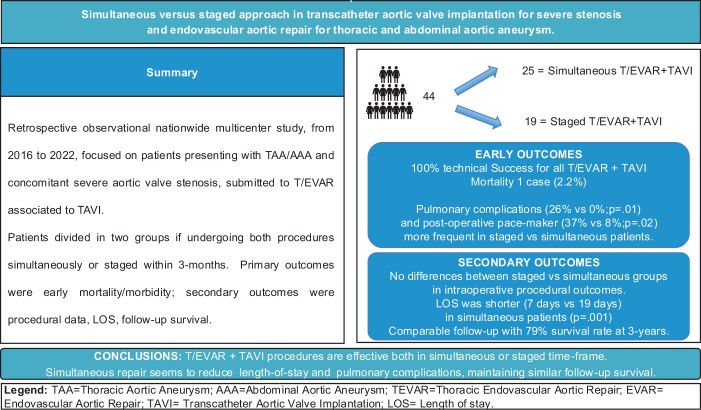
Abstract
OBJECTIVES
Thoracic/abdominal aortic aneurysms and aortic stenosis may be concomitant diseases requiring both transcatheter aortic valve implantation (TAVI) and endovascular aneurysm repair (T/EVAR) in high-risk patients for surgical approaches, but temporal management is not clearly defined yet. The aim of the study was to analyse outcomes of simultaneous versus staged TAVI and T/EVAR.
METHODS
Retrospective observational multicentre study was performed on patients requiring TAVI and T/EVAR from 2016 to 2022. Patients were divided into 2 groups: ‘Simultaneous group’ if T/EVAR + TAVI were performed in the same procedure and ‘Staged group’ if T/EVAR and TAVI were performed in 2 steps, but within 3 months. Primary outcomes were technical success, 30-day mortality/major adverse events and follow-up survival. Secondary outcomes were procedural metrics and length of stay.
RESULTS
Forty-four cases were collected; 8 (18%) had T/EVAR and 36 (82%) had EVAR, respectively. Upon temporal determination, 25 (57%) and 19 (43%) were clustered in Simultaneous and Staged groups, respectively. In Staged group, median time between procedures was 72 (interquartile range—IQR: 57–87) days. Preoperative and intraoperative figures were similar. There was no difference in 30-day mortality (Simultaneous: 0/25 versus Staged: 1/19; P = 0.43). Pulmonary events (Simultaneous: 0/25 versus Staged: 5/19; P = 0.01) and need of postoperative cardiac pacemaker (Simultaneous: 2/25 versus Staged: 7/19; P = 0.02) were more frequent in Staged patients. The overall length of stay was lower in the Simultaneous group [Simultaneous: 7 (IQR: 6–8) versus Staged: 19 (IQR: 15–23) days; P = 0.001]. The median follow-up was 25 (IQR: 8–42) months and estimated 3-year survival was 73% with no difference between groups (Simultaneous: 82% versus Staged: 74%; P = 0.90).
CONCLUSIONS
Both Simultaneous or Staged T/EVAR and TAVI procedures are effective with satisfactory outcomes. Despite the small numbers, simultaneous repair seems to reduce length of stay and pulmonary complications, maintaining similar follow-up survival.
Keywords: Transcatheter aortic valve implantation, Endovascular aortic repair, Abdominal aortic aneurysm, Transcatheter aortic valve implantation, Endovascular aortic repair, Thoracic endovascular aortic repair
According to the current international guidelines, transcatheter aortic valve implantation (TAVI) is the recommended option for treating patients with symptomatic and severe aortic valve stenosis (AS) in older patients (≥75 years) and at high-risk or anatomically unsuitable for surgical aortic valve replacement (SAVR).
Graphical Abstract
INTRODUCTION
According to the current international guidelines, transcatheter aortic valve implantation (TAVI) is the recommended option for treating patients with symptomatic and severe aortic valve stenosis (AS) in older patients (≥75 years) and at high-risk or anatomically unsuitable for surgical aortic valve replacement (SAVR). Trans-femoral (TF) approach is an available option with reduced perioperative morbidity and mortality when compared to transaxillary, transaortic and transapical routes [1–3].
The presence of concomitant aortic–iliac arterial diseases or vascular access complications during TF-TAVI may reduce the benefits of this approach as they are associated with prolonged hospitalization and postoperative increased mortality rates [4, 5]. Concomitant AS and thoracic or abdominal aortic aneurysms (TAAs/AAAs) are not uncommon [6], but no clear recommendations are reported into guidelines [1, 7, 8] and their ideal temporal management is yet to be defined since only anecdotal data are available about concomitant endovascular aneurysm repair (T/EVAR) and TF-TAVI [9]. From a hypothetical standpoint, a simultaneous repair may benefit exposing the patient to a single procedure; however, issues might be considered in combining 2 main interventions in the same setting. Therefore, the aim of the study was to report the results of the endovascular management of concomitant severe AS and TAAs or AAAs, both in simultaneous and staged approach.
METHODS
Study design/patient selections
It was a retrospective observational, nationwide study focused on patients with concomitant severe and symptomatic AS and presenting with symptomatic/asymptomatic TAAs or AAAs, undergoing TF-TAVI and T/EVAR, between 2016 and 2022.
Patients were divided into 2 groups:
Simultaneous group: T/EVAR + TF-TAVI in the same procedure.
Staged group: T/EVAR and TF-TAVI performed within 3 months.
Data from the Simultaneous and Staged groups were compared for the study’s outcomes [10].
Preoperative work-up
Patients were evaluated for an aortic valve replacement in case of severe and symptomatic AS, confirmed by transthoracic echocardiography (mean gradient >40 mmHg or aortic valve area <1.0 cm2) [1]. A multidisciplinary Heart Team, composed of Cardiologists, Interventional Cardiologists, Cardiac Anaesthetists and Cardiac Surgeons, was involved in the patient selection, and older patients (≥75 years) or high surgical risk for surgical aortic valve replacement (SAVR) were considered for TAVI [1]. An ECG-gated cardiac and thoraco-abdominal computed tomography angiography was evaluated for the valve-graft sizing and femoral/iliac or axillary access analysis. Patients were included in the study only if TAVI procedure was performed by transfemoral approach. In case of any vascular issue, an adjunctive preoperative consultation by Vascular Surgeons was performed. Indication for T/EVAR was considered by Vascular Surgeon according to the current guidelines [7, 8]. Patients were decided to undergo prior T/EVAR or TAVI or do both interventions in the same procedures, based on specific patients’ fitness, urgency of the repair per each pathology and institutional protocols. Patients with staged procedures with interval time longer than 3 months were arbitrary excluded from the study in order to reduce confounding factor in this specific fragile population that may interfere with the specific outcomes of the procedures. Aiming to analyse procedural outcomes, patients who did not perform both procedures due to clinical or other issues were excluded from the study.
Definitions and outcomes
Technical success, 30-day mortality/major adverse events and follow-up survival were assessed as primary outcomes. Procedure/fluoroscopy time, contrast media volume and hospitalization were evaluated as secondary outcomes. The cumulative data/events from both procedures for the Staged group were taken into account when comparing with the Simultaneous group.
Technical success was defined as the combination of successful deployment of the cardiac valve according to the Valve Academic Research Consortium (VARC)-3 definition and aortic endograft [11].
Thirty-day mortality and major adverse events were classified as by reporting standards [11]. Vascular complications were defined and classified according to the VARC 3 guidelines [12].
Statistical analysis
Continuous data were reported as a median and interquartile range (IQR). Categorical data were expressed as frequency. Differences between Simultaneous versus Staged groups were evaluated by Fisher’s exact test and Mann–Whitney test for categorical and continuous variables. Follow-up survival analysis was estimated by Kaplan–Meier analysis, and difference between Simultaneous versus Staged groups was evaluated by Log-Rank. Univariate analysis were performed and logistic regression multivariate analysis models were used to adjust for confounders. P value was considered significant when it was <0.05. Statistical analysis was performed by SPSS 28.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients selection
Forty-four patients required concomitant or early deferred aortic aneurysm repair, and TF-TAVI: 8 (18%) had a TAA and 36 (82%) an AAA, respectively. The median age and aneurysm diameter were 82 (IQR: 73–87) years and 58 (IQR: 50–71) mm, respectively. Three (7%) patients had a symptomatic aneurysm with abdominal pain and were treated by standard endovascular infrarenal repair 1st followed by staged TF-TAVI, 5 (11%) had acute heart failure at the moment of hospitalization and 14 (32%) had a history of acute heart failure within 3 preprocedural months. Twenty-five (57%) and 19 (43%) cases were grouped in Simultaneous and Staged groups, respectively. Demographics and preoperative data are reported in Table 1 and they were similar in the 2 groups, except for female gender (P = 0.001), more frequent in the Simultaneous group.
Table 1:
Demographics and preoperative risk-factors
| Overall—44, N (%) | Simultaneous—25, N (%) | Staged—19, N (%) | P-value | |
|---|---|---|---|---|
| Male | 30 (68) | 13 (52) | 17 (89) | 0.01 |
| Body mass index >31 | 8 (18) | 5 (20) | 3 (18) | 0.27 |
| Hypertension | 41 (93) | 24 (96) | 17 (89) | 0.57 |
| Dyslipidaemia | 40 (91) | 22 (88) | 18 (95) | 0.62 |
| Active smoker | 9 (20) | 5 (20) | 4 (21) | 0.53 |
| History of smoke | 22 (50) | 11 (44) | 11 (57) | 0.28 |
| Diabetes | 9 (21) | 5 (20) | 4 (21) | 1 |
| Chronic obstructive pulmonary disease | 15 (34) | 8 (32) | 7 (36) | 0.75 |
| Coronary artery disease | 27 (61) | 13 (52) | 14 (74) | 0.21 |
| Atrial fibrillation | 10 (23) | 6 (24) | 4 (21) | 0.47 |
| Cerebral vascular insufficiency | 7 (16) | 3 (12) | 4 (21) | 0.21 |
| Peripheral arterial occlusive disease | 8 (18) | 5 (20) | 3 (16) | 1.0 |
| Chronic renal failure | 20 (45) | 12 (48) | 8 (42) | 0.13 |
| Dialysis | 0 (0) | 0 (0) | 0 (0) | – |
| History of heart failure (within 3 months) | 14 (32) | 9 (36) | 5 (26) | 0.60 |
| Active heart failure | 5 (11) | 3 (12) | 2 (12) | 1 |
| Medical therapy | ||||
| Dual antiplatelet | 13 (30) | 6 (24) | 7 (36) | 0.57 |
| Anticoagulant therapy | 13 (30) | 8 (32) | 5 (26) | 0.74 |
| Statin | 42 (96) | 23 (92) | 19 (100) | 0.49 |
| Previous infrarenal aortic repair | 7 (16) | 3 (12) | 4 (21) | 0.44 |
| Surgical | 3 (7) | 1 (4) | 2 (12) | 0.60 |
| Endovascular | 5 (11) | 2 (8) | 3 (18) | 0.63 |
| American Score of Anesthesiologist | ||||
| 3 | 14 (32) | 6 (24) | 8 (42) | 0.32 |
| 4 | 30 (68) | 19 (76) | 11 (57) | 0.33 |
| Hostile bilateral femoral/iliac access | 11 (25) | 6 (24) | 5 (26) | 0.89 |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age (years) | 82 (78–86) | 81 (76–86) | 83 (79–87) | 0.32 |
| Preoperative creatinine (mg/dl) | 1.3 (0.9–1.7) | 1.2 (1.0–1.4) | 1.3 (1.1–1.5) | 0.43 |
| Preoperative eGFR (ml/min) | 59 (45–73) | 59 (44–73) | 58 (45–71) | 0.23 |
| Aneurysm diameter (mm) | 58 (55–61) | 57 (55–59) | 61 (57–65) | 0.07 |
IQR: interquartile-range; N: numbers.
Procedure
Table 2 summarizes the major procedural details. The median time between the T/EVAR and TAVI procedures in the Staged group was 72 (IQR: 57–87) days. Technical success (T/EVAR + TF-TAVI) was achieved in all cases. Details of the endograft used for T/EVAR procedures and type of valves used for TAVI are summarized in Supplementary Material, Table S1.
Table 2:
Procedural details
| Overall—44, N (%) | Simultaneous—25, N (%) | Staged—19, N (%) | P-value | |
|---|---|---|---|---|
| Anaesthesia for TEVAR/EVAR | ||||
| Local | 16 (34) | 9 (36) | 7 (37) | 1 |
| Loco-regional | 5 (11) | 2 (8) | 3 (16) | 0.63 |
| General | 23 (52) | 14 (56) | 9 (47) | 0.76 |
| Femoral access TEVAR EVAR | ||||
| Percutaneous | 27 (61) | 16 (64) | 11 (58) | 0.76 |
| Surgical cut down | 17 (39) | 9 (36) | 8 (42) | 0.92 |
| Femoral access TAVI | ||||
| Percutaneous | 30 (68) | 16 (64) | 14 (74) | 0.28 |
| Surgical cut down | 14 (32) | 9 (36) | 5 (26) | 0.63 |
| Aortic endograft configuration | ||||
| Tube | 7 (16) | 3 (12) | 4 (21) | 0.44 |
| Aortic–bi-iliac | 37 (84) | 22 (88) | 15 (79) | 0.44 |
| Iliac artery balloon angioplasty | 2 (5) | 1 (4) | 1 (5) | 1 |
| Iliac artery stenting | 2 (5) | 1 (4) | 1 (5) | 1 |
| Hypogastric artery embolization | 2 (5) | 0 (0) | 2 (11) | 0.10 |
| Blood transfusion | 12 (27) | 9 (36) | 3 (16) | 0.18 |
| Technical success | 44 (100) | 25 (100) | 19 (100) | 1 |
| Type II endoleak | 2 (5) | 1 (4) | 1 (5) | 1 |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Size of main access for T/EVAR (Fr) | 18 (16–20) | 18 (16–20) | 18 (16–20) | 1 |
| Size of main access for TAVI (Fr) | 14 (13–15) | 14 (12–16) | 14 (12–16) | 1 |
| Procedural time (min) | 181 (163–199) | 175 (156–194) | 190 (179–201) | 0.87 |
| Fluoroscopy time (min) | 38 (32–446) | 40 (36–44) | 42 (39–45) | 0.90 |
| Contrast media volume (ml) | 203 (181–223) | 202 (166–238) | 205 (187–223) | 0.20 |
EVAR: endovascular aortic repair; Fr: French; IQR: interquartile range; N: Numbers; T/EVAR: endovascular aneurysm repair; TAVI: transcatheter aortic valve implantation; TEVAR: thoracic endovascular aortic repair.
Early results
Table 3 summarizes adverse events within 30 postoperative days. Pulmonary adverse events (Simultaneous: 0/25 versus Staged: 5/19 versus; P = 0.01) and the need of postoperative cardiac pacemaker (Simultaneous: 2/25 versus Staged: 7/19; P = 0.02) were more frequent in the Staged group. One (2%) patient died within 30 days (Simultaneous: 0/25 versus Staged: 1/19; P = 0.43): an 84 year-old male who underwent EVAR 1st and TF-TAVI after 86 days; the 2nd postoperative course was complicated by urinary sepsis causing final exitus. The overall hospitalization was higher in the Staged group than the Simultaneous one [Simultaneous: 7 (IQR: 6–8) versus Staged: 19 (IQR: 15–23) days; P = 0.001].
Table 3:
Adverse events within 30 postoperative days
| Overall-44, N (%) | Simultaneous-5, N (%) | Staged-19, N (%) | P-value | |
|---|---|---|---|---|
| Cardiac adverse events | 2 (5) | 0 (0) | 2 (11) | 0.18 |
| Cerebrovascular adverse events | 2 (5) | 1 (4) | 1 (5) | 1 |
| Gastrointestinal adverse events | 0 (0) | 0 (0) | 0 (0) | – |
| Renal function worsening | 5 (11) | 2 (8) | 3 (16) | 0.64 |
| Dialysis | 0 | 0 | 0 | – |
| Pulmonary adverse events | 5 (11) | 0 (0) | 5 (26) | 0.01 |
| Need of postoperative cardiac pacemaker | 9 (21) | 2 (8) | 7 (37) | 0.02 |
| Reinterventions | 1 (2) | 0 (0) | 1 (5) | 0.43 |
| Vascular access complication | 2 (5) | 1 (4) | 1 (5) | 1 |
| Death | 1 (2) | 0 (0) | 1 (5) | 0.43 |
Among the 5 cases of renal function worsening reported at 24 postoperative days, 2 returned to baseline value within 30 days.
N: numbers.
Subgroups analysis
The aneurysm repair was performed before TF-TAVI in 18/25 (72%) cases in the Simultaneous group and in 9/19 (47%) in the Staged group, performing prior T/EVAR or prior TAVI (Table 4). Overall, 36 (82%) of patients received a EVAR and 8 (18%) a T/EVAR procedure for infrarenal or thoracic aortic pathology, respectively (Table 5).
Table 4:
Details of the procedures for both Simultaneous and Staged group
| Overall-44, N (%) | Simultaneous group |
Staged group |
|||||
|---|---|---|---|---|---|---|---|
| Overall-25, N (%) | EVAR first-18, N (%) | TAVI first-7, N (%) | Overall-19, N (%) | EVAR first-10, N (%) | TAVI first–9, N (%) | ||
| Preoperative factors | |||||||
| Male | 30 (68) | 13 (52) | 7 (39) | 5 (71) | 17 (89) | 9 (90) | 8 (89) |
| Urgent aneurysm | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (16) | 3 (30) | 0 (0) |
| Hostile bilateral femoral/iliac access | 11 (25) | 5 (20) | 4 (22) | 1 (14) | 6 (32) | 3 (30) | 3 (33) |
| TEVAR | 8 (57) | 3 (12) | 3 (17) | 0 (0) | 5 (26) | 2 (20) | 3 (33) |
| EVAR | 36 (43) | 22 (88) | 15 (83) | 7 (100) | 14 (74) | 8 (80) | 6 (67) |
| Days between procedures (Staged group) | 72 (IQR: 57–87) | 82 (IQR: 32–86) | 65 (IQR: 28–88) | ||||
| Intraoperative details | |||||||
| General Anaesthesia TEVAR/EVAR | 23 (52) | 14 (56) | 13 (72) | 1 (14) | 9 (47) | 3 (30) | 6 (67) |
| Percutaneous femoral access TEVAR/EVAR | 27 (61) | 16 (64) | 10 (56) | 6 (86) | 11 (58) | 4 (40) | 7 (78) |
| Percutaneous femoral access TAVI | 30 (68) | 16 (64) | 10 (56) | 6 (86) | 14 (74) | 7 (70) | 7 (78) |
| Need for iliac adjunctive procedures | 4 (9) | 1 (4) | 0 (0) | 1 (14) | 3 (16) | 2 (20) | 1 (11) |
| Technical Success | 44 (100) | 25 (100) | 18 (100) | 7 (100) | 19 (100) | 10 (100) | 9 (100) |
| Postoperative results | |||||||
| Cardiac adverse events | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (11) | 1 (10) | 1 (11) |
| Cerebrovascular adverse events | 2 (5) | 1 (4) | 0 (0) | 1 (14) | 1 (5) | 0 (0) | 1 (11) |
| Respiratory adverse events | 5 (11) | 0 (0) | 0 (0) | 0 (0) | 5 (26) | 2 (20) | 3 (33) |
| Reinterventions | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (10) | 0 (0) |
| Vascular access complication | 2 (5) | 1 (4) | 1 (6) | 0 (0) | 1 (5) | 0 (0) | 1 (11) |
| Death | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (10) | 0 (0) |
EVAR: endovascular aortic repair; IQR: interquartile range; N: numbers; TAVI: transcatheter aortic valve implantation; TEVAR: thoracic endovascular aortic repair.
Table 5:
Details of the procedures divided upon aortic repair both as endovascular aortic repair for infrarenal abdominal aorta (EVAR) and thoracic endovascular aortic repair (TEVAR)
| Overall-44, N (%) | EVAR-36, N (%) | TEVAR-8, N (%) | |
|---|---|---|---|
| Preoperative factors | |||
| Male | 30 (68) | 25 (69) | 5 (62) |
| Urgent aneurysm | 3 (7) | 3 (8) | 0 (0) |
| Hostile bilateral femoral/iliac access | 11 (25) | 10 (27) | 1 (12) |
| Iliac aneurysm | 3 (7) | 3 (8) | 0 (0) |
| Simultaneous group | 25 (57) | 22 (61) | 3 (37) |
| Staged group | 19 (43) | 14 (39) | 5 (62) |
| Days between procedures (Staged group) | 72 (IQR: 57–87) | – | 74 (IQR: 58–89) |
| Intraoperative details | |||
| General anaesthesia TEVAR/EVAR | 23 (52) | 15 (42) | 8 (100) |
| Percutaneous femoral access TEVAR/EVAR | 27 (61) | 24 (67) | 3 (37) |
| Percutaneous femoral access TAVI | 30 (68) | 26 (72) | 4 (60) |
| Need for iliac adjunctive procedures | 4 (9) | 4 (11) | 0 (0) |
| Technical success | 44 (100) | 36 (100) | 8 (100) |
| Postoperative results | |||
| Cardiac adverse events | 2 (5) | 1 (3) | 1 (12) |
| Cerebrovascular adverse events | 2 (5) | 1 (3) | 1 (12) |
| Respiratory adverse events | 5 (11) | 2 (6) | 3 (37) |
| Reinterventions | 1 (2) | 1 (3) | 0 (0) |
| Vascular access complication | 2 (5) | 2 (6) | 0 (0) |
| Death | 1 (2) | 0 (0) | 1 (12) |
IQR: interquartile range.
Follow-up results
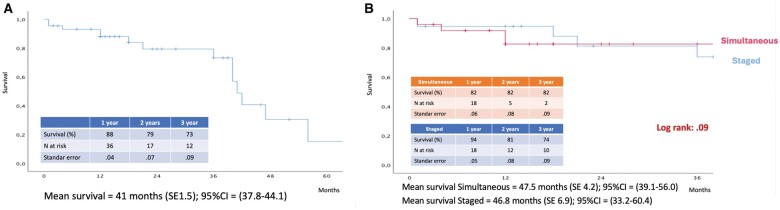
The median follow-up was 25 (IQR: 8–42) months. Estimated 3-year survival was 73% at Kaplan–Meier analysis, with no difference between groups (Simultaneous: 82% versus Staged: 74%; Log-Rank-P = 0.90; Fig. 1). Causes of mortality are summarized in Supplementary Material, Table S2. There was no difference in rehospitalization (Simultaneous: 5/25 versus Staged: 4/19; P = 0.30) and procedure-related reinterventions (Simultaneous: 1/25 versus Staged: 2/19; P = 1). Causes of rehospitalization and reinterventions are reported in Supplementary Material, Table S3. Two patients underwent reintervention due to iliac recoil after stenting and femoral pseudoaneurysm after percutaneous access.
Figure 1:
(A) Estimated overall survival by Kaplan–Meier analysis. (B) Estimated survival by Kaplan–Meier analysis in patients managed by simultaneous and staged approaches.
Univariate and multivariate analysis
Among primary end-points, staged repair appeared to be a risk factor for pulmonary adverse events [odds ratio = 7.4; 95% confidence interval 3.4–7.6; P = 0.006]. Multivariate analysis adjusted for potential confounders confirmed the independent role of the staged procedure (odds ratio = 15.2; 95% confidence interval 5.4–8.9; P ≤ 0.001). Follow-up survival was not impacted by staged versus simultaneous approach. The need for permanent cardiac pacemaker was the unique independent factor for follow-up mortality (hazard ratio = 6.3; 95% confidence interval 3.4–7.6; P = 0.012).
DISCUSSION
In the present manuscript, we report 44 patients with concomitant severe AS and T/AAAs, gathered from a multicentre nationwide experience within 7 years. Overall results were satisfactory in terms of technical success, early clinical results and a low number of vascular access complications. Follow-up mortality was also encouraging, especially if we consider high surgical-risk patients.
Concomitant AS and T/AAAs is not uncommon nowadays because of the increasing age of the population as well as multiple aortic comorbidities [1, 7, 13]. Until today, there are no definitive recommendations about the concomitant management of these diseases [1, 7, 8, 13].
Historically, in low-risk patients, the gold standard approach is surgical aortic valve replacement (SAVR) 1st performed by cardiac surgeons followed by aneurysm repair [1, 9]. However, SAVR is usually associated with postoperative increase of systolic blood pressure and risk of aneurysm rupture [14]. On the other hand, issues arise when performing an aneurysm repair as the 1st step due to severe fluctuation of blood pressure during aortic clamping [15].
In the last decades, the endovascular revolutions in both cardiac and vascular surgery allowed to guarantee mini-invasive solutions with effective and reproducible outcomes both for AS and T/AAAs [1, 7]. For these reasons, the current management of concomitant symptomatic and severe AS and T/AAAs is changing, and simultaneous endovascular repair could be feasible.
The 1st report of simultaneous TF-TAVI and EVAR was managed by Drury-Smith et al. in 2012 [16]. Table 6 provides a summary of the 25 cases reported in the literature about simultaneous TF-TAVI and EVAR procedures. Bramucci et al. [13] reported in 2023 the 1st case of simultaneous TF-TAVI and EVAR performed by total percutaneous approach under local anaesthesia.
Table 6:
Literature data about simultaneous TF-TAVI and EVAR
| Author | Year | Cases | VAC (n) | 30-day mortality (n) | Hospitalization (days) | Follow-up (months) |
|---|---|---|---|---|---|---|
| Naoum | 2023 | 6 | 2 | 0 | 8 | 19 |
| Bramucci | 2023 | 1 | 1 | 0 | 5 | 2 |
| Yammine | 2021 | 5 | 0 | 0 | 5 | 12 |
| Koutsias | 2020 | 2 | 0 | 0 | 9 | 18 |
| Mauri | 2019 | 2 | 1 | 0 | 10 | 9 |
| Sato | 2017 | 1 | 0 | 0 | 8 | 6 |
| Kawashima | 2016 | 1 | 0 | – | 9 | – |
| Koudoumas | 2015 | 1 | 0 | 0 | 3 | 3 |
| Binder | 2015 | 1 | 0 | 0 | – | 3 |
| Aluko | 2015 | 1 | 0 | 0 | 3 | 12 |
| Marchi | 2014 | 1 | 0 | – | 3 | – |
| Chakraborty | 2013 | 1 | 1 | 0 | – | – |
| Smith | 2012 | 1 | 0 | 0 | 5 | – |
| Smith | 2012 | 1 | 0 | 0 | 14 | 6 |
| Overall | 25 | 5 | 0 | 7 | 9 | |
| Present series | 2023 | 25 | 1 | 0 | 7 | 25 |
EVAR: endovascular aortic repair; TF-TAVI: trans-femoral transcatheter aortic valve implantation; VAC: vascular access complications.
In the present series, we have reported a wide series on this topic and compared cases treated in a single simultaneous procedure with cases managed by staged strategy. Preoperative clinical features were comparable between 2 groups, except for female gender, and more frequent in the Simultaneous group [17].
Even if concomitant TF-TAVI and T/EVAR may increase the complexity of a single procedure, our series demonstrates no differences in intraoperative figures as well as in postoperative mortality between groups. Specifically, postoperative pulmonary adverse events, the need of permanent cardiac pacemaker and length of stay resulted higher in the Staged group. We might speculate to address these findings with the need of multiple hospitalizations, especially in such a fragile population. Moreover, as resulted in the multivariate analysis as collateral finding, the permanent cardiac pacemaker was linked to a reduction in survival during follow-up.
Postoperative AKI is one of the most frequent complications after both T/EVAR and TAVI [18]. Tailored preoperative planning, automated CO2 angiography [19] and IVUS (intravascular ultrasound) play a crucial role in the reduction of renal toxicity guaranteeing non necessity of postoperative haemodialysis.
Follow-up results are currently lacking in literature because there are only few preliminary reports describing the feasibility/effectiveness [9, 13, 15, 20, 21]. In the present series, follow-up mortality is not negligible, but acceptable in consideration of the fragile patients’ population.
However, there are still open questions about timing/management, even in the case of concomitant TF-TAVI and T/EVAR. In the present series, numbers are too small to find any statistical association between preoperative morphological/clinical features and different timing of repair. Moreover, the retrospective and multicentre case enrolment plays a role in the heterogeneity of these different approaches, and since every multidisciplinary team based decision on the on specific patients fitness, urgency of the repair per each pathology and institutional protocols, no clear data on the indications are specified in this study, focusing on the procedural aspects. Future researches should better investigate specific morphological/clinical factors that may benefit for a staged or simultaneous approach, given the favourable results from this 1st experience.
Limitations
The present study has several design limits. It is a retrospective analysis, with small sample size and limited follow-up, with few events ranging from 0 to 5, so type 2 statistical error is to be taken into account, and complex statistical consideration should be considered in light of this.
Eventually, the retrospective design and the inclusion criteria, which were specifically considered just on patients who underwent both procedures, led us to have no data about patients managed by staged approach but unable to complete due to inter-procedural complications mortality.
The main advantage of a concomitant endovascular treatment of both AS and T/AAAs consists of using the same access for both procedures: both EVAR and TAVI require large femoral bore and a combination of both procedures may reduce the risk of vascular access complications, as suggested by the low numbers reported in our cohort, thanks to active hostile iliac vessel preparation [3]. Moreover, it allows to solve in a single procedure 2 different serious illness, avoiding any risk of mortality between therapeutic steps. At the same time, the combined procedure allows to face directly serious related complications: (i) the haemodynamic issues that might be relevant during aortic repair and may be highlighted due to the severe AS; (ii) the risk for aortic rupture or dissection that can arise while navigating TAVI in an aneurysmatic aorta that may suggest to use an alternative approach, such as transapical or axillary ones, none presented in our series of 100% TF-TAVI. (iii) Eventually, a simultaneous approach may also reduce the overall periprocedural costs due to a reduced pulmonary complication rate and shorter hospitalization period.
CONCLUSIONS
Simultaneous or staged thoracic/abdominal endovascular aortic repair and TAVI are effective with satisfactory outcomes with both strategies. Despite small numbers, simultaneous endovascular repair seems to offer significant reduction of overall hospitalization and pulmonary complications, while maintaining similar procedure-related follow-up outcomes. These data may be considered in the implementation of multidisciplinary teams of Cardiac, Vascular Surgeons and Interventional Cardiologists while evaluating high surgical risk patients presenting both pathologies.
Supplementary Material
ACKNOWLEDGEMENTS
Italian multicenter EVAR/TEVAR + TAVI study’s group collaborators are as follows: Antonello M., Bellosta R., Berti S., Bramucci A., Cappiello A., Cecere F., Di Marzo L., D’Oria M., Faggioli G.L., Freyrie A., Gallitto E., Gargiulo M,. Gelpi G., Gennai S., Grando B., Isernia G., Lepidi S., Lodato M., Marrozzini C., Palmerini T., Pratesi G., Piazza M., Mansour W., Mezzetto L., Piffaretti G., Rizza A., Saia F., Silingardi R., Simonte G., Squizzato F., Spath P., Tinelli G., Tozzi M., Trimarchi S. and Veraldi G.F.
Glossary
ABBREVIATIONS
- AAA
Abdominal aortic aneurysm
- AS
Aortic valve stenosis
- EVAR
Endovascular aortic repair
- IQR
Interquartile range
- SAVR
Surgical aortic valve replacement
- T/AAA
Thoracic abdominal aortic aneurysm
- TAVI
Transcatheter aortic valve implantation
- T/EVAR
Thoracic endovascular aortic repair
- TF
Trans-femoral
- VARC
Valve Academic Research Consortium
Contributor Information
Enrico Gallitto, Vascular Surgery, University of Bologna-DIMEC, Bologna, Italy; Vascular Surgery unit, IRCCS Azienda Ospedaliero-universitaria di Bologna.
Paolo Spath, Vascular Surgery, University of Bologna-DIMEC, Bologna, Italy; Vascular Surgery unit, IRCCS Azienda Ospedaliero-universitaria di Bologna.
Gian Luca Faggioli, Vascular Surgery, University of Bologna-DIMEC, Bologna, Italy; Vascular Surgery unit, IRCCS Azienda Ospedaliero-universitaria di Bologna.
Francesco Saia, Interventional Cardiology, IRCCS Azienda Ospedaliero-universitaria di Bologna.
Tullio Palmerini, Interventional Cardiology, IRCCS Azienda Ospedaliero-universitaria di Bologna.
Michele Piazza, Vascular Surgery, University of Padova, Padova, Italy.
Mario D’Oria, Vascular Surgery, University of Trieste, Trieste, Italy.
Gioele Simonte, Vascular and Endovascular Surgery Unit, Hospital S. Maria Misericordia, Perugia, Italy.
Antonio Cappiello, Vascular Surgery, University of Bologna-DIMEC, Bologna, Italy.
Giacomo Isernia, Vascular and Endovascular Surgery Unit, Hospital S. Maria Misericordia, Perugia, Italy.
Guido Gelpi, Cardiac Surgery, IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy.
Antonio Rizza, Cardiology Unit, Fondazione Toscana Gabriele Monasterio, Massa, Italy.
Gabriele Piffaretti, Vascular Surgery, Department of Medicine and Surgery, University of Insubria School of Medicine, ASST-Settelaghi Universitary Teaching Hospital, Varese, Italy.
Mauro Gargiulo, Vascular Surgery, University of Bologna-DIMEC, Bologna, Italy; Vascular Surgery unit, IRCCS Azienda Ospedaliero-universitaria di Bologna.
the Italian Multicenter T/EVAR + TAVI Study’s Group:
M Antonello, R Bellosta, S Berti, A Bramucci, A Cappiello, F Cecere, L Di Marzo, M D’Oria, G L Faggioli, A Freyrie, E Gallitto, M Gargiulo, G Gelpi, S Gennai, G Isernia, S Lepidi, M Lodato, C Marrozzini, T Palmerini, G Pratesi, M Piazza, W Mansour, L Mezzetto, G Piffaretti, A Rizza, F Saia, R Silingardi, G Simonte, F Squizzato, P Spath, G Tinelli, M Tozzi, S Trimarchi, and G F Veraldi
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
No funding was provided for this study.
Conflict of interest: none declared.
DATA AVAILABILITY
Data were retrospectively collected in each centre from clinical records, shared anonymously and analysed. Due to its retrospective nature, individual informed consent was waived and Institutional Review Board approval was obtained in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the observational studies. All relevant data are within the manuscript and its Supporting Information files. The data of this study are available from the corresponding author upon reasonable request to the corresponding author: DOI:10.5281/zenodo.13997887.
Author contributions
Enrico Gallitto: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Paolo Spath: Conceptualization; Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Gian Luca Faggioli: Conceptualization; Formal analysis; Supervision; Writing—review & editing. Francesco Saia: Conceptualization; Data curation; Investigation; Validation; Writing—review & editing. Tullio Palmerini: Conceptualization; Data curation; Validation; Writing—review & editing. Michele Piazza: Conceptualization; Data curation; Validation; Writing—review & editing. Mario D’Oria: Data curation; Writing—review & editing. Giole Simonte: Data curation; Writing—review & editing. Antonio Cappiello: Data curation. Giacomo Isernia: Data curation; Writing—review & editing. Guido Gelpi: Data curation; Writing—review & editing. Antonio Rizza: Data curation; Writing—review & editing. Gabriele Piffaretti: Data curation; Validation; Writing—review & editing. Mauro Gargiulo: Conceptualization; Data curation; Formal analysis; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Luca Di Marco, Sven Peterss, Mateo Marin-Cuartas and the other anonymous reviewers for their contribution to the peer review process of this article.
Presented at the 37th ESVS Annual Meeting, Belfast, UK, 26–29 September 2023.
REFERENCES
- 1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK. et al. ; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 3. Palmerini T, Saia F, Kim W-K, Renker M, Iadanza A, Fineschi M. et al. Vascular access in patients with peripheral arterial disease undergoing TAVR. JACC Cardiovasc Interv 2023;16:396–411. [DOI] [PubMed] [Google Scholar]
- 4. Raju S, Eisenberg N, Montbriand J, Cusimano RJ, Feindel C, Ouzounian M. et al. Vascular complications and procedures following transcatheter aortic valve implantation. Eur J Vasc Endovasc Surg 2019;58:437–44. [DOI] [PubMed] [Google Scholar]
- 5. Toggweiler S, Leipsic J, Binder RK, Freeman M, Barbanti M, Heijmen RH. et al. Management of vascular access in transcatheter aortic valve replacement. JACC Cardiovasc Interv 2013;6:767–76. [DOI] [PubMed] [Google Scholar]
- 6. Kurra V, Schoenhagen P, Roselli EE, Kapadia SR, Tuzcu EM, Greenberg R. et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1258–64. [DOI] [PubMed] [Google Scholar]
- 7. Czerny M, Grabenwöger M, Berger T, Aboyans V, Della Corte A, Chen EP. et al. ; EACTS/STS Scientific Document Group. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur J Cardiothorac Surg 2024;65:ezad426. [DOI] [PubMed] [Google Scholar]
- 8. Wanhainen A, Van Herzeele I, Bastos Goncalves F, Bellmunt Montoya S, Berard X, Boyle JR. et al. European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg 2024;67:192–331. [DOI] [PubMed] [Google Scholar]
- 9. Koutsias S, Karaolanis GI, Papafaklis MI, Peroulis M, Tzimas P, Lakkas L. et al. Simultaneous transcatheter aortic valve implantation and infrarenal aortic aneurysm repair for severe aortic stenosis and abdominal aortic aneurysm: report of 2 cases and literature review. Vasc Endovascular Surg 2020;54:544–8. [DOI] [PubMed] [Google Scholar]
- 10. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13:S31–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T et al; Writing Committee Group. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg 2021;73:4S–52S. [DOI] [PubMed] [Google Scholar]
- 12. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P. et al. ; VARC-3 WRITING COMMITTEE. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021;42:1825–57. [DOI] [PubMed] [Google Scholar]
- 13. Bramucci A, Vignali L, Tadonio I, Losi L, Freyrie A, Perini P.. Single-stage procedure of transcatheter aortic valve replacement and endovascular aneurysm repair under local anaesthesia and percutaneous access. Vasc Endovascular Surg 2023;57:949–53. [DOI] [PubMed] [Google Scholar]
- 14. Grinberg T, Aviv Y, Vaturi M, Perl L, Wiessman M, Vaknin‐Assa H. et al. Noninvasive hemodynamic evaluation following TAVI for severe aortic stenosis. J Am Heart Assoc 2023;12:e028479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mauri S, Bozzani A, Ferlini M, Aiello M, Gazzoli F, Pirrelli S. et al. Combined transcatheter treatment of severe aortic valve stenosis and infrarenal abdominal aortic aneurysm in increased surgical risk patients. Ann Vasc Surg 2019;60:480.e1–e5. [DOI] [PubMed] [Google Scholar]
- 16. Drury‐Smith M, Garnham A, Khogali S.. Critical aortic stenosis in a patient with a large saccular abdominal aortic aneurysm: simultaneous transcatheter aortic valve implantation and drive‐by endovascular aortic aneurysm repair. Catheter Cardiovasc Interv 2012;80:1014–8. [DOI] [PubMed] [Google Scholar]
- 17. Spath P, Campana F, Gallitto E, Pini R, Mascoli C, Sufali G. et al. Impact of iliac access in elective and non-elective endovascular repair of abdominal aortic aneurysm. J Cardiovasc Surg (Torino) 2024;65:85–98. [DOI] [PubMed] [Google Scholar]
- 18. Jhaveri KD, Saratzis AN, Wanchoo R, Sarafidis PA.. Endovascular aneurysm repair (EVAR)— and transcatheter aortic valve replacement (TAVR)—associated acute kidney injury. Kidney Int 2017;91:1312–23. [DOI] [PubMed] [Google Scholar]
- 19. Spath P, Caputo S, Campana F, Gallitto E, Pini R, Mascoli C. et al. CO2 angiography in the standard and complex endovascular repair of the abdominal aorta—a narrative review of the literature. JCM 2024;13:4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yammine H, Briggs CS, Rolle QV, Ballast JK, Frederick JR, Skipper E. et al. Simultaneous transcatheter aortic valve replacement and endovascular aortic aneurysm repair. J Am Coll Cardiol 2021;77:2156–7. [DOI] [PubMed] [Google Scholar]
- 21. Naoum I, Eitan A, Galili O, Hayeq H, Shiran A, Zissman K. et al. Strategy for totally percutaneous management of vascular injury in combined transfemoral transcatheter aortic valve replacement and endovascular aortic aneurysm repair procedures. Am J Cardiol 2023;207:130–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were retrospectively collected in each centre from clinical records, shared anonymously and analysed. Due to its retrospective nature, individual informed consent was waived and Institutional Review Board approval was obtained in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for the observational studies. All relevant data are within the manuscript and its Supporting Information files. The data of this study are available from the corresponding author upon reasonable request to the corresponding author: DOI:10.5281/zenodo.13997887.