Abstract
This guideline was developed according to the British Society for Rheumatology Guidelines Protocol by a Guideline Development Group comprising healthcare professionals with expertise in SSc and people with lived experience, as well as patient organization representatives. It is an update of the previous 2015 SSc guideline. The recommendations were developed and agreed by the group and are underpinned by published evidence, assessed by systematic literature review and reinforced by collective expert opinion of the group. It considers all aspects of SSc including general management, treatment of organ-based complications, including cardiopulmonary, renal and gastrointestinal tract manifestations, as well as broader impact of disease. Whilst it is focused on adults with SSc we expect that the guideline will be relevant to people of all ages and expert input and review by paediatric rheumatologists and other relevant specialists considered where the guideline was, or may not be, applicable to young people with SSc and juvenile-onset disease. In addition to providing guidance on disease assessment and management the full guideline also considers service organization within the National Health Service and future approaches to audit of the guideline. The lay summary that accompanies this abstract can be found in Supplemental information 1.
Keywords: scleroderma, systemic sclerosis, interstitial lung disease, guideline, management
Graphical abstract
This guideline was developed in line with the British Society for Rheumatology (BSR)’s Guidelines Protocol [1].


Background
Systemic sclerosis (SSc) is uncommon in the UK population, with around 1000 new cases per year in the UK [2] and is complex and diverse with limited treatment options [3]. It has the highest mortality of any of the autoimmune rheumatic diseases with approximately half of people affected by SSc eventually dying as a direct result of the disease or a related complication [4]. Around one in three people with SSc develops interstitial lung disease (ILD) and 1 in 10 may develop pulmonary hypertension and these are currently the most frequent direct causes of SSc related death [5]. One in five people with SSc develop overlap connective tissue diseases and this will require specific management in parallel with SSc [6].
Juvenile onset SSc (jSSc), presenting under the age of 18 years, is extremely rare, with an estimated prevalence of 3 in 1 000 000 [7]. Mortality is primarily from cardiopulmonary disease primarily, jSSc outcomes in prospective cohorts are generally favourable. ILD is reported in around half of jSSc whereas pulmonary hypertension is less common than in adults [7]. Overlap features are more common in childhood onset disease [8].
It is plausible that vigilant screening for organ-based complications and routine use of disease modifying immunosuppression in diffuse cutaneous SSc have improved overall survival and this is supported by single-centre observational cohort analysis [9].
Need for guideline
The current BSR Guideline for systemic sclerosis (SSc) was completed in 2015 [10] and represented an important step forward for management of this complex disease with high morbidity, mortality and unmet medical need. It provides a roadmap for best practice management to try and harmonise treatment and investigation of SSc and defines key quality and audit standards that can be used to assess practice and improve outcomes.
Updating the guideline is now required to reflect important changes in management of organ-based complications including pulmonary hypertension and to incorporate new trials and evidence-based therapies together with changes in NHS England prescribing policies (e.g. digital ulcers) that now mean that the 2015 guideline no longer reflects current best practice and does not reflect all the available high-quality evidence that can underpin management of SSc. There have been significant advances in treatment and clinical trials since 2015 with new drugs now used for treatment of complications of SSc, especially interstitial lung disease (e.g. nintedanib) and an enlarged evidence base supporting disease management.
Additionally, in line with other BSR guidelines and equality considerations, where possible and appropriate, the updated guideline includes consideration of all ages of people affected by SSc, with specific consideration of the relevance to children and adolescents with systemic sclerosis including transition into adult services.
Objectives of guideline
This guideline offers systematic and evidence-based recommendations to support UK clinicians in management of systemic sclerosis across the whole life course.
Target audience
The target readership is clinicians involved in management of people with systemic sclerosis. It will also be relevant to primary care clinicians, specialist nurses and other allied health professional involved in the management of SSc, and all people with SSc.
Areas the guideline does not cover
Diagnosis, classification, and investigation of localised scleroderma (morphoea) and of ‘scleroderma-like’ conditions (e.g. scleroedema, scleromyxeodema, fasciitis, nephrogenic systemic fibrosis) will not be considered in this guideline.
Stakeholder involvement
This project involved a multidisciplinary working group chaired by C.P.D. that included representation from SRUK, the relevant patient organisation (E.B., S.F.) as well as two people with SSc (K.F., G.P.) and rheumatologists (V.H.O., A.H., M.C., F.D.G., J.D.P., E.D.-S., J.R., M.S., M.H., A.P., K.D., B.G., M.H.B., L.G., C.C.), a paediatric rheumatologist (C.P.), dermatologist (N.OD.), respiratory physician (E.A.R.), stem cell transplant expert (J.S.), general practitioner (L.W.), specialist pharmacist (A.T.) and podiatrist/allied health professional (B.A.-P.). Three rheumatology clinical fellows were also included in the group (E.R., E.DL., N.G.). Literature reviews were carried out with expert assistance of UCL-Royal Free library services coordinated by two clinical fellows (E.R. and E.DL.) under supervision by C.P.D., V.H.O. and F.D.G. Stakeholder on the final draft guideline was solicited from a broader expert group of specialists in paediatric systemic sclerosis and adult cardiology and pulmonary hypertension (see Acknowledgements section).
Rigour of development
This guideline was developed in line with the BSR Creating Clinical Guidelines Protocol using AGREEII (Appraisal of Guidelines for Research and Evaluation II) methodology. Following two virtual meetings, the scope of the guideline was agreed and published [11]. The full guideline was then developed over a series of six virtual or hybrid meetings of the full GWG, and the final draft submitted to the BSR Guidelines Steering Group for stakeholder and internal review and feedback (https://rheumatology.org.uk/news/details/New-name-for-our-Guidelines-Steering-Group).
Literature search: scope and search strategy
Where topics or questions had already been considered in the previous guideline, the literature search was from 1 January 2014. New topics did not limit the dates for literature searching. The evidence used to develop this guideline was compiled from a systematic literature review (SLR), including electronic bibliographic databases (Medline and Embase) and the Cochrane Database of Systematic Reviews up to 31 July 2023. Key terms for the topic searches were agreed in discussion with UCL Library Services and included all relevant articles published in English or in languages other than English with an English translation available. All titles and abstracts were screened by the BSR systematic literature reviewer and full papers of relevant material were obtained. The BSR guideline protocol was used with a minimum of two assessors (including E.R., E.DL.) screening the search results with oversight and additional review by senior reviewers (C.P.D., V.H.O., F.DG.). Assessment and article selection and rating was agreed using the web based Rayyan systemic review software tool [12] [https://rayyan.ai/]. The literature review group prepared as summary of the quality of evidence following the GRADE approach (https://www.gradeworkinggroup.org/) that informed group review and discussion of the draft guideline.
Selection of key questions
Based upon review of the 2015 guideline by the GWG and a series of virtual meetings, the topics for this updated guideline were agreed by consensus and a series of PICO questions formulated that could then form the basis for literature review. The default approach was agreed as systematic literature review with recognition that there would be very limited literature or evidence for some topics despite prioritization by the GWG. In these cases, expert opinion would supplement the published literature.
Methods used to formulate recommendations
A draft document was then circulated to the full GWG for review. Each suggested recommendation in the final document was evaluated by all members and subjected to a vote relating to strength of agreement on, e.g., a scale of 1 [no agreement] to 100% [complete agreement]. The working group members then scored each recommendation on the same scale, and the mean was calculated to generate a strength of agreement (SoA) score. The wording of each recommendation was revised until all members were satisfied that they would score at least 80%.
In addition, and in accordance with the BSR protocol accompanying each recommendation in parenthesis is a statement reflecting the strength of recommendation and quality of supporting evidence.
Quality of evidence
Assessment of supporting evidence quality in GRADE reflects confidence in the estimates of benefits, harms and burdens. This guideline uses three levels and a letter (A, B, C) to reflect high, moderate or low/very low quality of evidence.
Strength of the recommendation
A strong recommendation to offer (or not to offer) something, where the benefits clearly outweigh the risks (or vice versa) for nearly all people with SSc, is denoted by the number 1 in the guideline. A conditional recommendation (to consider or not) is made either when the risks and benefits are more closely balanced or are more uncertain and is denoted by the number 2 in this guideline.
Therefore, detailed in parentheses, for each recommendation statement is a summary of:
strength of recommendation (1 or 2);
quality of supporting evidence/level of evidence (A, B, C); and
strength of agreement (SoA) score across the GWG (percentage).
Plan for review
The planned review date for this guideline will be in five years’ time. It is anticipated that important interim changes will be updated on the BSR website.
Guideline structure
The guideline builds on the previous recommendations for management and over four main themes that encompass the general approach to SSc, management of specific complications of the disease and organisation and delivery of excellent care within NHS, including suggestions for service specification and audit evaluation. The guideline comprises four subtopics listed below.
Early diagnosis, classification and stratification of risk.
Global management of systemic sclerosis.
Treatment of organ-based complications of systemic sclerosis: pharmacological and non-pharmacological.
Organisation of services for systemic sclerosis within NHS including paediatric services and transition of paediatric people into adult services.
Early diagnosis, classification and stratification of risk
Early accurate diagnosis is important so that treatment can be given, education provided and risk of complications assessed. There is evidence that detection and diagnosis of SSc is often delayed [13]. This may reflect the non-specific nature of many of the early symptoms [Raynaud’s phenomenon (RP), reflux oesophagitis, fatigue, arthralgia, peripheral oedema and carpal tunnel syndrome], the rarity of SSc compared with other causes of these symptoms and unavailability of more specialized assessment tools such as SSc-specific autoantibody testing and microvascular diagnostic testing (capillaroscopy) [13]. Thus, on average there is a delay of >10 years from onset of RP and over 12 months from non-Raynaud symptom onset to diagnosis. The working group considered that delays in diagnosis should be minimized, and patient organization initiatives can help [14]. Although not the most frequent disease subset, diffuse cutaneous SSc is a particularly important diagnosis because of the early risk of severe internal organ complications and need for specialist referral and initiation of disease-modifying treatment with immunosuppression. Risk markers for poor outcome include diffuse skin involvement, elevated acute phase markers and presence of certain SSc-associated antinuclear antibodies [5]. In addition, there are emerging new approaches to classification of SSc that may permit more precise or stratified approaches to investigation and treatment that will underpin individualized management.
Early diagnosis of SSc
The cardinal aspects of early diagnosis must incorporate history, examination and investigation. There are substantial implications for making a diagnosis of SSc and important considerations when the diagnosis is confidently excluded. There will be people where a risk of future development of SSc is identified. Making an early diagnosis includes the following:
History: Raynaud’s phenomenon (RP) provides a window of opportunity for early diagnosis although in early dcSSc where sometimes Raynaud’s onset is contemporaneous with, or even after, onset of non-Raynaud’s manifestations of SSc.
Examination: Puffy fingers.
Investigation: Includes ANA, nailfold capillaroscopy, SSc-specific autoantibodies.
Over the past few years, the concept of very early diagnosis of systemic sclerosis (VEDOSS) has emerged. This is important because emerging data suggest that significant complications of the disease may already be developing at this stage. It is notable that cases with all the ‘red flag’ VEDOSS features of RP, puffy fingers, ANA, SSc-specific antibody, abnormal nailfold capillaroscopy would already fulfil 2013 EULAR/ACR Classification criteria for SSc [15] but those with incomplete features have a high probability of progression to SSc [16].
Nailfold capillaroscopy is central to diagnosis, although a recent survey suggested 41% of UK rheumatologists do not access capillaroscopy on site and 27% do not use capillaroscopy at all [17].
Early onset features of SSc in children are much less defined and might be influenced by distinct features of jSSc such as greater frequency of overlap manifestations and different ANA profiles form adult-onset SSc. Expertise in paediatric nailfold capillaroscopy is limited with a UK survey showing 14% of paediatric rheumatologists did not undertake capillaroscopy [18]. Additionally, normative values for children are not yet established [19]. Assessment of nailfold capillaries is central to early diagnosis and differentiating primary from secondary RP and may be undertaken using a dermatoscope, or low-cost USB microscope if more sophisticated capillaroscopy is not available.
In considering early diagnosis, cases of SSc that present with organ-based complications without pre-existing diagnosis of SSc are important. These are seen in PH, ILD and gastroenterology clinics as well as in acute medicine and nephrology for cases of scleroderma renal crisis (SRC), where up to 20% may not have a pre-existing diagnosis. Classification criteria for SRC are being developed that will also assist with earlier diagnosis of SSc in these cases [20].
Current approach to classification and risk stratification
Subset and stage of SSc is relevant for management. Differentiation of limited or diffuse cutaneous subsets based upon extent of skin involvement has been central to management historically. While this classification has some practical value, it is now recognized that other factors should be considered in global management, and that subset independent factors also should be considered. Thus, an outcome-based classification incorporating skin subset and ANA subgroup has been proposed [5]. This reflects the current approach used in many SSc centres. Importantly, it highlights that risk of certain complications crosses skin subset boundaries and may be more linked to ANA specificity. Unbiased data-driven approaches reinforce the need for individualized patient assessment beyond skin subset [21]. Modern molecular analytic methods are being developed to help with classification in the future and may lead to better targeting of future therapies and more precise risk stratification [22, 23].
From a paediatric rheumatology perspective regarding diagnosis and classification of juvenile onset SSc, an international study is currently validating the 2013 EULAR/ACR classification criteria in jSSc. Until this work is complete, either the 2013 EULAR/ACR classification criteria or the PRES/ACR/EULAR provisional classification criteria for jSSc can be used [24].
What is the best approach to early diagnosis and classification?
Guideline recommendation for diagnosis and classification of SSc:
Clinical diagnosis of SSc should be guided by validated classification criteria (1A, 96%).
Skin subset and SSc-associated autoantibody subset should be used to stratify for risk of specific organ-based complications (1B, 97%).
Assessment of nailfold microcirculation (capillaroscopy) should be performed as part of initial SSc assessment and when a diagnosis of SSc is suspected (2C, 96%).
Global management of systemic sclerosis
Overarching principles of management of all people with SSc
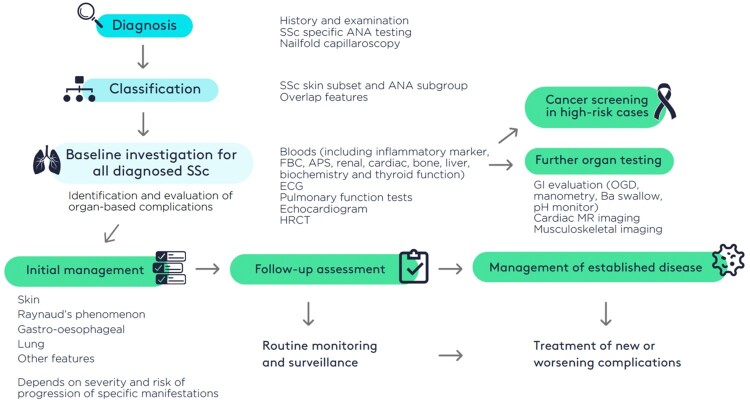
Systemic sclerosis is heterogeneous and can be classified as outlined above into subsets based upon the extent of skin involvement. The major subsets are diffuse and limited cutaneous SSc. Symptomatic management is required in all cases together with investigation and treatment of specific complications [3]. It is an important principle that any of the organ-based complications may develop in all subsets including overlap SSc and SSc sine scleroderma. The principles of management are outlined in Fig. 1. Diffuse cutaneous SSc is a particularly important subgroup as these cases should be assessed comprehensively and urgently due to the risk of early severe or progressive organ-based complications and because cases should be managed in collaboration with an expert SSc centre. Cases of limited cutaneous SSc often develop major organ-based complications that should be managed as outlined in the third topic of this guideline [5].
Figure 1.
Overarching principles for management of systemic sclerosis
This guideline considers all age groups that may develop SSc including juvenile onset disease (jSSc) defined as <18 years at onset of the first non-Raynaud manifestation [7]. These cases will be managed by paediatric rheumatologist multidisciplinary teams along with organ-based specialist paediatricians. Transitional care into adult services and long-term management of adults with juvenile onset SSc are also considered. An overarching goal is to comment on where adult recommendations may be applied to those with juvenile onset SSc at all life stages. Progression of disease, particularly as defined by subset, is less well studied in juvenile SSc but does show differences compared with adult-onset disease [25].
General management of early diffuse cutaneous SSc
What are the best treatments for early diffuse cutaneous SSc?
Early-stage diffuse cutaneous SSc deserves particular attention because of the high risk of early progression and development of organ-based complications and because treatment interventions may be more effective at this stage, the group consider that separating this stage and subset out from others is no longer central to overall SSc management. This is highlighted in Fig. 1. The concept of early SSc differs according to disease subset, but for dcSSc the first 3 years from onset of first non-RP manifestation has been used. In lcSSc there is more consistent change over time, but 5–7 years has been used operationally. However, if first non-RP manifestation is considered there may be less difference between accrual of organ-based disease between subsets and timing or development of complications such as ILD may be similar for high-risk ANA subgroups such as anti-Scl-70 [26].
Guideline recommendations for treatment of early diffuse SSc:
Early dcSSc is defined by disease duration from first non-RP manifestation of less than 3 years, although cases may show improvement in skin from 18 months, and some have clinical features of skin worsening and progression over more than 5 years (2C, 94%).
All early dcSSc cases should be considered for immunosuppression with MMF as treatment for skin fibrosis. Alternatively, methotrexate may be used for skin fibrosis (1C, 96%).
Multi-disciplinary and multi-speciality care should be available. All early dcSSc cases should be assessed in a specialist centre for consideration of clinical trials and specialised treatments including biological agents such as rituximab or tocilizumab and autologous haematopoietic stem cell transplant (AHSCT) (2B, 97%).
All paediatric SSc should be managed in a tertiary paediatric rheumatology service with multi-disciplinary and multi-speciality input (1C, 99%).
Cancer and screening for malignancy in SSc
When and how should people with SSc be screened for malignant disease?
After cardiac and pulmonary disease, cancer accounts for the highest proportion of deaths in SSc (∼16%), exceeding deaths attributed to renal disease and/or infection [27]. Registry analyses have identified a history of cancer in 7.1–14.2% of people with SSc, with the most commonly occurring cancers being breast, haematological, skin and lung cancer [28, 29]. Several studies have reported the close temporal relationship between SSc and cancer, particularly in the context of ARA and ATA [28, 30]. Overexpression of mutated forms of RNA polymerase in tumour tissue has provided an attractive mechanistic link between cancer occurrence, autoimmunity and the development of SSc [31]. The overall burden of cancer may also be greater in SSc, with relative risks (RR) ranging from 1.55–2.15 [30, 32].
The safety, efficacy and cost-effectiveness of malignancy screening in SSc has not yet been confirmed in robust studies and so evidence is limited. Nonetheless, clinicians should be aware of the potential increased risk of cancer in SSc and ensure people are up to date with age-specific cancer screening (e.g. mammography, faecal occult blood testing). Moreover, a detailed systems review should include enquiry around possible neoplastic symptoms (constitutional and organ-specific) which should be actively followed up with necessary investigation where relevant, particularly in ‘high risk’ situations such as newly diagnosed dcSSc in the context of anti-RNA Pol III antibodies. Lung malignancy is a particular concern in later stage interstitial lung disease, where diagnosis can also be difficult or delayed in the context of a background of interstitial lung disease on cross-sectional imaging. Recommended baseline cross-sectional CT imaging in all people with SSc should identify lung tumours or nodules that require further protocolized follow-up. Barrett’s metaplasia in the oesophagus if a frequent finding in SSc and alongside other recognized risk factors should direct follow-up OGD. There is currently no evidence that ARA positivity is a risk for malignancy in children with SSc and cancer screening is not routinely recommended.
Guideline recommendation for cancer screening in adults with SSc:
Cases over 65 years or with a clinical phenotype of paraneoplastic SSc [overlap dermatomyositis; anti-RNA polymerase III (ARA), palmar fibrosis; red flag symptoms of malignancy] should have baseline screening with breast examination, lymphoreticular assessment, faecal immunochemical testing (FIT) and endoscopy if indicated (2C, 95%).
In addition, chest, abdomen and pelvis (CAP) CT scan with contrast, and/or 18F-FDG PET/CT scanning should be considered on an individual basis (2C, 95%).
Follow-up screening should be guided by clinical suspicion and in high-risk cases with history of Barrett’s oesophagus or previous treatment with high cumulative dose of cyclophosphamide (2C, 94%).
Treatment of organ-based complications of systemic sclerosis
Key therapies and treatment of organ-based disease in SSc
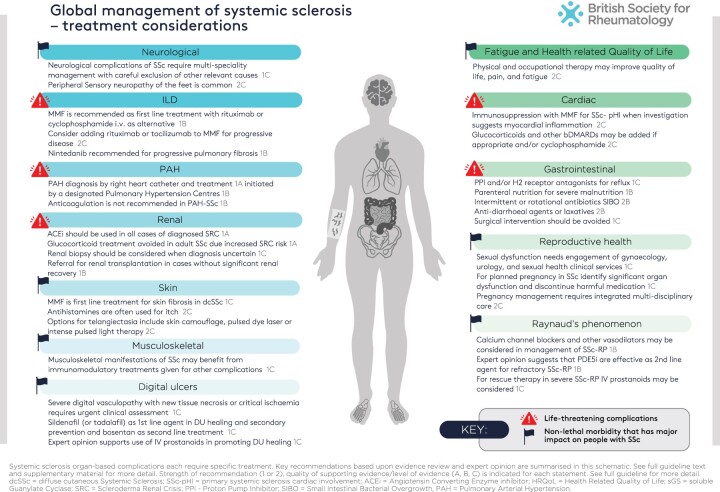
For all people diagnosed with SSc, there should be a focus on identification and treatment of specific complications and organ-based disease. In considering management, it is important to recognise that many of the very troublesome symptoms of SSc are treatable but despite this the burden of disease can be considerable. Some aspects such as fatigue, calcinosis and functional impact of skin and musculoskeletal disease are hard to quantify and manage and these, together with gastrointestinal tract complications, are frequently identified as the most difficult aspects of SSc. The following section provides a summary of guidance for managing common aspects of SSc that are summarised in Fig. 2. The grade of recommendation and level of evidence is considered for each topic. The topics are informed by key questions defined during the scoping of this guideline and results of associated systematic literature reviews.
Figure 2.
Global management of systemic sclerosis: treatment considerations
Cardio-pulmonary complications
Cardio-pulmonary manifestations are critical in managing SSc, accounting for most SSc-related deaths as well as major morbidity. There has been progress in management of all three aspects since the previous BSR guideline. In the area of ILD, there are now approved therapies supported by robust clinical trials. There has also been progress in understanding classification and diagnosis of SSc-ILD although many findings inform the research agenda. Evidence-based recommendations have been published by organisations including the American Thoracic Society (ATS) [33]. For PH there have been more trials and studies that definitively show improved outcome and survival for PAH in SSc. Other forms of PH have also been considered in expert and evidence-based recommendations published by ERS/ESC and from WSPH meeting proceedings [34]. Cardiac SSc is at an earlier stage of understanding but there has been progress in managing cardiac failure that can be directly extrapolated to SSc. Group projects have started to better define primary SSc cardiac disease and refine the recommendations included in the previous BSR guideline.
Interstitial lung disease (lung fibrosis)
What is the best management for interstitial lung disease in SSc?
At the time of publication of the last BSR guideline, only cyclophosphamide was an evidence-based treatment for SSc-ILD. The SLSII trial [35] showed that mycophenolate mofetil was equally effective to cyclophosphamide but much better tolerated. Although generally well tolerated, experts agree that MMF is teratogenic and requires double barrier contraception. In people with SSc wishing to have a planned pregnancy, alternatives such as azathioprine are to be considered. Beyond these immunosuppressive treatments, nintedanib, a tyrosine kinase inhibitor, is an oral antifibrotic drug first used in IPF that is now approved in UK for progressive phenotype SSc-ILD, based on the results of the INBUILD trial that included non-IPF progressive pulmonary fibrosis despite optimal management [36]. Nintedanib was also shown to reduce the rate of decline in FVC in a robust phase 3 trial in SSc-ILD with at least 10% extent of fibrosis on CT, although progression was not required for entry into the trial [37]. A post hoc analysis suggested there may be an additive benefit of MMF and Nintedanib in slowing down FVC decline [38]. Nintedanib has received endorsement from a positive NICE Health Technology Appraisal, where progressive pulmonary fibrosis over the previous 24 months is defined as a drop in FVC by at least 10% or decline in FVC between 5% and 10% and worsening in symptoms or CT or worsening on CT and worsening symptoms. Nintedanib can be associated with appetite loss, GI symptoms and weight loss. People should be informed to monitor their weight and inform their healthcare team if significant weight loss occurs. In adults the dose can be reduced from 150 mg twice daily to 100 mg twice daily if side effects are not tolerated. Loperamide can be used to treat diarrhoea caused by nintedanib. In general immunosuppression (e.g. MMF) treatment is optimised prior to starting nintedanib.
Smoking cessation is also important in the management of SSc-ILD both in terms of preserving lung health but also because smoking reduces the efficacy of nintedanib.
Tocilizumab, an IL6 receptor blocking biologic, is approved in North America by the Food and Drug Administration (FDA) based on post hoc analysis of two clinical trials that included SSc-ILD [39]. Rituximab was also found to have a beneficial impact on lung function and symptoms in a small RCT focused on SSc (DESIRES) [40] and in a larger trial including SSc-ILD, mixed connective tissue disease and myositis associated ILD (RECITAL) [41]. A recent trial suggested that rituximab combined with MMF may be superior to MMF alone in a mixed cohort of NSIP [42]. Experts consider that AHSCT may be considered for refractory cases [43].
It is recommended that all adults with SSc should be screened at diagnosis for the presence of interstitial lung disease by HRCT and pulmonary function testing. This is important as the PFT can then be used more reliably for longer-term follow-up and cases of early ILD will not be missed.
Consensus guidelines for jSSc recommend HRCT and PFT as sensitive tests to detect presence and severity of jSSc-ILD and repeat PFT at least 6 monthly are recommended [44]. PFT with diffusion capacity can be performed from around age 6–7 years in most children. FVC under-performs in detection of ILD in jSSc and can miss up to 60% of cases. Sensitivity is improved with addition of DLco but could still miss jSSc-ILD and thus the results of this study support HRCT in all jSSc at baseline [45]. Apart from a dose-response RCT of nintedanib in childhood ILD (including seven with SSc-ILD), there have been no paediatric studies in jSSc-ILD. Like adults, GI symptoms were the most common adverse events with nintedanib. However, paediatric consensus and best practice recommendations agree that where paediatric specific evidence is lacking, evidence can be extrapolated from adult trials where paediatric dosing and safety for treatments exists from other conditions [44, 46].
Guideline recommendation for screening and monitoring of SSc-ILD:
All SSc cases should be screened for ILD with baseline chest HRCT and PFTs (including spirometry and gas transfer) (1B, 97%).
In confirmed SSc-ILD, PFTs should be repeated every 3–6 months in recently diagnosed SSc (first 3–5 years) and considered every 6–12 months thereafter (1B, 96%).
Chest HRCT should be repeated to evaluate ILD progression if worsening symptoms/PFTs and to exclude alternative causes of worsening. Consider repeating chest HRCT to compare with baseline after 1–3 years, or pre-treatment changes (2B, 97%).
Guideline recommendation for treatment of SSc-ILD:
Treatment is determined by risk factors associated with extensive or progressive ILD including recent SSc diagnosis, diffuse skin disease, raised inflammatory markers, ATA positive, CT extent and lung function impairment together with longitudinal behaviour. Informed choice should be considered in selecting treatment (1B, 99%).
MMF is recommended as first-line treatment. Rituximab and/or cyclophosphamide by i.v. infusion may be used as an alternative (1B, 97%).
Consider tocilizumab as first-line treatment in early dcSSc with raised inflammatory markers and ATA positivity, independent of the extent of ILD on CT (1A, 92%).
Consider adding rituximab or tocilizumab to background treatment with MMF or other immunosuppressant, as rescue immunomodulatory therapy (2C, 96%).
Nintedanib is recommended in progressive pulmonary fibrosis despite immunosuppressant treatment, dependent on tolerability and may be considered as first-line treatment in combination with MMF in extensive fibrosis (1B, 98%).
Consider reducing/stopping immunosuppression in severe fibrosis experiencing recurrent infections particularly if elderly/frail. Consider nintedanib as sole treatment in extensive fibrosis (extensive traction bronchiectasis/bronchial dilatation and/or honeycombing) and with recurrent infections on immunosuppressants (2C, 95%).
Supportive treatment is important including pulmonary rehabilitation and management of infection, gastro-oesophageal reflux, nutrition, resting hypoxaemia or severe exertional hypoxaemia (long term oxygen and/or ambulatory oxygen therapy) (1C, 99%).
Vaccination against SARS-CoV-2, influenza, Streptococcus pneumoniae and herpes zoster (using a non-live vaccine) is recommended and consider antibiotic prophylaxis for recurrent infections and Pneumocystis jirovecii pneumonia (PJP) prevention, especially on RTX and with combination immunosuppression (2B, 99%).
Referral for lung transplantation is appropriate in some cases although comorbidity, particularly oesophageal involvement, may limit eligibility (2C, 96%).
Pulmonary hypertension
What is the best management for pulmonary hypertension in SSc?
Routine investigation and screening for the presence of pulmonary hypertension is a cornerstone of management for SSc [47]. Pulmonary hypertension (PH) is defined by a mean pulmonary arterial pressure (mPAP) >20 mmHg at rest [48]. In SSc, pulmonary hypertension develops in 1–2% per year of follow-up [49]. It can be due to pre-capillary pulmonary vasculopathy PAH (group 1) or be secondary to lung complications, especially SSc-ILD, when it is classified as group 3, or it may result from left heart disease, leading to post-capillary PH (group 2), confirmed at RHC by PAWP >15 mm. In addition, thromboembolic PH can occur (group 4) and studies have shown intravascular thrombosis in the context of PAH. In the UK, these therapies are approved for the treatment of PAH-SSc provided specified treatment criteria defined by the NHS are fulfilled. The process varies slightly between the devolved nations. In SSc-PAH anticoagulation is not recommended, unless CTEPH is diagnosed [50]. It is important to recognise that PH may also be mixed in origin and that this can represent a therapeutic and investigational challenge. Evidence supporting use of therapies outside PAH is more limited. Observational cohort studies have confirmed improved survival from SSc-PAH but not from PH due to lung disease in SSc. It can be difficult to discern whether in SSc-ILD the PH is proportionate to the severity of the ILD or whether it is out of keeping with the extent of ILD, in which case anti-PAH drugs may be considered. Furthermore, inhaled treprostinil was the first agent shown to be effective in fibrotic ILD-associated PH, although CTD-ILD was excluded from this trial [51]. Whether similar benefits are seen in SSc-ILD with associated PH remains to be established. Finding effective treatments for SSc-ILD-associated PH is crucial as this group has the highest mortality. Studies also confirm that precapillary PH likely develops slowly over several years in many cases but that current approaches for screening and diagnosis are limited by intrinsic variability in assessment techniques [52]. There is a need for improved evidence-based screening and detection especially for PAH as the risk of development is comparable to people with a susceptibility allele for familial PAH and so there is the possibility of earlier detection and treatment for SSc-PAH compared with idiopathic PAH. The DETECT tool may be used in adults with DLco <60% predicted and >3 years disease duration to identify need for echocardiography and right heart catheterisation [53]. Sometimes, people with SSc-PAH will also have other manifestations of SSc, such as interstitial lung disease, or features of overlap CTD, such as SLE. Treatment of the underlying condition according to current guidelines—e.g. with intravenous cyclophosphamide, rituximab or MMF—is recommended [54]. In the case of concomitant ILD, if the PH is considered disproportionate to the severity of ILD (as judged by extent of ILD on CT, DLco markedly reduced compared with FVC), treatment with PAH therapies can be considered.
In jSSc, the rates of pulmonary hypertension are poorly defined. Evaluation and treatment of PH is extrapolated from other causes of PH in children and from adult PAH guidelines. Highly specialised management and treatment is essential. There is a single NHSE designated specialist centre for childhood onset pulmonary hypertension at Great Ormond Street Hospital.
Guideline recommendation for screening and management of SSc-PAH:
Screening for PAH should be undertaken in all people with SSc on an annual basis. This would typically comprise pulmonary function tests, echocardiography, NTproBNP and use of the DETECT tool in appropriate people (1B, 98%).
In children, the need for RHC for diagnosis of PAH is made on a case-by-case basis. Diagnosis and treatment initiation should be through the designated paediatric Pulmonary Hypertension Centre (1C, 99%).
Several classes of drugs have shown a beneficial effect in randomized controlled trials for the treatment of Group I precapillary pulmonary arterial hypertension (PAH) with mPAP ≥25 mmHg and this is generally used as the threshold for initiation of PAH drug therapy (1A, 94%). PAH therapies should be initiated and monitored by a designated PH centre.
The following classes of drug are used to treat PAH-SSc: phosphodiesterase type 5 inhibitors (PDE5i) (tadalafil, sildenafil), endothelin receptor antagonists (ambrisentan, macitentan, bosentan), prostaglandins (e.g. inhaled iloprost, IV epoprostenol, SC or inhaled treprostinil), prostacyclin receptor agonist (selexipag), riociguat (sGC stimulator). Combining riociguat and any PDE5i is contraindicated due to risk of hypotension (1A, 99%).
For adults living in England, the diagnosis must be made by right heart catheter and treatments are initiated through one of the designated Pulmonary Hypertension Centres (see NHS England A11/S/a) and according to the national commissioning policy for targeted therapies for the treatment of PAH in adults (NHS England/A11/P/b and NHSCB/A11/P/a) (1B, 99%).
People should also receive supportive medical treatment, such as diuretic therapy, specialist input for management of arrhythmia, correction of iron deficiency, supervised exercise training, oxygen [long-term oxygen for at least 15 hours a day if they are hypoxic at rest with an arterial partial pressure of O2 <8 kPa (SaO2 <92%) and/or ambulatory oxygen if they experience exertional desaturation of SpO2 ≤88% on a six-minute walk test], vaccination against SARS-CoV-2, influenza and Streptococcus pneumoniae, contraception and pregnancy counselling for women of child-bearing age, and psychosocial support (2B, 99%).
Anticoagulation with warfarin is not recommended in SSc-PAH (1B, 98%).
SSc cardiac involvement
What is the best management for cardiac involvement in SSc?
Clinically evident cardiac involvement is associated with a poor prognosis, and a large proportion of SSc-related fatalities are attributable to cardiac causes. It is important to carefully consider non-SSc cardiac disease in all cases before focusing on potential SSc primary cardiac involvement (SSc-pHI). SSc-pHI should be considered particularly in the early stages of the disease, but it may also be present and develop throughout the disease course SSc [55]. Subclinical cardiac involvement [usually employing cardiac magnetic resonance imaging (CMR)] is commonly observed but prognostic relevance is not fully established. While the natural history and outcomes of cardiac involvement have not been fully elucidated, certain SSc features and blood-based measures are associated with higher risk for development. Although fibrosis is a central feature of SSc, clinical, imaging and pathological evidence suggests that microvascular dysfunction and myocardial inflammation are primary processes and one of the earliest features of cardiac involvement. Myocardial inflammation and fibrosis can affect the endocardium, myocardium and pericardium, explaining the varied clinical presentations. Of note, these recommendations relate to primary heart (myocardial) involvement (pHI) as opposed to right heart involvement and PAH, which are discussed elsewhere in this guideline. Recent expert consensus has defined the entity of SSc-pHI and recommended how people with SSc should be assessed [56]. These concepts and related guidance are not yet fully validated but provide a framework for improved and more consistent clinical practice. Although the published evidence base remains limited, significant insights from cardiovascular imaging (predominantly CMR) studies alongside expert opinion have recommended the following management and treatment approach for SSc-pHI [56]. A meta-analysis suggested ERA associated with increased cardiovascular events but RCTs are lacking, warranting caution in the interpretation [57]. In jSSc, cardiac involvement is recognized as an important cause for mortality and expert guidance from paediatric cardiology services is important.
In children, other causes of heart failure with reduced ejection fraction that should be excluded are genetic, metabolic, and post-inflammatory causes. As well as CMR, blood panel and genetic testing for inherited causes should be considered. Cardiomyopathy associated with jSSc should be diagnosed by paediatric multidisciplinary consensus. While the below recommendations apply to all ages, implantable devices in children have associated complications and are rarely used. Some of the medical therapies for heart failure are not used in children (e.g. SGLT2) or are in trial [ARNI (angiotensin receptor-neprilysin inhibitor)].
Guideline recommendation for screening and diagnosis of SSc-pHI:
A multi-speciality team should inform the management and treatment of possible SSc-pHI and other cardiovascular pathology should be excluded (1C, 100%).
Screening for pHI (in asymptomatic individuals) should be undertaken in all people with SSc on an annual basis. This would typically comprise ECG, ECHO and serum troponin (ideally, I or T) and NTpro BNP (or BNP in renal disease) (2C, 96%).
Where pHI is suspected, diagnostic work up should include CMR. Endomyocardial biopsy should only be considered in selected cases, after exclusion of coronary artery disease. Holter monitor should be performed to detect arrhythmic burden (2C, 96%).
Screening with CMR (or other sensitive cardiovascular imaging) may be considered in high-risk individuals (male gender, diffuse cutaneous skin subset, anti-topoisomerase I, early disease, presence of interstitial lung disease, peripheral myositis, and other inflammatory manifestations) (2C, 95%).
In jSSC with suspected pHI, a formal assessment by a paediatric cardiologist is recommended (1C, 100%).
Guideline recommendations on the use of immunosuppressive treatment of SSc-pHI:
Immunosuppression with MMF should be considered in SSc-pHI when investigation suggests myocardial inflammation. Glucocorticoids may also be added to MMF (although risk of SRC in adults should warrant caution) (2C, 95%).
Other bDMARDs (rituximab, tocilizumab,) may be added to MMF therapy if appropriate and/or cyclophosphamide (2C, 89%).
Immunosuppression with MMF may be considered for SSc-pHI with evidence of myocardial fibrosis although robust studies are lacking. Evidence of myocardial fibrosis may support additional treatment as indicated in (ii) (2C, 92%).
Guideline recommendations for heart failure with reduced ejection fraction (HFrEF) in SSc:
First exclude other aetiologies for HFrEF including coronary artery disease and perform CMR to confirm diagnosis attributable to SSc (1C, 95%).
Having excluded other aetiologies, consider immunosuppression as detailed above with observed reduction in EF (2C, 91%).
Medical therapy should be undertaken collaboratively with a heart failure team and the following treatments considered: (a) Loop diuretics (for fluid retention); (b) the ‘four pillars of heart failure’, namely: (i) beta blockers; (ii) angiotensin converting enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARB)/angiotensin II receptor-neprilysin inhibitor (ARNI); (iii) mineralocorticoid receptor antagonists (MRA); (iv) sodium glucose co-transporter-2 inhibitors (SGLT2i, such as dapagliflozin, empagliflozin). Consider increased risk of urinary tract infection with SGLT2 inhibitors and worsening of digital vasculopathy with beta blockers (1C, 98%).
Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure should be considered as per NICE Technology Appraisal Guidance [TA314] and ESC guidelines; those not fulfilling criteria may warrant discussion with the multi-disciplinary team (2C, 95%).
Calcium channel blockers may reduce the frequency of systolic heart failure in SSc with evidence of vasodilator therapy and low-dose aspirin reducing manifestations of pHI in individuals with LVEF<55% (2C, 85%).
Guideline recommendations for heart failure with preserved ejection fraction (HFpEF) in SSc:
First exclude other aetiologies for HFpEF and apply CMR to confirm diagnosis secondary to SSc (1C, 99%).
Medical therapy should be undertaken with a heart failure team and using standard treatments including diuretics for management of fluid overload (e.g. furosemide and spironolactone). SGLT2 inhibitors (dapagliflozin, empagliflozin) may improve outcome and are recommended. Increased risk of urinary tract infection with SGLT2 inhibitors should be considered in the context of immunosuppression, with appropriate counselling (1C, 95%).
Autologous haematopoietic stem cell transplantation (AHSCT) as a treatment for SSc
Which people with SSc should be considered for autologous stem cell transplantation?
AHSCT as a treatment for adult SSc has been shown to be superior to treatment with intravenous cyclophosphamide in high-quality randomized controlled trials that have been assessed in a meta-analysis and Cochrane review [58]. There is support for long-term benefit in adults from many registry studies and observational cohorts [59]. The efficacy is well shown but studies have also defined potential treatment-related toxicity and mortality and this together with the invasive and intense nature of the procedure and resource requirements limit applicability. A key outstanding question for the future will be the extent to which there is an advantage over increasingly effective standard of care immunosuppression including early use of drugs such as MMF and how to best make treatment decisions about suitability, need and timing of AHSCT. The ongoing UPSIDE clinical trial (NCT04464434) [60] will help to better define potential benefit of AHSCT as an early treatment as it introduces AHSCT as a first-line treatment for dcSSc and directly compares with intravenous cyclophosphamide and MMF together with rescue AHSCT. In this way the feasibility and efficacy of AHSCT as an initial treatment in appropriate cases will be defined, together with applicability as a rescue therapy in refractory cases. There is also ongoing exploration of the specific regimens used for mobilisation and conditioning; it is possible that reduced toxicity conditioning may further improve safety and tolerability. Therefore, basing practice on comparative trials with IV cyclophosphamide may not be appropriate to current standard of care. For example, a recent case series comparing AHSCT with RTX-MMF in combination provides an alternative comparator. At the time of writing the previous guideline there was much less evidence and experience available for review. There is now support for superiority of AHSCT compared with some other treatment regimens and it is a widely used therapy. It is, however, important to consider risk vs benefit at an individual level to make an informed decision following appropriate assessment, investigation and discussion. Furthermore, AHSCT should only be offered in centres with experienced multidisciplinary teams and an intensive care unit. AHSCT may be considered for refractory SSc-ILD although caution needs to be taken as adults with DLco below 40% are believed to have increased risk of treatment-related complications. For a summary of when to consider AHSCT in adults and recommended screening prior to AHSCT, see Boxes 1 and 2.
Box 1.
Considerations in selecting the most appropriate cases to be considered for AHSCT and situations where the risk of the procedure is likely to exceed benefit and so AHSCT is not recommended.
When to consider AHSCT in adults
People with:
early diffuse cutaneous SSc;
rapidly progressive skin involvement and/or kidney, cardiac or lung involvement;
younger than 65 years.
Because of increased treatment-related risks, AHSCT is not recommended in people with:
severe ILD with DLco % of predicted below 40%;
decreased cardiac function with left ventricular ejection fraction below 45%;
pulmonary hypertension;
active/uncontrolled scleroderma renal crisis;
poor kidney function, creatinine clearance <40 ml/min;
poor liver function (sustained 3-fold increase in serum transaminase or bilirubin);
active/uncontrolled infection;
untreated severe arrhythmia;
bone marrow insufficiency;
concurrent neoplasms.
Box 2.
Investigations that should be performed during work-up to assess treatment-associated risk of AHSCT. Comprehensive cardiopulmonary assessment is paramount.
Recommended screening prior to AHSCT
Cardiac function tests
Electrocardiography and 24-hour Holter (in case of abnormal ECG or palpitations).
NT ProBNP, high sensitivity troponin, to monitor cardiac stress during the procedure.
Cardiac echo.
Right heart catheterisation with fluid challenge.
Cardiac MR with contrast.
Pulmonary function test
Lung function with FVC and diffusion capacity (DLco).
HRCT chest.
General
Liver and kidney function.
Screen for infections.
Endoscopy in case of anaemia or history of GAVE.
Trials to firmly place AHSCT in the treatment pathway are ongoing (e.g. UPSIDE) and the procedural aspects of this treatment are also being carefully reviewed and considered. There is currently robust evidence to support many of the statements made below and collective expert opinion related to AHSCT in SSc. Management of post-AHSCT relapse should be considered and is currently explored in ongoing studies.
Expert opinion is that AHSCT can be considered in selected cases of severe or progressive jSSc as there is some case-based positive experience. The possibility of remodelling and recovery of damage may be higher in jSSc. A current clinical trial, Autologous Stem Cell Transplantation with CD34-Selected Peripheral Blood Stem Cells (PBSC) in Treatment Resistant Systemic Sclerosis (SSc) (ClinicalTrials.gov Identifier: NCT03630211) has included seven jSSc, four of whom would not have met the inclusion criteria for previous adult-based RCTs, the main reason being an FVC and/or DLco lower than typical adult SSc AHSCT criteria. The possibility of remodelling and recovery of damage may be higher in jSSc allowing them to tolerate AHSCT regimen and gain clinically significant recovery of musculoskeletal function and composite ADL function. AHSCT in young people with SSc needs specialist consideration within a paediatric multidisciplinary expert centre and delivery in a centre with expertise in both jSSc and AHSCT for autoimmune disease.
Guideline recommendation for AHSCT in SSc:
AHSCT may be considered in selected diffuse cutaneous systemic sclerosis (dcSSc) where benefit is likely to be greater than treatment-related risk. Severe internal organ disease precludes AHSCT and should be carefully evaluated before considering this treatment in adults (1B, 96%).
AHSCT should be delivered within an experienced specialized centre for both adults and children (1B, 98%).
Use of AHSCT in adults with later-stage dcSSc and in lcSSc requires further data and is not recommended (2C, 90%).
AHSCT may be considered in children and young people with SSc who have severe or refractory disease, regardless of disease subset (2C, 92%).
Digital vasculopathy
Digital vasculopathy leads to Raynaud’s and digital ischaemia with development of complications of severe vasculopathy including gangrene, ulceration and infection of superficial and deep tissues [61]. Management of Raynaud’s is central to the symptomatic treatment of SSc. Since 2015 there have been several studies exploring new treatments, but these have not led to new approved therapies. The challenge of outcome assessment in RP is recognized and new outcome measures are currently being validated. Digital ulceration almost always reflects vasculopathy even when there are other contributory factors such as calcinosis or trauma. Treatment in adults is supported by clinical trial evidence including two robust trials showing significant benefit for the non-selective endothelin antagonist bosentan in reducing the number of new DU in adults with SSc [62]. Digital ulcers and severe ischaemia require urgent prioritized management and establishment of ‘hot clinics’ analogous to those for giant cell arteritis may be considered. Benefit from the selective phosphodiesterase inhibitor (PDE5i) tadalafil is considered by experts to be similar to sildenafil and may be considered as an alternative in those who are having difficulty with sildenafil. Experts agree that PDE5i and bosentan may be used in combination in severe cases, especially those being considered for intravenous prostanoids. Despite a lack of paediatric-specific trials, in children and adolescents similar approaches are used and are recommended by international paediatric consensus [44, 46]. However, it is important to consider the availability of paediatric dosing and safety data for specific therapies, e.g. currently available for sildenafil but not for tadalafil.
Raynaud’s phenomenon (RP) is the most common disease-specific manifestation of SSc. Virtually all people with SSc will experience cold sensitivity and exhibit other features of digital vascular compromise [63].
Self-management in the form of cold avoidance and effective measures to promote re-warming forms the mainstay of management. Most people with SSc can predict the occurrence of RP symptoms based on relevant environmental and/or contextual factors. Many people with SSc become adept at managing their symptoms, although this can be at the expense of social participation or rely on the support of others [64]. Smoking is associated with worse digital vascular outcomes and support should be made available to achieve smoking cessation, including nicotine replacement therapy.
Pharmacological intervention should be considered for RP symptoms inadequately controlled through self-management. Registry analyses have identified wide variation in prescribing practices for SSc and indicate clinicians are not fully exploiting the range of therapeutic options available to them [65, 66]. Many pharmacological interventions have been assessed in RP and a recent network meta-analysis suggests the efficacy of treatments for SSc-RP are modest at best [67]. Potential side effects common to all treatments designed to promote vasodilation include headaches (which often regress with repeated dosing) and hypotensive symptoms. Low resting blood pressure may influence the choice of treatment. It is prudent to commence a low dose of any vasoactive treatment for RP and increase gradually depending on tolerability and efficacy. Calcium channel blockers (CCBs) are generally considered the first line [68]. Selective phosphodiesterase (PDE5i) inhibitors are increasingly being positioned as a second-line treatment for SSc-RP [69], and their use has been supported by the falling cost of generic non-proprietary brands. Such treatments may exert benefits on other aspects of SSc. Registry evidence has suggested treatments for RP such as CCBs may reduce the likelihood of developing PAH and left ventricular dysfunction in SSc [70].
Several complementary treatments including low-level light therapy, antioxidants, gingko biloba, acupuncture, L-arginine and essential fatty acids have been studied, but their impact on RP severity has been modest at best [71]. Similarly, there is a strong therapeutic rationale for anti-platelet, anticoagulant therapy, and statins for SSc-related digital vasculopathy, and while the evidence base for such interventions is not strong, they have been proposed as useful adjunct therapies for refractory RP in SSc. Anti-platelet agents may also prevent new DU occurrence [72]. Botulinum toxin has an attractive therapeutic rationale, and may avert unwanted systemic vasodilating adverse effects, but is expensive to administer and the trial results have been conflicting [73]. Surgical intervention is seldom required for RP symptoms alone but may have a role in the context of critical digital ischaemia or refractory ulceration in SSc.
Paediatric specific consensus and best practice guidelines based on expert opinion and extrapolation from adult studies also recommend CCBs as first line and PDE5i [44, 46]. IV prostanoids are used for rescue treatment and bosentan may be considered, but local expert guidance should be sought and dosing in children should be informed by use in PAH, including availability of smaller tablet and liquid formulations for appropriate dosing. Antiplatelet therapy and statins are likely to have less of a role in jSSc but could be considered, particularly in severe SSc-RP or critical ischaemia.
Digital ulcer disease in SSc
What is the best management for digital ulceration in SSc?
Guideline recommendation for digital ulcers in SSc:
Severe digital vasculopathy with new tissue necrosis or critical ischaemia is an emergency requiring urgent clinical assessment (preferably within 48 hours) (1C, 95%).
Sildenafil (or tadalafil) is recommended as first-line agent in DU healing and secondary prevention and bosentan as second-line treatment in line with NHS England policy 210302P [1911] (1C, 99%).
Intravenous prostanoids may be considered to promote DU healing (1C, 98%).
Consider anti-platelet therapy in DU disease (particularly if local necrosis) (2C, 94%).
Digital (palmar) sympathectomy (with or without botulinum toxin injection) may be considered in those intolerant to systemic vasodilator meds and recurrent DU at a single site (2C, 95%).
Debridement of DU may promote healing (2C, 93%).
There should be access to SSc/CTD specialist wound care services to prevent and treat skin ulcers (1C, 98%).
What is the best management for Raynaud’s phenomenon?
Guideline recommendation for RP in SSc:
Although therapeutic benefits appear modest, calcium channel blockers and other vasodilators should be considered in management of SSc-RP (1B, 100%).
Phosphodiesterase 5 inhibitors (PDE5i such as sildenafil, tadalafil) and intravenous prostanoids are effective as second-line agents for refractory SSc-RP (1B, 99%).
Consider anti-platelet therapy (aspirin, clopidogrel) and statins in refractory SSc-RP given strong therapeutic rationale despite limited evidence (2C, 93%).
For rescue therapy in severe SSc-RP IV, prostanoids may be considered (1C, 99%).
Digital (palmar) sympathectomy (with or without botulinum toxin injection) which may be considered in severe and/or refractory cases of SSc-RP, particularly if systemic vasodilator therapies are poorly tolerated, e.g. low basal BP (2C, 93%).
Gastrointestinal tract disease
What is the best management for gastrointestinal complications of SSc?
Gastrointestinal tract manifestations are the most frequent organ-based complication of SSc. The GI symptoms are also consistently reported as some of the most burdensome by people with SSc, significantly impacting HRQoL in both adult and children and can be extraordinarily difficult to manage [74]. While some drugs are effective in milder cases such as acid suppressive treatments for reflux oesophagitis, dysmotility and anorectal disease leading to incontinence are much more intractable [75]. People with SSc may nevertheless benefit substantially from treatment approaches used in other medical contexts and incorporation of expert gastroenterology approaches and where necessary GI surgical expertise can be transformative. It is critical to consider nutritional aspects of SSc and recognise that many people have difficulties with adequate oral nutrition and that dietary adjustments may significantly benefit GI and other aspects of the disease [76].
Nutritional management is particularly important in the growing/developing child with jSSc. Growth should be routinely monitored in clinic and proactively managed, including the use of nutritional supplements where indicated, in consultation with a paediatric dietician. Liaison with a paediatric gastroenterologist is recommended to guide investigation and management of GI symptoms in jSSc.
The following recommendations represent components of current best practice approaches for GI SSc. Despite the benefit as first-line treatment of small intestinal bacterial overgrowth, experts agree that fluoroquinolone treatment should be discontinued at the first signs of a serious adverse reaction, including tendon pain or inflammation.
Guideline recommendation for gastrointestinal manifestations in SSc:
The following therapeutic approaches and drugs are considered by experts to be of value in treatment of GI tract complications of SSc.
Optimise and ensure compliance to general/lifestyle measures in SSc with oesophageal symptoms (e.g. gastro-oesophageal reflux) (1C, 99%).
Proton pump inhibitors and/or histamine H2 receptor antagonists are recommended for treatment of symptomatic gastro-oesophageal reflux and dysphagia (1C, 99%).
Promotility agents including prokinetic dopamine antagonists may be used for dysphagia and reflux (1C, 98%).
In refractory gastro-oesophageal reflux disease, consider examination of upper gastrointestinal tract structure and motility, and confirmation of acid reflux (e.g. pH testing) (2C, 96%).
Parenteral nutrition should be considered for those with severe weight loss and/or malnutrition (including high-risk), which is refractory to enteral supplementation (1B, 97%).
In jSSc, nutrition, growth and pubertal status should be actively assessed, monitored and pro-actively managed if faltering growth is noted, which includes liaison with tertiary paediatric gastroenterology and paediatric dietetic expertise (1C, 99%).
Intermittent broad-spectrum oral antibiotics (e.g. ciprofloxacin) are recommended for symptomatic small intestinal bacterial overgrowth, and rotational regimes may be helpful. Rifaximin may be an effective alternative in refractory cases (2B, 95%).
Anti-diarrhoeal agents (e.g. loperamide) or laxatives may be used for symptomatic management of diarrhoea or constipation, which often alternate as clinical problems, and non-SSc causes should be excluded (1C, 96%).
Surgical intervention for gastrointestinal complications of SSc should generally only be considered when essential and no alternative (1C, 97%).
Pelvic floor physiotherapy including anorectal biofeedback training may be considered in selected cases with incontinence (2C, 91%).
Renal complications
What is the best management for scleroderma renal crisis?
While the most serious renal complication of SSc is scleroderma renal crisis, it is important to consider the broader impact and management of renal disease in SSc. Renal crisis is appropriately described as thrombotic microangiopathy with AKI in the context of systemic sclerosis, generally associated with significant new onset hypertension [20]. There are similarities with other forms of TMA. However, it is important to consider longer-term post-SRC management that included management of residual CKD after acute treatment as well as acute management of SRC. The cornerstone of acute management remains prompt initiation of angiotensin-converting enzyme inhibitors (ACEi). Timely diagnosis of SRC is critical and this is facilitated by appreciation of the risk phenotypes including ARA positivity, early diffuse, active disease with TFR and elevated ESR, prior high dose steroids, proteinuria and hypertension. In adults, there should be caution in using prednisolone at doses above 10 mg prednisolone equivalent and calcineurin antagonists [77, 78]. Education in high-risk cases in adults should be undertaken including self-monitoring of BP in early-stage disease.
In addition to SRC, CKD appears to be a significant prognostic factor for long-term outcome in SSc and may reflect broader renal and vascular pathology than SRC. Management of renal disease in SSc should include consideration of renal comorbidity, drug toxicity and overlap rheumatic disease such as SLE or vasculitis that may occur in SSc. It should be noted that SRC is very rare in jSSc and alternative diagnoses should be considered. Because of the rarity of SRC in jSSc, we do not know if ARA are associated with an increased risk of SRC in children. Paediatric consensus and best practice do not suggest minimising glucocorticoid treatment in jSSc due to the extreme rarity of SRC in this group and the frequent overlap features in children with jSSc, which often requires glucocorticoid treatment [44, 46].
Guideline recommendations for treatment of SRC:
ACEi should be initiated or continued in all cases of diagnosed SRC and up titrated to maximum therapeutic dose (GRADE 1A, 100%).
Other antihypertensive drugs are often required to control hypertension and can be added based on clinical need (1B, 99%).
In adults, glucocorticoid treatment should be minimized in SSc due to association with increased SRC (1A, 96%).
When required, renal replacement therapy should initially use the least haemodynamically demanding approach (e.g. haemofiltration or peritoneal dialysis) (1C, 99%).
Renal biopsy should be considered when diagnosis is uncertain (especially if substantial proteinuria, ANCA+, overlap serology SLE, etc) (1C, 99%).
Referral for renal transplantation may be considered after 12 months in cases without features suggesting significant renal recovery (1B, 99%).
Skin complications
What is the best management for non-fibrotic skin manifestations in SSc?
As well as skin thickening and fibrosis, there are many other dermatological aspects of systemic sclerosis, and these require expert management. It is essential to involve dermatologists and other health professionals in management. While the evidence base is relatively poor, there is self-evident benefit from the approaches outlined in the recommendations below.
Telangiectasia are visible permanently dilated postcapillary venules that blanch under pressure and are present in ∼80% of SSc people with SScs, second only to RP in terms of the most common disease-specific manifestation of SSc. The presence and burden of telangiectasias are associated with the presence of RHC-confirmed PAH, as well as increased estimated right ventricular systolic pressure on Doppler echocardiography [53]. There is also an independent relationship between telangiectasias and DU disease, even after correcting for relevant confounders such as disease duration and serology. Those with telangiectasias report significantly higher ‘dissatisfaction with appearance’ scores, which can lead to distress and anxiety.
For many people with SSc, the use of concealment approaches, such as make-up camouflage, provides an acceptable long-term approach to management of SSc-Tel. Attempts at eradication using ablative techniques such as injected sclerosing agents or thermocoagulation methods such as pulsed dye laser therapy would be more desirable for others. Both pulsed dye laser (PDL) and intense pulsed light (IPL) are effective treatments for telangiectasias with PDL having better outcomes in terms of appearance and IPL associated with fewer side effects [79].
Other non-fibrotic skin manifestations include itch, which can be associated with early stage dcSSc but is also a major cause of morbidity in some cases of established SSc across all disease subgroups. Treatment is symptomatic.
Fat loss and atrophic changes in the subcutaneous tissues are a feature of later stage SSc of both subsets and cause secondary cosmetic and functional effects on skin. Autologous fat transfer is beneficial and may give sustained benefit [80].
Guideline recommendation for non-fibrotic skin manifestations in SSc:
Practical approaches, maintaining adequately moisturized skin, are essential. It is strongly recommended to avoid frequent bathing with harsh deodorant soaps, and emollients should be used as soap substitutes where possible (2C, 97%).
Itch is associated with disease activity and so other disease-targeted treatment may result in improvement. Anti-pruritic moisturizers and antihistamines are often used for itch, and the sedative effects of the latter agents may be beneficial at night-time. In adults, expert opinion suggests low-dose opioid antagonists such as naloxone and naltrexone, and other options including gabapentin and pregabalin and low-dose antidepressants such as mirtazapine may be considered (2C, 89%).
Current management options for telangiectasia include (green) skin camouflage and injected sclerosing agents or thermocoagulation methods such as pulsed dye laser or intense pulsed light therapy (2C, 95%).
Consider autologous fat transfer for facial fat loss (2C, 89%).
Musculoskeletal disease, fatigue and quality of life
SSc has a major impact on daily activity. Key to this is disease-related fatigue as well as musculoskeletal complications including arthropathy, contractures, muscle weakness and debilitating mechanical musculoskeletal pain [81]. These non-lethal burdensome aspects of the disease have substantial negative impact on quality of life. In this guideline we have considered management of musculoskeletal disease. It is noted that in overlap jSSc-arthritis may be more frequent than in adult-onset SSc [8].
Musculoskeletal manifestations
What is the management for musculoskeletal manifestations of SSc?
Musculoskeletal involvement includes tendinopathy, joint contractures and, in some cases, overlap arthritis. Chronic widespread pain and associated features are not uncommon. Management advice is to consider and treat both inflammatory tendon and joint disease and non-inflammatory causes.
Guideline Recommendation for musculoskeletal manifestations in SSc:
Musculoskeletal manifestations of SSc may benefit from immunomodulatory treatments given for other complications, such as skin disease (1C, 95%).
When arthritis or myositis (or non-inflammatory musculoskeletal pain) is more severe, generally in the context of an overlap SSc syndrome, management is in line with similar clinical conditions occurring outside the context of SSc (1C, 93%).
Calcinosis in SSc
What is the management for calcinosis in SSc?
There is a very limited evidence base to guide clinicians on the management of calcinosis in SSc, but practical approaches are considered important to mitigate impact. This is a key area for the research agenda (see below).
Guideline Recommendation for calcinosis in SSc:
Superadded infection of calcinosis should be recognized early and treated with appropriate antibiotic therapy (1C, 99%).
Surgical intervention should be considered in severe, refractory calcinosis, which is severely impacting upon functional ability and quality of life (1C, 95%).
Fatigue, and quality of life
What are the best interventions for general impact of SSc on health status and quality of life, including fatigue?
Fatigue is a major unmet need that is generally managed as for other disorders [82]. While evidence of benefit for exercise specific to SSc is not available, this is generally encouraged. Treatments that target inflammation may also improve symptoms although this has not been clearly shown in trials. Quality of life measures are routinely assessed in interventional studies, and some have shown improvement but in such a multicompartment disease that includes damage that is very hard to reverse it is challenging to significantly improve standard measures. It was recognised that self-help information and resources are important for people with SSc. These are often available from support organisations and can include valuable guidance. In addition, the psychological impact of SSc is considered important and initiatives to support self-management as well as structured psychological assessment and intervention has relevance when considering disease impact on everyday life and function as outlined below.
Quality of life and non-pharmacological treatment
Health-related quality of life (HRQoL) refers to functioning and well-being in physical, emotional and social domains. SSc has a major negative impact on HRQoL and is associated with fatigue and anxiety and depression [81].
Impaired HRQoL spans disease groups but those with diffuse disease are most severely affected. In SSc the key clinical burdens contributing to worsening of HRQoL over time include digital ulcers, Raynaud’s phenomenon and gastrointestinal involvement. Children with jSSc have more disability than children with other rheumatic diseases with GI involvement having the greatest impact on quality of life [8]. The impact on education, peer and family relationships, mental health and social activities should be explored in jSSc with a multidisciplinary approach to support individual needs. It is important to encourage physical activity and consider occupational and physical therapy to maintain range of motion in a growing child.
There are several valid measures of HRQoL and function that can be used, including HAQ-DI/CHAQ-DI, UK Functional Score, SF-36, ScleroID, PCS and PROMIS physical functioning domain [81]. Not all tools are validated in jSSc.
The evidence of the effectiveness of non-pharmacological interventions (e.g. the effects of exercise on pain and fatigue as well as potential benefit for cardiorespiratory fitness, vascular function and quality of life) is limited but promising.
Palliative care
Involvement of palliative care teams should be considered for symptom control in severe cases and in end-of-life scenarios. This is an area for future development and engagement. In progressive systemic disease, discussion of prognosis is important. In those reaching the end of their life, collaborative support should be provided between the healthcare professionals involved in the person’s care, community services and the palliative care team.
Guideline recommendation for fatigue and quality of life in SSc:
Consider the impact of diagnosis and disease on HRQoL in all people with SSc (2C, 99%).
Physical and occupational therapy are recommended for the management of musculoskeletal impairment in SSc to improve function and may have a role in improving quality of life, pain and fatigue (2C, 99%).
Neurological complications
What is the best management for neurological complications of SSc?
A range of neurological complications of systemic sclerosis have been reported. Some of these relate to secondary effects of scleroderma renal crisis including hypertensive encephalopathy and posterior reversible encephalopathy syndrome (PRES). Peripheral nerve complications can be the presenting feature including carpal tunnel syndrome most often in early diffuse cutaneous systemic sclerosis. Cranial nerve involvement is well recognised and includes trigeminal neuropathy as well as trigeminal neuralgia. Involvement of other cranial nerves, particularly the glossopharyngeal nerve, also occurs [83].
Peripheral neuropathy has also been observed in case series including most often neuronal patterns of neuropathy by electrophysiological, quantitative sensory testing and pathological investigation.
There have been additional reports of autonomic and peripheral sensory neuropathy. Autonomic neuropathy in SSc involves the enteric, sympathetic and parasympathetic nervous systems affecting the function of the gastrointestinal, cardiovascular, urinary, skin and ocular systems. Depending on the system affected, dysautonomia in SSc may underlie several symptoms. Although some reported associations include restless leg syndrome, erythromelalgia and regional pain syndromes, albeit of infrequent occurrence [84].
Large fibre neuropathy is increasingly recognised in SSc [85]. In addition, peripheral sensory neuropathy (PSN) has been reported mostly affecting cranial, truncal, upper and lower extremities’ nerves. The latter was recently reported to be of high prevalence in a larger cohort, where PSN assessed by quantitative sensory testing (QST) was present in the feet in 85.3% of 109, with 80% reporting at least one neuropathic symptom. When PSN was present it was reported as a disabling manifestation, with paraesthesia, numbness or stabbing pain. PSN in the feet involved both large and small neural fibres, often co-existing. Clinically, the presence of neuropathic symptoms might serve as an indicator of PSN, although it can have a subclinical presentation. Therefore, it is important to screen for PSN with tests that capture both small and large fibre neuropathy. Polyneuropathy can also be demyelinating in the context of the scleroderma-like POEMS syndrome.
At present, robust clinical trial data are lacking for treatment of neurological complications and these should be managed in line with current practice for the specific neurological conditions. Additional research studies have suggested potential impact on central nervous system perfusion perhaps related to microvascular disease, but this is something that requires additional research. Neurological complications have not been reported in the larger jSSc cohorts and are less likely to affect children with jSSc [8, 46].
In conclusion, while neurological complications occur in systemic sclerosis, disease-specific recommendations for investigation and treatment are not appropriate at present.
Guideline recommendation for neurological complications in SSc:
Neurological complications of SSc require multi-speciality management with careful exclusion of other relevant causes (1C, 97%).
Peripheral nerve abnormalities occur in SSc including carpal tunnel syndrome, peripheral neuropathy and cranial nerve dysfunction that can be neuralgia or neuropathy most often affecting the trigeminal nerve (1C, 97%).
Peripheral sensory neuropathy of the feet is common and disabling in adults with SSc and investigation should be prompted by clinical suspicion (2C, 93%).
Pregnancy and reproductive health
What is the management for pregnancy and reproductive health in SSc?
Systemic sclerosis has a major impact on reproductive health. There is substantial unmet need related to sexual dysfunction in male and females [86]. Pregnancy raises important issues relevant to the mother and foetus and newborn [87]. There is the need for more research in this area and recommendations can be made based upon available literature and expert consensus.
Guideline recommendation for pregnancy and reproductive health in SSc:
Sexual dysfunction should be sensitively discussed with engagement of specialist gynaecology, urology, and sexual health clinical services (1C, 98%).
When considering planned pregnancy in SSc it is important to identify any significant renal, cardiac or lung complications as well as discontinue medication that may be harmful and replace with safer alternatives (e.g. azathioprine) if necessary (1C, 99%).
SSc should be judged stable and pregnancy management should occur within the context of robust medical support and integrated multi-disciplinary care (2C, 100%).
Organisation or services for systemic sclerosis within NHS
Service organization and delivery within NHS England and UK devolved nations including paediatric and transitional services
The working group recognizes that there are challenges in delivering high-quality equitable care for SSc across England and the devolved nations. This reflects the infrequency of SSc in primary and secondary care and its clinical diversity as well as the need for comprehensive multi-specialist and interdisciplinary clinical care, including access to specialist diagnostic investigation such as nailfold capillaroscopy that currently may have limited availability outside specialist centres. In addition, while treatment options are increasing it is recognized that clinical impact can be limited and so even with optimal management there is significant unmet medical need for people with SSc.
It is likely that SSc will provide a template that may be relevant to other uncommon multisystem autoimmune rheumatic diseases managed across the NHS. It is important to ensure that adult services link to paediatric, adolescent and transitional services for SSc. All children with jSSc should be managed by tertiary paediatric services with multidisciplinary and multi-specialist expertise. Given the rarity of jSSc and jSSc organ complications, close links with adult SSc centres have benefits for sharing expertise and allowing smooth transition of young people to adult services. Transition should be developmentally appropriate and follow a person-centred approach. Point of transfer to adult services should ideally occur when disease is relatively stable and the young person is ready rather than at a specific age. Transition from paediatric to adult services should be carefully planned and in line with NICE guideline NG43 [https://www.nice.org.uk/guidance/ng43]. The NCEPOD report of transition from child to adult healthcare is a useful resource [https://www.ncepod.org.uk/2023transition.html]. Transition tools are available to help services such as the Ready, Steady, Go tool [https://www.readysteadygo.net/].
Approaches to audit of the guideline
This guideline offers opportunity for audit to assess management practice and to monitor quality of services. The individual measures that comprise the audit tool are congruent with the metric definition set out in the Specialized Services Quality Dashboard for connective tissue disease and these measures may be considered as quality standards for scleroderma as part of the extended scope for the National Early Inflammatory Arthritis Audit.
The following are some topics that may be audited
Service delivery
Time to assessment in a specialist SSc clinic after referral: 6 weeks from GP referral to specialist and 18 weeks for specialist scleroderma review. In children this should be in a paediatric tertiary rheumatology service. Standard 100%.
Nominated lead clinician for each person with SSc. Standard 100%.
Access to multidisciplinary team (in children this should be with paediatric expertise). Standard 100%.
Availability of full range of SSc-specific ANA testing and screening investigations (echo, lung function and HRCT Chest). Standard 100%.
Screening for malignancy in high-risk individuals including ARA specificity. Standard 100%.
Access to cardiac MRI for diagnosis of cardiac involvement. Standard 100%.
Links with specialized commissioning centre for access to treatment for lung fibrosis (e.g. nintedanib). Standard 100%.
Access to i.v. prostanoids for critical digital ischaemia or severe DU disease. Standard 100%.
Defined referral pathway for AHSCT therapy. Standard 80%.
Links with local NHS England networks for specialised rheumatology and advanced drug access. Standard 100%.
Patient access to specialist SSc/CTD wound care services. Standard 80%.
Mapping all AHP SSc specialist services in the UK (i.e., wound care specialist, physio, OT, dietician, psychologist and podiatrist) with clear and functional referral pathways. Standard 80%.
Defined transition services from paediatric to adult care. Standard 80%.
Patient-specific audit
Documented management plan for each person with SSc. Standard 60–90%.
Access for annual review in specialist clinic with monitoring with lung function and Echo. Standard 50–70%.
Documentation of explanation of risks of immunosuppressive and other SSc therapies for childbearing in appropriate cases. Standard 100%.
Proportion of people with SSc having phosphodiesterase type 5 inhibitor (e.g. sildenafil) for DU disease. Standard 10–25%.
Proportion of people with SSc receiving bosentan for treatment of DU. Standard 5–10%.
Proportion of people with SSc with progressive ILD referred for anti-fibrotic therapy (nintedanib) for progressive fibrotic ILD. Standard 10–30%.
Proportion referred for expert evaluation of suspected pulmonary hypertension. Standard 10–40%.
Proportion of people with SSc taking immunosuppressive treatment (skin, ILD, musculoskeletal involvement). Standard 10–40%.
Proportion of people with SSc receiving biologics (rituximab, tocilizumab). Standard 10–40%.
Proportion of cases enrolled into observational clinical studies or interventional clinical trials. Standard 5–40%.
Documented transition plan for children with SSc 14 years and above. Standard 60%.
Applicability and utility
This updated guideline provides an overview of current best practice and evidence-based management of systemic sclerosis with a particular focus on delivering care within the NHS in England and the devolved nations. It is important to also consider expert recommendations and guidelines from other relevant organizations that relate to systemic sclerosis and its organ-based complications such as interstitial lung disease and pulmonary hypertension. It is recognized that there may be local challenges accessing unlicensed therapies that fall outside current NHS England prescribing policies but strongly consider these recommendations to align with current best practice and hope that they will provide a roadmap to harmonize and improve management of people with SSc. Whilst some aspects of SSc are treatable and the evidence based for drugs is increasingly robust, there is tremendous unmet need. As treatment of life-threatening aspects of the disease improves, there is the potential for greater long-term morbidity and burden.
Research recommendations
In preparing the guideline a large and important research agenda has been identified:
There is a notable lack of research into all aspects of juvenile SSc including only one RCT (nintedanib). This is a marked unmet need leading to poor understanding of the outcomes and prognosis of juvenile onset SSc and inequity of access to care and treatments. Inclusion of paediatric participants in SSc studies should be considered.
There is profound lack of understanding and very inadequate treatment for aspects of SSc such as calcinosis, fatigue and GI disease that have very high priority for people with SSc.
Better definition of priorities and approach for early diagnosis, classification, and stratification of risk in SSc, including molecular classification.
There is need to establish the best evidence-based management and treatment for critical digital ischaemia.
Detection, diagnosis and treatment of primary heart involvement in SSc requires much greater understanding to improve outcome.
Treatments to reverse or reduce established fibrosis in affected organs are lacking.
Supplementary Material
Acknowledgements
Scleroderma and Raynaud’s UK and the Primary Care Rheumatology and Musculoskeletal Medicine Society endorse the British Society for Rheumatology guideline for management of systemic sclerosis, which was developed in line with the BSR Creating Clinical Guidelines Protocol using AGREEII (Appraisal of Guidelines for Research and Evaluation II) methodology.
We are very grateful to BSR for supporting and managing the guideline development process, especially Lindsay Turner. UCL-Royal Free Medical Library staff provided valuable assistance and guidance for systematic literature review strategy and retrieval. Outstanding administrative and technical support from Ms Millie Williams is gratefully acknowledged. The following cardiology, pulmonary hypertension, paediatric transplant haematology and juvenile SSc experts reviewed the near final draft guideline to provide additional expert opinion and are gratefully acknowledged: Dr Daniel Knight, Dr Phuoc Duong, Dr Shahin Moledina, Dr Juliana Silva, Dr Kathryn Torok, Dr Eslam Al-Abadri, Dr Clarissa Pilkington, Dr Sunil Sempath, Dr Sam Deepak, Dr Hanna Lythgoe, Dr Emily Willis.
Appendix: Glossary of Terms
ACR: American College of Rheumatology
AGREEII: Appraisal of Guidelines for Research and Evaluation II
AHSCT: Autologous haematopoietic stem cell transplantation
ANA: antinuclear autoantibody
APS: anti-phospholipid screen
ARA: anti-RNA polymerase III autoantibody
ARNI: angiotensin receptor-neprilysin inhibitor
ATA: anti-topoisomerase-1 autoantibody
ATS: American Thoracic Society
bDMARD: biological disease-modifying anti-rheumatic drug
BSR: British Society for Rheumatology
CCB: calcium channel blocker
CD34: cluster differentiation antigen 34 (stem cell marker)
CKD: chronic kidney disease
CMR: cardiac magnetic resonance imaging
CTEPH: chronic thromboembolic pulmonary hypertension
DESIRES: trial of safety and efficacy of rituximab in systemic sclerosis
DETECT: evidence-based detection of pulmonary arterial hypertension in SSc
DLco: diffusing capacity for carbon monoxide (transfer factor)
ERS: European Respiratory Society
ESC: European Society of Cardiology
EULAR: European Alliance of Associations for Rheumatology
FBC: full blood count
FVC: forced vital capacity
GAVE: gastric antral vascular ectasia
GI: gastrointestinal
GWG: Guideline Working Group
HRCT: high-resolution computerised tomography
HRQoL: health-related quality of life
IL6: interleukin 6
ILD: interstitial lung disease
jSSc: juvenile onset systemic sclerosis
MMF: mycophenolate mofetil
MRA: mineralocorticoid receptor antagonists
NCEPOD: National Confidential Enquiry into Patient Outcome and Death
NICE: National Institute for Health and Care Excellence
NTproBNP: N-terminal pro-brain natriuretic peptide
OGD: upper GI endoscopy
PAH: pulmonary arterial hypertension
PDE5i: phosphodiesterase type 5 inhibitor
PFT: pulmonary function test
PH: pulmonary hypertension
pHI: primary heart involvement
PJP: Pneumocystis jirovecii pneumonia
RCT: randomised controlled trial
RECITAL: trial of rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease
RP: Raynaud’s phenomenon
SGLT2i: sodium-glucose co-transporter-2 inhibitors
SRC: scleroderma renal crisis
SSc: systemic sclerosis
VEDOSS: very early diagnosis of systemic sclerosis
WSPH: World Symposium for Pulmonary Hypertension
Contributor Information
Christopher P Denton, Division of Medicine, University College London, London, UK.
Enrico De Lorenzis, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Elen Roblin, Centre for Rheumatology, Royal Free London NHS Foundation Trust, London, UK.
Nina Goldman, Division of Medicine, University College London, London, UK.
Begonya Alcacer-Pitarch, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Emma Blamont, Scleroderma and Raynaud’s UK (SRUK), London, UK.
Maya H Buch, Department of Rheumatology, University of Manchester, Manchester, UK.
Maresa Carulli, Department of Rheumatology, Hammersmith Hospitals NHS Foundation Trust, London, UK.
Caroline Cotton, Department of Rheumatology, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Francesco Del Galdo, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK.
Emma Derrett-Smith, Department of Rheumatology, University of Birmingham, Birmingham, UK.
Karen Douglas, Department of Rheumatology, The Dudley Group NHS Foundation Trust, Dudley, UK.
Sue Farrington, Scleroderma and Raynaud’s UK (SRUK), London, UK.
Kim Fligelstone, Centre for Rheumatology, Royal Free London NHS Foundation Trust, London, UK.
Luke Gompels, Department of Rheumatology, Somerset NHS Foundation Trust, Taunton, UK.
Bridget Griffiths, Department of Rheumatology, Freeman Hospital, Newcastle, UK.
Ariane Herrick, Department of Rheumatology, University of Manchester, Manchester, UK.
Michael Hughes, Department of Rheumatology, University of Manchester, Manchester, UK.
Clare Pain, Department of Rheumatology, Alder Hey Childrens Hospital, Liverpool, UK.
Georgina Pantano, Lived Experience, London, UK.
John D Pauling, Department of Rheumatology, North Bristol NHS Foundation Trust, Bristol, UK.
Athiveeraramapandian Prabu, Department of Rheumatology, University of Birmingham, Birmingham, UK.
Nuala O’Donoghue, Department of Dermatology, Northern Care Alliance, Salford Royal, UK.
Elisabetta A Renzoni, Interstitial Lung Disease Unit, Royal Brompton NHS Foundation Trust, London, UK.
Jeremy Royle, Department of Rheumatology, University Hospitals NHS Foundation Trust, Leicester, UK.
Muditha Samaranayaka, Department of Rheumatology, Northern Care Alliance, Salford Royal, UK.
Julia Spierings, Department of Rheumatology, University of Utrecht, Utrecht, Netherlands.
Aoife Tynan, Centre for Rheumatology, Royal Free London NHS Foundation Trust, London, UK.
Louise Warburton, Primary Care Sciences, Keele University, Keele, UK.
Voon H Ong, Division of Medicine, University College London, London, UK.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
No new data were generated in support of this work.
Funding
There was no external funding for this work but logistical support and advice was provided by the British Society for Rheumatology.
Disclosure statement: C.P.D. has received clinical trial support as investigator for trials for Roche, Janssen, Boehringer Ingelheim, CSL Behring, GSK; consulting fees related to clinical trials from Roche, Janssen, Boehringer Ingelheim, CSL Behring, GSK, Astra Zeneca; and speaker fees for CME events from Janssen, Boehringer Ingelheim, GSK. E.B. is employed by SRUK that has received funding from Janssen, Boehringer Ingelheim. S.F. has received honoraria from Boehringer Ingelheim, Janssen. B.G. was Chair of NHS England's Specialised Rheumatology Clinical Reference group 2016–2022. A.H. has received research funding from Gesynta Pharma, consultancy fees from Arena, Boehringer-Ingelheim, Camurus, Galderma, Gesynta Pharma and Janssen, and speaker fees from Janssen. E.D.-S. has received consultancy/speaker fees from Janssen, Boehringer Ingelheim, Galapagos. M.H. has received speaker fees from Janssen; research funding to institution from Janssen. MH leads the World Scleroderma Foundation Digital Ulcer Ad Hoc Committee and co-leads the World Scleroderma Foundation Gastrointestinal Ad Hoc Committee. J.D.P. has received an unrestricted grant from Janssen received circa 2015 and was invited to speak at Janssen Sponsored SSc Masterclass in 2023 and has received fees for consultancy work for Janssen, Astra Zeneca, Boehringer Ingelheim, Sojournix Pharma, Permeatus Inc and IsoMab, and is co-chair for the Scleroderma Clinical Trials Consortium Industry Roundtable meeting and does not receive any direct payments for this role (beyond reimbursement of costs for meeting attendance). E.A.R. has received research funding to institution from Boehringer Ingelheim and honoraria for lectures and for participation in advisory boards and clinical trial steering committees by Boehringer Ingelheim, and lecture fees by Mundipharma (received by employer). J.R. has received conference fees from Roche for ANCA vasculitis conference 2022 and from UCB for BSR Annual Conference 2023. A.T. has received funding from UCB pharma for organising a Rheumatology education day for Pharmacists. L.W. has received honorarium from Novartis and is Co-President elect of the Primary Care Rheumatology and MKS Medicine Society whose annual conference is supported by various Pharma companies. V.H.O. has received speaker fees from Boehringer Ingleheim. F.DG. has received consultancy fees and research support from Abbvie, Argenx, Arxx, AstraZeneca, Boehringer-Ingelheim, DeepCure, GSK, Janssen, Mitsubishi-Tanabe, MSD, Novartis, Ventus. M.H.B. has received consultancy/speaker fees (all paid to host institution) from Abbvie, Alfasigma, Arxx Therapeutics, Boehringer Ingelheim, Galapagos, Pfizer Ltd. C.C. has received sponsorship from Novartis to attend EULAR in 2024. The remaining authors have declared no conflicts of interest.
References
- 1. British Society for Rheumatology. British society for rheumatology’s guidelines protocol. https://www.rheumatology.org.uk/Portals/0/Documents/Guidelines/Guidelines%20Protocol%20edited%20-%20Dec23%20FINAL.pdf?ver=-Kk1kW7Pat7ncErckPrzTw%3d%3d (13 August 2024, date last accessed).
- 2. Royle JG, Lanyon PC, Grainge MJ, Abhishek A, Pearce FA. The incidence, prevalence, and survival of systemic sclerosis in the UK Clinical Practice Research Datalink. Clin Rheumatol 2018;37:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 4. Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2012;51:1017–26. [DOI] [PubMed] [Google Scholar]
- 5. Nihtyanova SI, Sari A, Harvey JC et al. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol 2020;72:465–76. [DOI] [PubMed] [Google Scholar]
- 6. Pakozdi A, Nihtyanova S, Moinzadeh P et al. Clinical and serological hallmarks of systemic sclerosis overlap syndromes. J Rheumatol 2011;38:2406–9. [DOI] [PubMed] [Google Scholar]
- 7. Foeldvari I, Klotsche J, Kasapcopur O et al. Differences Sustained Between Diffuse and Limited Forms of Juvenile Systemic Sclerosis in an Expanded International Cohort. Arthritis Care Res (Hoboken) 2022;74:1575–84. [DOI] [PubMed] [Google Scholar]
- 8. Stevens BE, Torok KS, Li SC et al. ; Childhood Arthritis and Rheumatology Research Alliance Registry Investigators. Clinical characteristics and factors associated with disability and impaired quality of life in children with juvenile systemic sclerosis: results from the childhood arthritis and rheumatology research alliance legacy registry. Arthritis Care Res (Hoboken) 2018;70:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nihtyanova SI, Tang EC, Coghlan JG et al. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. QJM 2010;103:109–15. [DOI] [PubMed] [Google Scholar]
- 10. Denton CP, Hughes M, Gak N et al. ; BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology (Oxford) 2016;55:1906–10. [DOI] [PubMed] [Google Scholar]
- 11. Denton CP, De Lorenzis E, Roblin E et al. Management of systemic sclerosis: british Society for Rheumatology guideline scope. Rheumatol Adv Pract 2023;7:rkad022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pauling JD, McGrogan A, Snowball J, McHugh NJ. Epidemiology of systemic sclerosis in the UK: an analysis of the Clinical Practice Research Datalink. Rheumatology (Oxford) 2021;60:2688–96. [DOI] [PubMed] [Google Scholar]
- 14. Hughes M, Baker A, Farrington S, Pauling JD. Patient organisation-led initiatives can play an important role in raising awareness about Raynaud’s phenomenon and encourage earlier healthcare utilisation for high-risk groups. Ann Rheum Dis 2019;78:439–41. [DOI] [PubMed] [Google Scholar]
- 15. van den Hoogen F, Khanna D, Fransen J et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellando-Randone S, Del Galdo F, Lepri G et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Lancet Rheumatol 2021;3:e834–43. [DOI] [PubMed] [Google Scholar]
- 17. Eden M, Wilkinson S, Murray A et al. Nailfold capillaroscopy: a survey of current UK practice and ‘next steps’ to increase uptake among rheumatologists. Rheumatology (Oxford) 2022;62:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson C, Leone V, Pain C. Comment on: nailfold capillaroscopy: a survey of current UK practice and ‘next steps’ to increase uptake among rheumatologists. Rheumatology (Oxford) 2023;62:e303–e304. [DOI] [PubMed] [Google Scholar]
- 19. Melsens K, Cutolo M, Schonenberg-Meinema D, EULAR Study Group on Microcirculation in Rheumatic Diseases et al. Standardized nailfold capillaroscopy in children with rheumatic diseases: a worldwide study. Rheumatology (Oxford) 2023;62:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butler EA, Baron M, Fogo AB, Scleroderma Clinical Trials Consortium Scleroderma Renal Crisis Working Group et al. Generation of a Core Set of Items to Develop Classification Criteria for Scleroderma Renal Crisis Using Consensus Methodology. Arthritis Rheumatol 2019;71:964–71. [DOI] [PubMed] [Google Scholar]
- 21. Sobanski V, Giovannelli J, Allanore Y et al. ; EUSTAR Collaborators. Phenotypes determined by cluster analysis and their survival in the prospective European scleroderma trials and research cohort of patients with systemic sclerosis. Arthritis Rheumatol 2019;71:1553–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark KEN, Csomor E, Campochiaro C et al. Integrated analysis of dermal blister fluid proteomics and genome-wide skin gene expression in systemic sclerosis: an observational study. Lancet Rheumatol 2022;4:e507–e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franks JM, Toledo DM, Martyanov V et al. A genomic meta-analysis of clinical variables and their association with intrinsic molecular subsets in systemic sclerosis. Rheumatology (Oxford) 2022;62:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zulian F, Woo P, Athreya BH et al. The Pediatric Rheumatology European Society/American College of Rheumatology/European League against Rheumatism provisional classification criteria for juvenile systemic sclerosis. Arthritis Rheum 2007;57:203–12. [DOI] [PubMed] [Google Scholar]
- 25. F, oeldvari I, Klotsche J, Torok KS et al. Are diffuse and limited juvenile systemic sclerosis different in clinical presentation? Clinical characteristics of a juvenile systemic sclerosis cohort. J Scleroderma Relat Disord 2019;4:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. N, ihtyanova SI, Denton CP. Pathogenesis of systemic sclerosis associated interstitial lung disease. J Scleroderma Relat Disord 2020;5:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elhai M, Meune C, Boubaya M et al. ; EUSTAR Group. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 28. Moinzadeh P, Fonseca C, Hellmich M et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther 2014;16:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pauling JD, Snowball J, McHugh N, McGrogan A. OA30 Systemic sclerosis and cancer in the UK: an epidemiological analysis using the Clinical Practice Research Datalink. Rheumatology 2023;62:ii17. [DOI] [PubMed] [Google Scholar]
- 30. Shah AA, Hummers LK, Casciola-Rosen L et al. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis Rheumatol 2015;67:1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah AA, Rosen A. Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications. Curr Opin Rheumatol 2011;23:530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Derk CT, Rasheed M, Artlett CM, Jimenez SA. A cohort study of cancer incidence in systemic sclerosis. J Rheumatol 2006;33:1113–6. [PubMed] [Google Scholar]
- 33. Raghu G, Montesi SB, Silver RM et al. Treatment of systemic sclerosis-associated interstitial lung disease: evidence-based recommendations. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2023;209:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Humbert M, Kovacs G, Hoeper MM et al. ; ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. [DOI] [PubMed] [Google Scholar]
- 35. Tashkin DP, Roth MD, Clements PJ et al. ; Sclerodema Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flaherty KR, Wells AU, Cottin V et al. ; INBUILD Trial Investigators. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019;381:1718–27. [DOI] [PubMed] [Google Scholar]
- 37. Distler O, Highland KB, Gahlemann M et al. ; SENSCIS Trial Investigators. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 38. Highland KB, Distler O, Kuwana M et al. ; SENSCIS Trial Investigators. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. [DOI] [PubMed] [Google Scholar]
- 39. Khanna D, Lin CJF, Furst DE et al. ; focuSSced investigators. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. [DOI] [PubMed] [Google Scholar]
- 40. Ebata S, Yoshizaki A, Oba K et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. The Lancet Rheumatology 2021;3:e489–97. [DOI] [PubMed] [Google Scholar]
- 41. Maher TM, Tudor VA, Saunders P et al. ; RECITAL Investigators. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med 2023;11:45–54. [DOI] [PubMed] [Google Scholar]
- 42. Mankikian J, Caille A, Reynaud-Gaubert M et al. ; EVER-ILD investigators and the OrphaLung network. Rituximab and mycophenolate mofetil combination in patients with interstitial lung disease (EVER-ILD): a double-blind, randomised, placebo-controlled trial. Eur Respir J 2023;61:2202071. [DOI] [PubMed] [Google Scholar]
- 43. Khanna D, Lescoat A, Roofeh D et al. Systemic sclerosis-associated interstitial lung disease: how to incorporate two food and drug administration-approved therapies in clinical practice. Arthritis Rheumatol 2022;74:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foeldvari I, Culpo R, Sperotto F et al. Consensus-based recommendations for the management of juvenile systemic sclerosis. Rheumatology (Oxford) 2021;60:1651–8. [DOI] [PubMed] [Google Scholar]
- 45. Foeldvari I, Klotsche J, Hinrichs B et al. Underdetection of interstitial lung disease in juvenile systemic sclerosis. Arthritis Care Res (Hoboken) 2022;74:364–70. [DOI] [PubMed] [Google Scholar]
- 46. Foeldvari I, Torok KS, Antón J et al. Best clinical practice in the treatment of juvenile systemic sclerosis: expert panel guidance—the result of the International Hamburg Consensus Meeting December 2022. Expert Rev Clin Immunol 2023;20:387–404. [DOI] [PubMed] [Google Scholar]
- 47. Álvarez-Hernández MP, Allanore Y, Andrade I et al. Attitudes and barriers to pulmonary arterial hypertension screening in systemic sclerosis patients: a survey of UK-based rheumatologists. J Scleroderma Relat Disorders 2024;9:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simonneau G, Montani D, Celermajer DS et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nihtyanova SI, Schreiber BE, Ong VH et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 50. Olsson KM, Delcroix M, Ghofrani HA et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014;129:57–65. [DOI] [PubMed] [Google Scholar]
- 51. Waxman A, Restrepo-Jaramillo R, Thenappan T et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021;384:325–34. [DOI] [PubMed] [Google Scholar]
- 52. Nihtyanova SI, Schreiber BE, Ong VH et al. Dynamic prediction of pulmonary hypertension in systemic sclerosis using landmark analysis. Arthritis Rheumatol 2023;75:449–58. [DOI] [PubMed] [Google Scholar]
- 53. Coghlan JG, Denton CP, Grünig E et al. ; DETECT Study Group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jais X, Launay D, Yaici A et al. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum 2008;58:521–31. [DOI] [PubMed] [Google Scholar]
- 55. Bruni C, Buch MH, Furst DE et al. Primary systemic sclerosis heart involvement: a systematic literature review and preliminary data-driven, consensus-based WSF/HFA definition. J Scleroderma Relat Disord 2022;7:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruni C, Buch MH, Djokovic A et al. Consensus on the assessment of systemic sclerosis-associated primary heart involvement: world Scleroderma Foundation/Heart Failure Association guidance on screening, diagnosis, and follow-up assessment. J Scleroderma Relat Disord 2023;8:169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Valentini G, Huscher D, Riccardi A et al. Vasodilators and low-dose acetylsalicylic acid are associated with a lower incidence of distinct primary myocardial disease manifestations in systemic sclerosis: results of the DeSScipher inception cohort study. Ann Rheum Dis 2019;78:1576–82. [DOI] [PubMed] [Google Scholar]
- 58. Bruera S, Sidanmat H, Molony DA et al. Stem cell transplantation for systemic sclerosis. Cochrane Database Syst Rev 2022;7:CD011819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henes J, Oliveira MC, Labopin M et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica 2021;106:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spierings J, van Rhenen A, Welsing PM et al. A randomised, open-label trial to assess the optimal treatment strategy in early diffuse cutaneous systemic sclerosis: the UPSIDE study protocol. BMJ Open 2021;11:e044483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hughes M, Ong VH, Anderson ME et al. Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis. Rheumatology (Oxford) 2015;54:2015–24. [DOI] [PubMed] [Google Scholar]
- 62. Matucci-Cerinic M, Denton CP, Furst DE et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2011;70:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hughes M, Snapir A, Wilkinson J et al. Prediction and impact of attacks of Raynaud’s phenomenon, as judged by patient perception. Rheumatology (Oxford) 2015;54:1443–7. [DOI] [PubMed] [Google Scholar]
- 64. Pauling JD, Domsic RT, Saketkoo LA et al. Multinational qualitative research study exploring the patient experience of Raynaud's Phenomenon in systemic sclerosis. Arthritis Care Res (Hoboken) 2018;70:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herrgott I, Riemekasten G, Hunzelmann N, Sunderkotter C. Management of cutaneous vascular complications in systemic scleroderma: experience from the German network. Rheumatol Int 2008;28:1023–9. [DOI] [PubMed] [Google Scholar]
- 66. Moinzadeh P, Riemekasten G, Siegert E et al. ; German Network for Systemic Scleroderma. Vasoactive Therapy in Systemic Sclerosis: real-life Therapeutic Practice in More Than 3000 Patients. J Rheumatol 2016;43:66–74. [DOI] [PubMed] [Google Scholar]
- 67. Khouri C, Lepelley M, Bailly S et al. Comparative efficacy and safety of treatments for secondary Raynaud's phenomenon: a systematic review and network meta-analysis of randomised trials. Lancet Rheumatol 2019;1:e237–e46. [DOI] [PubMed] [Google Scholar]
- 68. Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud's phenomenon: a meta-analysis. Rheumatology (Oxford) 2005;44:145–50. [DOI] [PubMed] [Google Scholar]
- 69. Roustit M, Blaise S, Allanore Y et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud's phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis 2013;72:1696–9. [DOI] [PubMed] [Google Scholar]
- 70. Allanore Y, Meune C, Vonk MC et al. ; EUSTAR Co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69:218–21. [DOI] [PubMed] [Google Scholar]
- 71. Malenfant D, Catton M, Pope JE. The efficacy of complementary and alternative medicine in the treatment of Raynaud's phenomenon: a literature review and meta-analysis. Rheumatology (Oxford) 2009;48:791–5. [DOI] [PubMed] [Google Scholar]
- 72. Garaiman A, Steigmiller K, Gebhard C et al. ; EUSTAR Collaborators. Use of platelet inhibitors for digital ulcers related to systemic sclerosis: EUSTAR study on derivation and validation of the DU-VASC model. Rheumatology (Oxford) 2023;62:SI91–SI100. [DOI] [PubMed] [Google Scholar]
- 73. Żebryk P, Puszczewicz MJ. Botulinum toxin A in the treatment of Raynaud's phenomenon: a systematic review. Arch Med Sci 2016;12:864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bandini G, Alunno A, Ruaro B et al. Significant gastrointestinal unmet needs in patients with systemic sclerosis: insights from a large international patient survey. Rheumatology (Oxford) 2023;63:e92–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maclean RH, Ahmed F, Ong VH, Murray CD, Denton CP. A phenome-wide association study of drugs and comorbidities associated with gastrointestinal dysfunction in systemic sclerosis. J Rheumatol 2023;50:907–15. [DOI] [PubMed] [Google Scholar]
- 76. Massat B, McCarthy J. Systemic sclerosis, malnutrition, and small bowel obstruction: why clinicians should consider early total parenteral nutrition in systemic sclerosis with severe gastrointestinal involvement. Cureus 2022;14:e27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hofmann NN, Ambühl RA, Jordan S, Distler O. Calcineurin inhibitors in systemic sclerosis—a systematic literature review. Ther Adv Musculoskelet Dis 2022;14:1759720X221092374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Griffiths-Jones DJ, Garcia YS, Ryder WD et al. A Phase II randomized controlled trial of oral prednisolone in early diffuse cutaneous systemic sclerosis (PRedSS). Rheumatology (Oxford) 2023;62:3133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dinsdale G, Murray A, Moore T et al. A comparison of intense pulsed light and laser treatment of telangiectases in patients with systemic sclerosis: a within-subject randomized trial. Rheumatology (Oxford) 2014;53:1422–30. [DOI] [PubMed] [Google Scholar]
- 80. Almadori A, Griffin M, Ryan CM et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS One 2019;14:e0218068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nagy G, Dobrota R, Becker MO et al. Characteristics of ScleroID highlighting musculoskeletal and internal organ implications in patients afflicted with systemic sclerosis. Arthritis Res Ther 2023;25:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Santos EJF, Farisogullari B, Dures E, Geenen R, Machado PM; EULAR Taskforce on Recommendations for the Management of Fatigue in People with Inflammatory Rheumatic Diseases. Efficacy of non-pharmacological interventions: a systematic review informing the 2023 EULAR recommendations for the management of fatigue in people with inflammatory rheumatic and musculoskeletal diseases. RMD Open 2023;9:e003350. Erratum in: RMD Open 2023;94.37604639 [Google Scholar]
- 83. Maltez N, Choi MY, Troyanov Y et al. ; Canadian Scleroderma Research Group. Trigeminal neuralgia in systemic sclerosis. Semin Arthritis Rheum 2021;51:318–23. [DOI] [PubMed] [Google Scholar]
- 84. Di Battista M, Wasson CW, Alcacer-Pitarch B, Del Galdo F. Autonomic dysfunction in systemic sclerosis: a scoping review. Semin Arthritis Rheum 2023;63:152268. [DOI] [PubMed] [Google Scholar]
- 85. Raja J, Balaikerisnan T, Ramanaidu LP, Goh KJ. Large fiber peripheral neuropathy in systemic sclerosis: a prospective study using clinical and electrophysiological definition. Int J Rheum Dis 2021;24:347–54. [DOI] [PubMed] [Google Scholar]
- 86. Dai L, Xu D, Li X et al. Reproductive health in female patients with systemic sclerosis: a cross-sectional study. Rheumatology (Oxford). 2023 20;63:1911–6. [DOI] [PubMed] [Google Scholar]
- 87. Braun J, Balbir-Gurman A, Toledano K, Tavor Y, Braun-Moscovici Y. Favourable outcome of planned pregnancies in systemic sclerosis patients during stable disease. Scand J Rheumatol 2022;51:513–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated in support of this work.