10.1016/0006-8993(77)90650-3. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Dreifuss J., Harris M. C., Orci L. Secretion-related uptake of horseradish peroxidase in neurohypophysial axons. J Cell Biol. 1976 Aug;70(2 Pt 1):294–303. doi: 10.1083/jcb.70.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp-Pedersen C., Saermark T., Bundgaard M., Thorn N. A. ATP-dependent Ca2+ accumulation by microvesicles isolated from bovine neurohypophyses. J Neurochem. 1980 Sep;35(3):552–557. doi: 10.1111/j.1471-4159.1980.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Evidence for dynamic interactions between pituicytes and neurosecretory axons in the rat. Neuroscience. 1980;5(3):661–671. doi: 10.1016/0306-4522(80)90063-9. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977 Jun 20;181(1):59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- Vilhardt H., Hope D. B. Adenosine triphosphatase activity in the neural lobe of the bovine pituitary gland. Biochem J. 1974 Oct;143(1):181–190. doi: 10.1042/bj1430181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Rutherford A. V., Gallo M. G., Wehland J., Dickson R. B., Schlegel R., Pastan I. H. Receptor-mediated endocytosis in cultured fibroblasts: cryptic coated pits and the formation of receptosomes. J Histochem Cytochem. 1981 Sep;29(9):1003–1013. doi: 10.1177/29.9.6169759. [DOI] [PubMed] [Google Scholar]