Abstract
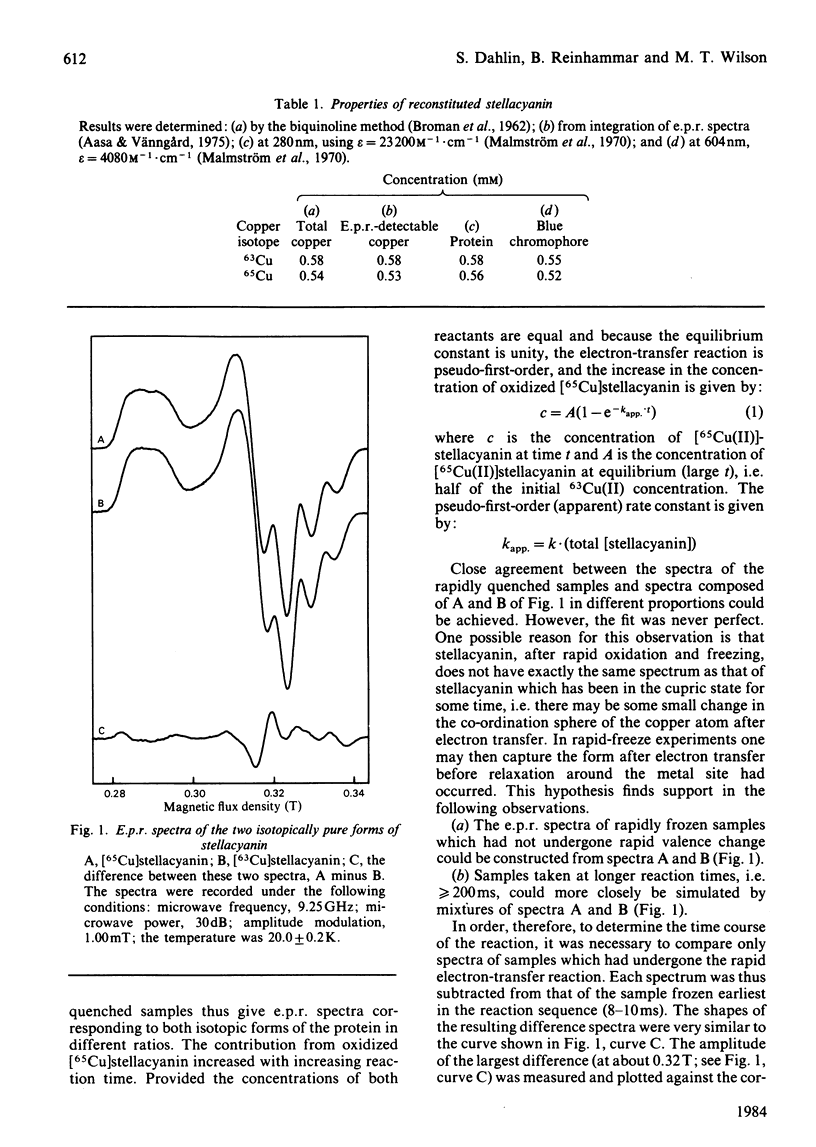
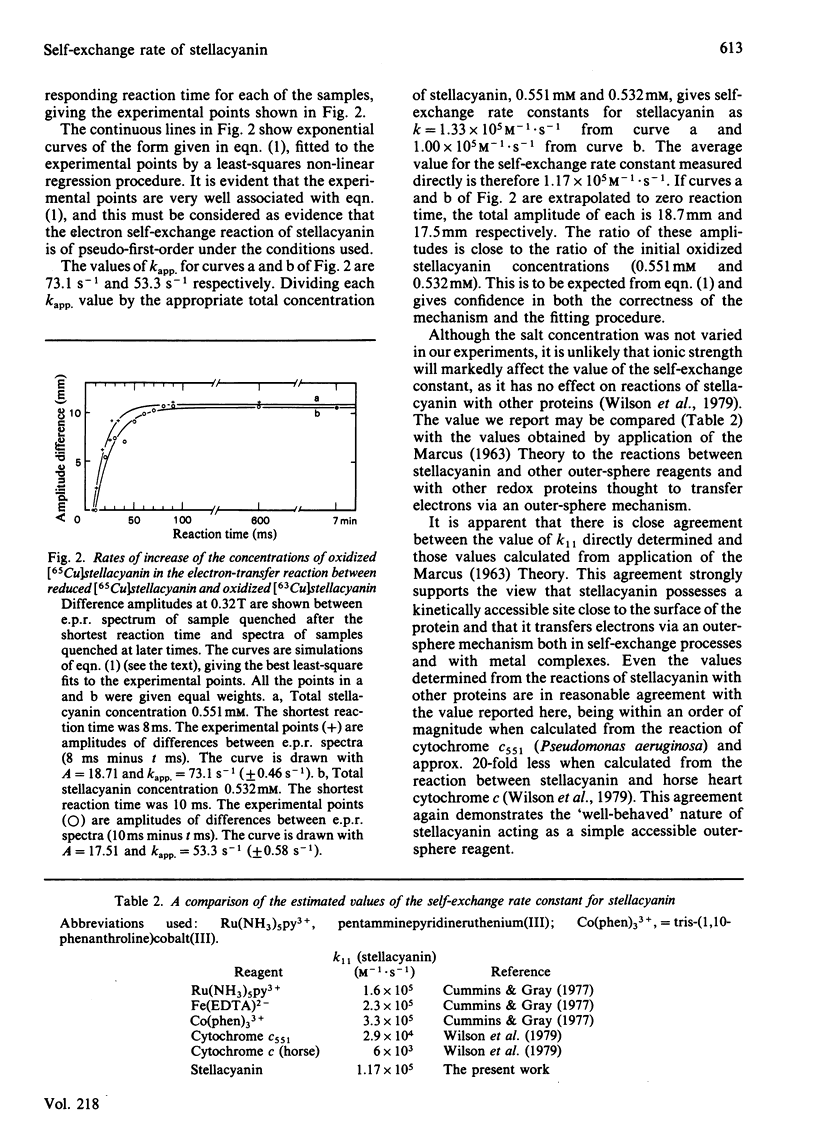
A method for reconstituting the blue copper protein stellacyanin with the stable copper isotopes 63Cu and 65Cu is reported. Small differences in the e.p.r. spectra of the two isotopic forms of stellacyanin have been used to monitor the electron self-exchange reaction of stellacyanin by rapid-freeze e.p.r. methods. The self-exchange rate constant (k11) for stellacyanin has been determined as 1.2 X 10(5) M-1 X S-1 at 20 degrees C. This value is in close agreement with values obtained from less-direct methods.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROMAN L., MALMSTROM B. G., AASA R., VANNGARD T. Quantitative electron spin resonance studies on native and denatured ceruloplasmin and laccase. J Mol Biol. 1962 Sep;5:301–310. doi: 10.1016/s0022-2836(62)80074-6. [DOI] [PubMed] [Google Scholar]
- Cummins D., Gray H. B. Electron-transfer protein reactivities. Kinetic studies of the oxidation of horse heart cytochrome c, Chromatium vinosum high potential iron-sulfur protein, Pseudomonas aeruginosa azurin, bean plastocyanin, and Rhus vernicifera stellacyanin by pentaamminepyridineruthenium(III). J Am Chem Soc. 1977 Jul 20;99(15):5158–5167. doi: 10.1021/ja00457a042. [DOI] [PubMed] [Google Scholar]
- Gupta R. Electron transfer in cytochrome c: role of the polypeptide chain. Biochim Biophys Acta. 1973 Jan 18;292(1):291–295. doi: 10.1016/0005-2728(73)90274-0. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. M., Wüthrich K., Pecht I. Structural studies of cytochrome c-551 by 1H NMR spectroscopy at 360 MHz. FEBS Lett. 1976 Nov;70(1):180–184. doi: 10.1016/0014-5793(76)80753-3. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Wherland S., Gray H. B. Metalloprotein electron transfer reactions: analysis of reactivity of horse heart cytochrome c with inorganic complexes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2950–2954. doi: 10.1073/pnas.73.9.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherland S., Pecht I. Protein-protein electron transfer. A Marcus theory analysis of reactions between c type cytochromes and blue copper proteins. Biochemistry. 1978 Jun 27;17(13):2585–2591. doi: 10.1021/bi00606a020. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Silvestrini M. C., Morpurgo L., Brunori M. Electron transfer kinetics between Rhus vernicifera stellacyanin and cytochrome c (horse heart cytochrome c and Pseudomonas cytochrome c551). J Inorg Biochem. 1979 Oct;11(2):95–100. doi: 10.1016/s0162-0134(00)80175-0. [DOI] [PubMed] [Google Scholar]


