Abstract
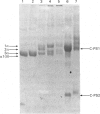
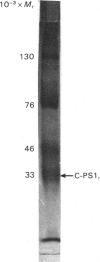
The collagens of bovine vitreous-humour and nasal-septum cartilage have been extracted, fractionated and compared. Both tissues show the same heterogeneity of collagen types, consisting of type II, 1 alpha, 2 alpha, 3 alpha and C-PS collagens. The type II collagen of the vitreous humour was significantly more hydroxylated both in the lysine and proline residues than was that of cartilage. C-PS1 collagen, together with higher-Mr forms were present in the vitreous humour, but the higher-Mr forms were not seen in cartilage. Both C-PS1 and C-PS2 were present in vitreous humour and cartilage, but vitreous humour contained three times more of these collagens than did cartilage. Despite the difference in amount, the molar ratio C-PS1/C-PS2 was approx. 1 in both tissues, suggesting that they are components of a larger molecule. The 1 alpha, 2 alpha, 3 alpha collagens were present in the same concentration in both tissues. These three chains co-precipitated on dialysis against phosphate-buffered saline, pH 7.2, in a manner analogous to type V collagen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abedin M. Z., Ayad S., Weiss J. B. Isolation and native characterization of cysteine-rich collagens from bovine placental tissues and uterus and their relationship to types IV and V collagens. Biosci Rep. 1982 Jul;2(7):493–502. doi: 10.1007/BF01115247. [DOI] [PubMed] [Google Scholar]
- Abedin M. Z., Ayad S., Weiss J. B. Type V collagen: the presence of appreciable amounts of alpha 3(V) chain in uterus. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1237–1245. doi: 10.1016/s0006-291x(81)80144-1. [DOI] [PubMed] [Google Scholar]
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Ayad S., Abedin M. Z., Weiss J. B., Grundy S. M. Characterisation of another short-chain disulphide-bonded collagen from cartilage, vitreous and intervertebral disc. FEBS Lett. 1982 Mar 22;139(2):300–304. doi: 10.1016/0014-5793(82)80875-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Hebda P. A., Morris N. P., Hollister D. W. Human cartilage collagens. Comparison of cartilage collagens with human type V collagen. J Biol Chem. 1982 Jul 10;257(13):7852–7856. [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Burke J. M. An analysis of rabbit vitreous collagen. Connect Tissue Res. 1980;8(1):49–52. doi: 10.3109/03008208009152121. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Mainardi C. L., Seyer J. M., Kang A. H. Collagen-platelet interaction. Type V(A-B) collagen induced platelet aggregation. J Lab Clin Med. 1980 Jan;95(1):99–107. [PubMed] [Google Scholar]
- Evans H. B., Ayad S., Abedin M. Z., Hopkins S., Morgan K., Walton K. W., Weiss J. B., Holt P. J. Localisation of collagen types and fibronectin in cartilage by immunofluorescence. Ann Rheum Dis. 1983 Oct;42(5):575–581. doi: 10.1136/ard.42.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., MATOLTSY G., COHEN C. Vitrosin: a member of the collagen class. J Biophys Biochem Cytol. 1955 May 25;1(3):215–220. doi: 10.1083/jcb.1.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Grant M. E., Freeman I. L., Schofield J. D., Jackson D. S. Variations in the carbohydrate content of human and bovine polymeric collagens from various tissues. Biochim Biophys Acta. 1969 May 6;177(3):682–685. doi: 10.1016/0304-4165(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Kalebic T., Reese C. A., Mayne R. Protease susceptibilities of HMW, 1 alpha, 2 alpha, but not 3 alpha cartilage collagens are similar to type V collagen. Biochem Biophys Res Commun. 1982 Jan 29;104(2):500–506. doi: 10.1016/0006-291x(82)90664-7. [DOI] [PubMed] [Google Scholar]
- MATOLTSY A. G., GROSS J., GRIGNOLO A. A study of the fibrous components of the vitreous body of the electron microscope. Proc Soc Exp Biol Med. 1951 Apr;76(4):857–860. doi: 10.3181/00379727-76-18655. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Newsome D. A., Linsenmayer T. F., Trelstad R. L. Vitreous body collagen. Evidence for a dual origin from the neural retina and hyalocytes. J Cell Biol. 1976 Oct;71(1):59–67. doi: 10.1083/jcb.71.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. IV. Structure of vitrosin fibrils and interaction properties of vitrosin molecules. J Ultrastruct Res. 1965 Aug;13(1):172–191. doi: 10.1016/s0022-5320(65)80095-8. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of a new disulphide bonded collagen from cartilage. FEBS Lett. 1980 Nov 17;121(1):51–54. doi: 10.1016/0014-5793(80)81265-8. [DOI] [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of two further collagenous fractions from articular cartilage. Biosci Rep. 1981 Jul;1(7):561–570. doi: 10.1007/BF01116305. [DOI] [PubMed] [Google Scholar]
- Snowden J. M., Eyre D. R., Swann D. A. Vitreous structure. VI. Age-related changes in the thermal stability and crosslinks of vitreous, articular cartilage and tendon collagens. Biochim Biophys Acta. 1982 Sep 7;706(2):153–157. doi: 10.1016/0167-4838(82)90481-2. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Swann D. A., Caulfield J. B., Broadhurst J. B. The altered fibrous form of vitreous collagen following solubilization with pepsin. Biochim Biophys Acta. 1976 Mar 18;427(1):365–370. doi: 10.1016/0005-2795(76)90312-3. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Constable I. J., Caulfield J. B. Vitreous structure, IV. Chemical composition of the insoluble residual protein fraction from the rabbit vitreous. Invest Ophthalmol. 1975 Aug;14(8):613–616. [PubMed] [Google Scholar]
- Swann D. A., Sotman S. S. The chemical composition of bovine vitreous-humour collagen fibres. Biochem J. 1980 Mar 1;185(3):545–554. doi: 10.1042/bj1850545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of new collagens from chick cartilage. Eur J Biochem. 1982 May;124(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05905.x. [DOI] [PubMed] [Google Scholar]