Abstract
Background
Certified peer recovery specialists (CPRS) and licensed clinical social workers (LCSWs) can facilitate substance use disorder (SUD) treatment engagement for emergency department (ED) patients at risk for overdose. Predictors of treatment engagement after such behavioral services are unknown.
Methods
This secondary analysis included Rhode Island ED patients at high risk for opioid overdose participating in a randomized controlled trial comparing the effectiveness of CPRS and LCSWs services (2018–2021). SUD treatment engagement within 90 days post-discharge was identified using statewide administrative data. Potential predictors were obtained from baseline questionnaires. Classification and regression trees (CART) were used to identify predictors of treatment engagement.
Results
In the ED, 323 and 325 participants received CPRS and LCSWs services, respectively, among whom 141 (43.7 %) and 137 (42.2 %) engaged in SUD treatment within 90 days post-discharge. For the CPRS group, predictors of treatment engagement included unhealthy alcohol use, prescription opioid or benzodiazepine use in past 6 months, and lifetime history of: unstable housing, barriers to treatment, bipolar disorder diagnosis, addiction treatment, and recovery services. In the LCSW group, predictors included health insurance, current pain, opioid overdose in past year, and lifetime history of anxiety disorder diagnosis and mental illness treatment. However, CART had low predictive accuracy (CPRS: 60.9 %, LCSW: 54.8 %).
Conclusions
Among ED patients at high risk of opioid overdose receiving behavioral services, 90-day SUD treatment engagement was high. Sociobehavioral and clinical patient characteristics did not accurately predict treatment engagement. Behavioral services should be offered to all ED patients at high risk of opioid overdose.
Keywords: Substance use disorder treatment, Opioid overdose, CART prediction analysis, Emergency medicine, Peer recovery specialists, Social work
Highlights
-
•
SUD treatment engagement was high (43 %) for patients after ED behavioral service.
-
•
Time to SUD treatment did not differ by ED behavioral service (CPRS vs. LCSWs).
-
•
A non-parametric prediction algorithm did not identify predictors of SUD treatment.
-
•
ED patients should receive behavioral services to promote SUD treatment.
1. Introduction
Opioid overdose-related emergency department (ED) visits in the United States increased by 29 % from 2018 to 2020, despite a decrease in all-cause ED visits during this period (Soares et al., 2022). Many but not all of these visits are among persons with a substance use disorder (SUD), for whom the ED is often the first or only point of contact for opioid-related care (Langabeer et al., 2021). The ED has, therefore, been identified as a critical access point for interventions to reduce the risks of drug-related harms and mortality among populations at high risk of overdose (Rodda et al., 2020).
Behavioral services during drug-related ED visits are frequently provided by licensed clinical social workers (LCSWs) and, more recently, by certified peer recovery specialists (CPRS) (Moore et al., 2016, McGuire et al., 2020). LCSWs typically employ patient-centered approaches to provide counseling about substance use and link persons with SUD to community-based treatment (Auerbach and Mason, 2010). CPRS have lived experiences of recovery and typically offer non-clinical support, coaching, mentoring, and education in navigation of treatment and recovery (Serrano and Conley, 2021, Liebling et al., 2021). In addition to ED-based services, CPRS aim to provide ongoing support following discharge.
Recently, a large randomized controlled trial in Rhode Island (RI) evaluated the effectiveness of ED behavioral interventions delivered by CPRS compared to LCSWs among persons at high risk of opioid overdose (Beaudoin et al., 2022). Although no overall difference in SUD treatment engagement within 30 days post-discharge was observed by behavioral service, it remains unknown whether certain subgroups of patients might benefit most from either CPRS or LCSW services. This evidence gap limits implementation of evidence-based, personalized support for ED patients at high risk of opioid overdose. For example, consideration of patients’ SUD treatment history and prior experience with healthcare access barriers might aid in connecting them with the appropriate ED behavioral services and increase their likelihood of engaging in SUD treatment post-discharge (Madras et al., 2020, Volkow and Blanco, 2021).
Therefore, the objective of this study was to identify predictors of post-discharge SUD treatment engagement among ED patients at high risk of opioid overdose, separately for those who received CPRS versus LCSW services in the ED. We hypothesized, due to prior experiences with substance use, navigating psychosocial and structural barriers to recovery, and the ability to communicate treatment plans using nonmedical terminology, CPRS services would be more likely to increase treatment engagement for patients with a history of healthcare access barriers, unstable housing, arrests, mental health comorbidities, and use of both prescribed and non-prescribed drugs (Cos et al., 2020, Blakeman et al., 2006, Reblin and Uchino, 2008, Substance Abuse and Mental Health Services Administration (SAMHSA), 2024, Sells et al., 2020, Laudet and Humphreys, 2013, Abbott et al., 2019, Davidson et al., 2012, Chinman et al., 2024, Boisvert et al., 2008). On the other hand, LCSW services would be more effective for patients with prior SUD treatment experience due to adherence to a traditional clinical care model (Selby et al.,). A secondary objective was to evaluate whether time to SUD treatment engagement post-discharge differed for ED patients who received CPRS and LCSW services. We hypothesized that patients who received CPRS services would have a shorter time to treatment engagement than those who received LCSW services.
2. Material and methods
2.1. Study population
This study involved secondary analyses of data collected from ED patients at high risk for opioid overdose enrolled in a randomized controlled trial in RI. The trial evaluated the effectiveness of ED-based behavioral interventions delivered by CPRS compared to LCSWs. The trial protocol (ClinicalTrials.gov identifier: NCT03684681) and interventions have previously been described (Beaudoin et al., 2022, Goedel et al., 2019). Briefly, from November 2018 to May 2021, RI residents presenting to one of two study EDs for treatment of an opioid overdose or opioid use disorder (OUD), or who had an opioid overdose within the past year, were enrolled and randomized to receive a behavioral intervention from either a CPRS or LCSW. A baseline questionnaire was administered to obtain sociobehavioral and clinical information. Post ED discharge, participants were followed for 18 months to evaluate health outcomes. Participants provided informed consent and permission for linkage of their study data to statewide administrative data, including SUD treatment. The study sites, Brown University, and RI Department of Health Institutional Review Boards approved the trial protocol.
2.2. Outcome
The primary outcome was engagement in any SUD treatment within 90 days of ED discharge (yes, no). Treatment engagement was identified through deterministic linkage to statewide administrative data from the Rhode Island Behavioral Health Online Database (BHOLD) and Prescription Drug Monitoring Program (PDMP) (Eddie et al., 2019). BHOLD includes all SUD treatment episodes at publicly-licensed facilities in RI, including methadone, detoxification, outpatient, intensive outpatient, and residential treatment. The PDMP database includes all buprenorphine prescriptions dispensed to RI residents by retail pharmacies in RI and most out-of-state retail pharmacies (State of Rhode Island Department of Health, 2024). SUD treatment engagement within 90 days post-discharge was defined as any: (1) new SUD treatment episode at a publicly-licensed program; or (2) fill of a buprenorphine prescription for OUD. Participants currently or recently (in the two weeks before) engaged in SUD treatment at baseline were included in the study and considered to have the study outcome if they transitioned to a new SUD treatment type, level of care, or provider, or continued buprenorphine via prescription during follow-up.
2.3. Baseline characteristics hypothesized to influence SUD treatment engagement
The Behavioral Model of Healthcare Utilization Framework was used to identify the baseline characteristics considered in the prediction analysis. Within this framework, four overarching factors influence engagement in care: predisposing factors (individual characteristics), enabling factors (social/structural barriers to care), need factors (diagnosed and perceived health conditions), and health behavior factors (steps taken to manage substance use or co-morbid conditions) (Heidari et al., 2022). Baseline characteristics hypothesized to influence SUD treatment engagement were selected within this framework based on content expertise (Mauro et al., 2022, Macmadu et al., 2021, Wyse et al., 2022, Ober et al., 2018, Gideon,). Most characteristics were obtained from study questionnaires, with a few others based on administrative data.
Predisposing factors included age, race, ethnicity, and sex assigned at birth. Enabling factors included baseline health insurance, monthly income (including public assistance or family support), employment (full-time or part-time), receipt of disability benefits, and highest education attained, as well as lifetime history of unstable housing, barriers to treatment access, and arrest. Need factors included baseline unhealthy alcohol use, pain, and reason for the ED visit; use of specific substances in the past 6 months (P6M) (prescription opioids, prescription benzodiazepines, marijuana, crystal methamphetamine, cocaine, heroin, and club drugs); previous opioid overdose in past 12 months (P12M); and lifetime history of injecting drugs, any mental health diagnosis, and specific mental health diagnoses (depressive disorder, bipolar disorder, psychosis, and anxiety disorder). Health behavior factors included a lifetime history of receiving mental illness treatment, addiction treatment, and recovery services.
Of note, unhealthy alcohol use was measured with the Alcohol Use Disorders Identification Test (AUDIT-C) (U.S., 2024). Opioid overdose as the reason for ED visit was defined as having decreased levels of consciousness or respiratory depression after consuming opioids which resolved after naloxone administration. Prescription opioid and benzodiazepine use in the P6M included use without a prescription or not as prescribed. Club drug use in the P6M included any of the following: molly, MDMA, ecstasy, mushrooms, GHB, or ketamine.
2.4. Statistical methods
Analyses were completed using STATA® Version 16.0 with significance level p<0.05, unless otherwise specified (StataCorp, 2021). The time to engagement and the type of SUD treatment received were compared between participants who received CPRS and LCSW services in the ED using t-tests and χ2 tests, respectively. Differences in the time to engagement by behavioral service were also assessed using Kaplan Meier curves and Cox proportional hazards (Cox-PH) models, with and without adjustment for SUD treatment in the two weeks before or at the baseline ED visit. All baseline characteristics hypothesized to influence SUD treatment engagement were compared between participants who did and did not engage in SUD treatment within 90 days post-discharge using χ2 tests (categorical variables) and t-tests (continuous variables), stratified by receipt of CPRS and LCSW services. Any characteristic associated with 90-day SUD treatment engagement (p≤0.10) was considered as a “potential predictor” and included in prediction analyses.
The primary prediction analyses used classification and regression trees (CART) performed in R (v4.2.2). CART is a “decision tree" algorithm that employs a non-parametric approach to create nested trees with binary splits that group participants into increasing homogenous groups based on “risk” of the outcome (Chambers et al., 2018). The recursive nature of CART makes it ideal for considering complex relations between predictors (Morgan, 2014). For binary outcomes, the Gini index and entropy values are used to define each split point (Strobl et al., 2009). Cross-validation with the optimal complexity parameter was used to build the classification tree and split the data into training (90 % for LCSW, 80 % for CPRS) and test (10 % for LCSW, 20 % for CPRS) sets (Bertsimas and Dunn, 2017, Statistical tools for high-throughput data analysis (STHDA), 2024). Training and test splits were chosen to yield CART models with highest predictive accuracy, based on best practice guidance (Bertsimas and Dunn, 2017). Training sets were used to create the nested decision trees. To avoid overfitting the data, the decision tree was “pruned” by selecting the tree that minimized cross-validation error (James et al., 2021). The test sets were then used to validate the selected decision tree. Performance of the selected tree was evaluated using: predictive accuracy (difference between observed and predicted values), sensitivity, specificity, positive predictive value, and negative predictive value.
Of note, for categorical variables used in the bivariate and CART analyses, participants with unknown information (“Don’t Know/Refused” or missing responses) were included in the “No” or “Never” group. We assumed this would not considerably impact the findings because ≤10 % of participants had unknown information for each variable. To maintain clinically meaningful interpretation, several categorical variables were collapsed into binary variables (Supplemental Table 1), and race was collapsed into fewer categories (“Black, African, Haitian, or Cape Verdean”, “White”, and “Other”).
2.5. Sensitivity analyses
To evaluate the robustness of the primary analysis, two sensitivity analyses were conducted. The prediction process was repeated using: (1) the adaptive least absolute shrinkage and selection operator (adaptive LASSO) and (2) stepwise regressions. LASSO is an extension of ordinary least squares regression that selects predictors that minimize mean squared errors and balances bias and variance in prediction models using a penalizing [tuning] parameter (λ) (Columbia University Mailman School of Public Health, 2024). Adaptive LASSO is an improvement of the LASSO variable selection process that uses adaptive penalty weights specific to each predictor, thus avoiding overfitting of large coefficients (Zou, 2006, Courtois et al., 2021). Ten-fold cross-validation was employed during the adaptive LASSO process (95 % training and 5 % test sets for both CPRS and LCSW) to identify the optimal tuning parameter that produced the best-fitting parsimonious model with minimal out-of-sample prediction error (Stata 16, 2024). The model’s predictive performance was evaluated using deviance ratios, which are analogous to the R2 for linear models and measure the relative difference of the likelihoods between the fitted adaptive LASSO models and a fully-saturated abstract model (García-Portugués, 2024, StataCorp, 2023). Deviance ratios between 0 and 1 indicate a good model fit (StataCorp, 2023).
In contrast, stepwise regression uses a stepwise process at pre-specified significance levels to add and remove potential predictors and build a model with a final set of predictors. The significance level was set at p≤ 0.15 for potential predictors to be included in the stepwise regression model and p>0.1 for removal from the model. These significance levels are the default in most statistical software and have shown reasonable bias-variance trade-off in prior simulation studies (Bursac et al., 2008).
3. Results
3.1. Sample characteristics
In total, 325 and 323 participants at high risk of opioid overdose were randomized to receive ED-based behavioral services from LCSW and CPRS, respectively, after enrollment in the trial. Due to randomization, the characteristics of participants who received LCSW and CPRS services were similar (Supplemental Table 2). In each group, the median age of participants was about 34–35 years, and most participants identified as White (LCSW=69.5 %, CPRS=67.5 %), non-Hispanic/Latino(a) (LCSW=82.8 %, CPRS=80.2 %), and male (LCSW=68.9 %, CPRS=67.5 %). At the time of the ED visit, 26.8 % (n=87) and 27.6 % (n=89) of participants who received LCSW and CPRS services, respectively, were currently or recently in SUD treatment.
Overall, 137 (42.2 %) and 141 (43.7 %) participants who received LCSW and CPRS services, respectively, engaged in SUD treatment within the 90 days post-discharge (Table 1).
Table 1.
Characteristics of SUD treatment engagement among Navigator trial participants who engaged in SUD treatment 90 days post ED discharge, stratified by behavioral service (n = 278).
| Licensed clinical social workers (n = 137) | Certified peer recovery specialists (n = 141) | p-value | |
|---|---|---|---|
| Time to SUD engagement in days, median (IQR) | 30.0 (9.0 – 62.0) | 30.0 (7.0 – 63.0) | 0.50 |
| Type of SUD treatment, n (%) | 0.88 | ||
| Methadone | 25 (18.2) | 32 (22.7) | |
| Buprenorphine | 75 (54.7) | 73 (51.8) | |
| Residential | 19 (13.9) | 20 (14.2) | |
| Detoxification | 11 (8.0) | 11 (7.8) | |
| Intensive outpatient or outpatient | 7 (5.1) | 5 (3.6) |
Baseline characteristics stratified by 90-day SUD treatment engagement and behavioral service are summarized in Table 2. Among participants who engaged in SUD treatment within 90 days, some baseline characteristics that were prevalent among participants who received CPRS services compared LCSW services included: Hispanic/Latina(o) ethnicity (20.6 % vs. 14.6 %), monthly income between $1 and $500 (18.4 % vs. 13.9 %), and a lifetime history of barriers to treatment (46.1 % vs. 40.9 %). Some baseline characteristics that were prevalent among participants who received LCSW services compared to CPRS services, among those who engaged in SUD treatment within 90 days, were: employment (29.2 % vs. 18.4 %), unhealthy alcohol use (40.2 % vs. 29.8 %), ED visit unrelated to opioids (27.7 % vs. 20.6 %), and pain (67.9 % vs. 63.1 %).
Table 2.
Baseline characteristics hypothesized to influence SUD treatment engagement among Navigator trial participants, stratified by behavioral service and 90-day SUD treatment engagement post ED discharge (n = 648).
| Licensed clinical social workers | Certified peer recovery specialists | |||
|---|---|---|---|---|
| Baseline characteristics, n (%) | Not engaged (n = 188) |
Engaged (n =137) |
Not engaged (n = 182) |
Engaged (n =141) |
| Predisposing factors | ||||
| Age, median (IQR) | 35 (29 – 46) | 34.5 (30 – 44.5) | 35.0 (29 – 44) | 34 (29 – 41) |
| Race | ||||
| American Indian or Alaska Native | 6 (3.2) | 3 (2.2) | 4 (2.2) | 4 (2.8) |
| Asian | 1 (0.5) | 1 (0.7) | 0 (0.0) | 1 (0.7) |
| Black, African, Haitian, or Cape Verdean | 11 (5.9) | 9 (6.6) | 12 (6.6) | 7 (5.0) |
| Native Hawaiian or other Pacific Islander | 1 (0.5) | 1 (0.7) | 1 (0.6) | 0 (0.0) |
| White | 132 (71.0) | 94 (68.6) | 126 (69.2) | 92 (65.3) |
| Mixed, bi-racial, or multi-racial | 16 (8.5) | 11 (8.0) | 20 (11.0) | 14 (9.9) |
| Other | 13 (6.9) | 16 (11.7) | 14 (7.7) | 19 (13.5) |
| Don't Know/Refused | 6 (3.2) | 1 (0.7) | 2 (1.1) | 4 (2.8) |
| Missing | 2 (1.1) | 1 (0.7) | 3 (1.7) | 0 (0.0) |
| Hispanic/Latina(o) | ||||
| Yes | 29 (15.4) | 20 (14.6) | 29 (15.9) | 29 (20.6) |
| No | 156 (83.0) | 113 (82.5) | 149 (81.9) | 110 (78.0) |
| Don’t Know/Refused | 1 (0.5) | 4 (2.9) | 1 (0.6) | 1 (0.7) |
| Missing | 2 (1.1) | 0 (0.0) | 3 (1.7) | 1 (0.7) |
| Female sex at birth | 60 (31.9) | 41 (29.9) | 60 (33.0) | 45 (31.9) |
| Enabling factors | ||||
| Baseline health insurance | ||||
| Yes | 164 (87.2) | 125 (91.2) | 157 (86.3) | 130 (92.2) |
| No | 17 (9.0) | 8 (5.8) | 19 (10.4) | 4 (2.8) |
| Don’t Know/Refused | 3 (1.6) | 2 (1.5) | 3 (1.7) | 4 (2.8) |
| Missing | 4 (2.1) | 2 (1.5) | 3 (1.7) | 3 (2.1) |
| Baseline monthly income | ||||
| $0 | 41 (21.8) | 40 (29.2) | 36 (19.8) | 41 (29.1) |
| $1 - $500 | 23 (12.2) | 19 (13.9) | 20 (11.0) | 26 (18.4) |
| $501 - $1500 | 61 (32.5) | 42 (30.7) | 60 (33.0) | 30 (21.3) |
| $1501 - $3000 | 26 (13.8) | 23 (16.8) | 32 (17.6) | 18 (12.8) |
| > $3000 | 17 (9.0) | 7 (5.1) | 22 (12.1) | 9 (6.4) |
| Don't Know/Refused | 16 (8.5) | 6 (4.4) | 7 (3.9) | 15 (10.6) |
| Missing | 4 (2.1) | 0 (0.0) | 5 (2.8) | 2 (1.4) |
| Baseline employment | ||||
| Yes | 55 (29.3) | 40 (29.2) | 60 (33.0) | 26 (18.4) |
| No | 127 (67.6) | 97 (70.8) | 116 (63.7) | 110 (78.0) |
| Don’t know/Refused | 2 (1.1) | 0 (0.0) | 2 (1.1) | 2 (1.4) |
| Missing | 4 (2.1) | 0 (0.0) | 4 (2.2) | 3 (2.1) |
| Baseline highest education attained | ||||
| Elementary or grade school | 8 (4.3) | 7 (5.1) | 6 (3.3) | 6 (4.3) |
| Some high school | 46 (24.5) | 41 (29.9) | 38 (20.9) | 33 (23.4) |
| Finished high school or GED | 70 (37.2) | 47 (34.3) | 62 (34.1) | 52 (36.9) |
| Some college | 49 (26.1) | 28 (20.4) | 42 (23.1) | 29 (20.6) |
| Trade or technical school | 4 (2.1) | 2 (1.5) | 7 (3.9) | 5 (3.6) |
| College or university degree or higher | 8 (4.3) | 12 (8.8) | 23 (12.6) | 13 (9.2) |
| Don't Know/Refused | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Missing | 3 (1.6) | 0 (0.0) | 4 (2.2) | 2 (1.4) |
| Lifetime history of unstable housing | 121 (64.4) | 103 (75.2) | 113 (62.1) | 104 (73.8) |
| Lifetime history of barriers to treatment access | ||||
| Yes | 57 (30.2) | 56 (40.9) | 48 (26.4) | 65 (46.1) |
| No | 124 (66.0) | 79 (57.7) | 126 (69.2) | 72 (51.1) |
| Don’t know/Refused | 3 (1.6) | 2 (1.5) | 5 (2.8) | 2 (1.4) |
| Missing | 4 (2.1) | 0 (0.0) | 3 (1.7) | 2 (1.4) |
| Lifetime history of arrest | 154 (81.9) | 117 (85.4) | 149 (81.9) | 117 (83.0) |
| Baseline receipt of disability benefits | ||||
| Yes | 51 (27.1) | 30 (21.9) | 47 (25.8) | 31 (22.0) |
| No | 132 (70.2) | 107 (78.1) | 130 (71.4) | 107 (75.9) |
| Don’t know/Refused | 1 (0.5) | 0 (0.0) | 1 (0.6) | 1 (0.7) |
| Missing | 4 (2.1) | 0 (0.0) | 4 (2.2) | 2 (1.4) |
| Need factors | ||||
| Baseline unhealthy alcohol use | ||||
| Yes | 85 (45.2) | 55 (40.2) | 78 (42.9) | 42 (29.8) |
| No | 85 (45.2) | 72 (52.6) | 79 (43.4) | 77 (54.6) |
| Don’t know/Refused | 1 (0.5) | 2 (1.5) | 1 (0.6) | 4 (2.8) |
| Missing | 17 (9.0) | 8 (5.8) | 24 (13.2) | 18 (12.8) |
| Reason for ED visit | ||||
| Opioid overdose | 95 (50.5) | 53 (38.7) | 90 (49.5) | 59 (41.8) |
| Infectious complication of OUD | 5 (2.7) | 10 (7.3) | 12 (6.6) | 9 (6.4) |
| Opioid withdrawal | 6 (3.2) | 8 (5.8) | 9 (5.0) | 11 (7.8) |
| Seeking treatment/detox for opioids | 17 (9.0) | 15 (11.0) | 9 (5.0) | 20 (14.2) |
| Other | 16 (8.5) | 12 (8.8) | 14 (7.7) | 13 (9.2) |
| Not opioid related | 49 (26.1) | 38 (27.7) | 47 (25.8) | 29 (20.6) |
| Missing | 0 (0.0) | 1 (0.7) | 1 (0.6) | 0 (0.0) |
| Baseline pain | 108 (57.5) | 93 (67.9) | 115 (63.2) | 89 (63.1) |
| Prescription opioid or benzodiazepine use in P6Mb | 120 (63.8) | 99 (72.3) | 109 (59.9) | 94 (66.7) |
| Marijuana use in P6M | 121 (64.4) | 86 (62.8) | 114 (62.6) | 82 (58.2) |
| Illicit substance use in P6Mc | 144 (76.6) | 112 (81.8) | 128 (70.3) | 107 (75.9) |
| Previous opioid overdose in P12Ma | ||||
| Yes | 17 (9.0) | 21 (15.3) | 15 (8.2) | 19 (13.5) |
| No | 171 (91.0) | 115 (83.9) | 166 (91.2) | 122 (86.5) |
| Missing | 0 (0.0) | 1 (0.7) | 1 (0.6) | 0 (0.0) |
| Lifetime history of depressive disorder diagnosis | 89 (47.3) | 77 (56.2) | 90 (49.5) | 81 (57.5) |
| Lifetime history of anxiety disorder diagnosis | 88 (46.8) | 81 (59.1) | 97 (53.3) | 85 (60.3) |
| Lifetime history of bipolar disorder diagnosis | 42 (22.3) | 45 (32.9) | 46 (25.3) | 44 (31.2) |
| Lifetime history of psychosis diagnosis | 21 (11.2) | 17 (12.4) | 13 (7.1) | 14 (9.9) |
| Lifetime history of injecting drugs | 89 (47.3) | 75 (54.7) | 82 (45.1) | 76 (53.9) |
| Lifetime history of any mental health diagnosis | 133 (70.7) | 105 (76.6) | 133 (73.1) | 100 (70.9) |
| Health behavior factors | ||||
| Lifetime history of mental illness treatment | ||||
| Yes | 83 (44.2) | 74 (54.0) | 67 (36.8) | 70 (49.7) |
| No | 85 (45.2) | 48 (35.0) | 87 (47.8) | 55 (39.0) |
| Don’t know/Refused | 3 (1.6) | 2 (1.5) | 3 (1.7) | 3 (2.1) |
| Missing | 17 (9.0) | 13 (9.5) | 25 (13.7) | 13 (9.2) |
| Lifetime history of addiction treatment | 138 (73.4) | 114 (83.2) | 125 (68.7) | 115 (81.6) |
| Lifetime history of recovery services | ||||
| Yes | 54 (28.7) | 55 (40.2) | 60 (33.0) | 52 (36.9) |
| No | 129 (68.6) | 80 (58.4) | 119 (65.4) | 87 (61.7) |
| Don’t know/Refused | 1 (0.5) | 2 (1.5) | 0 (0.0) | 0 (0.0) |
| Missing | 4 (2.1) | 0 (0.0) | 3 (1.7) | 2 (1.4) |
P12M: past 12 months.
P6M: past 6 months.
Crystal methamphetamine, cocaine, heroin, or club drugs in the P6M.
3.2. Time to SUD treatment engagement
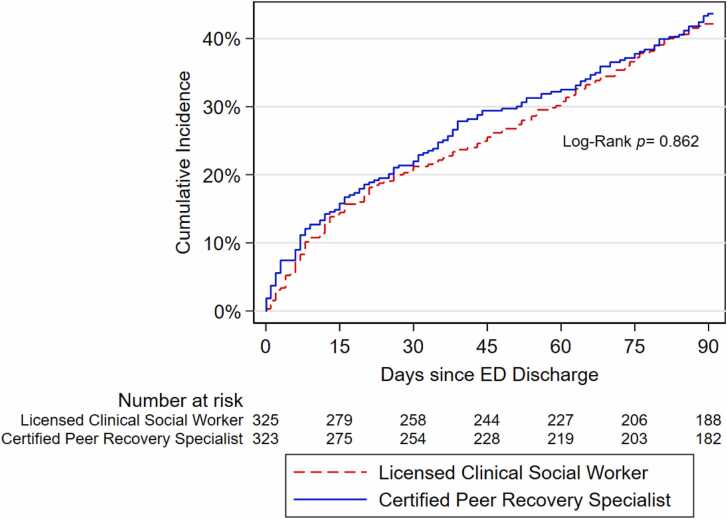
Median time to SUD treatment engagement post ED discharge was similar for participants who received LCSW and CPRS services (median=30 days, interquartile range [IQR]=9.0–62.0 days for LCSW, and median=30 days, IQR=7.0–63.0 days for CPRS; p-value=0.50). In Cox-PH analyses, likelihood of SUD treatment engagement was also similar for participants who received CPRS compared to LCSW services, without (hazard ratio [HR]=1.06, 95 % confidence interval [CI]=0.84–1.34) and with (HR=1.08, 95 %CI=0.85–1.37) adjustment for SUD treatment engagement in the two weeks before or at the ED visit. Fig. 1 depicts the cumulative incidence of SUD treatment engagement within 90 days post-discharge by behavioral service. SUD treatment engagement occurred at a similar rate over time in the CPRS and LCSW groups, with higher engagement by participants who received CPRS services between 30 and 60 days post-discharge. After 75 days, there was no difference in the percentage of participants who had engaged in SUD treatment between the groups.
Fig. 1.
Cumulative incidence curve for time to engagement in SUD treatment 90 days post ED discharge by behavioral service among Navigator trial participants (n = 648).
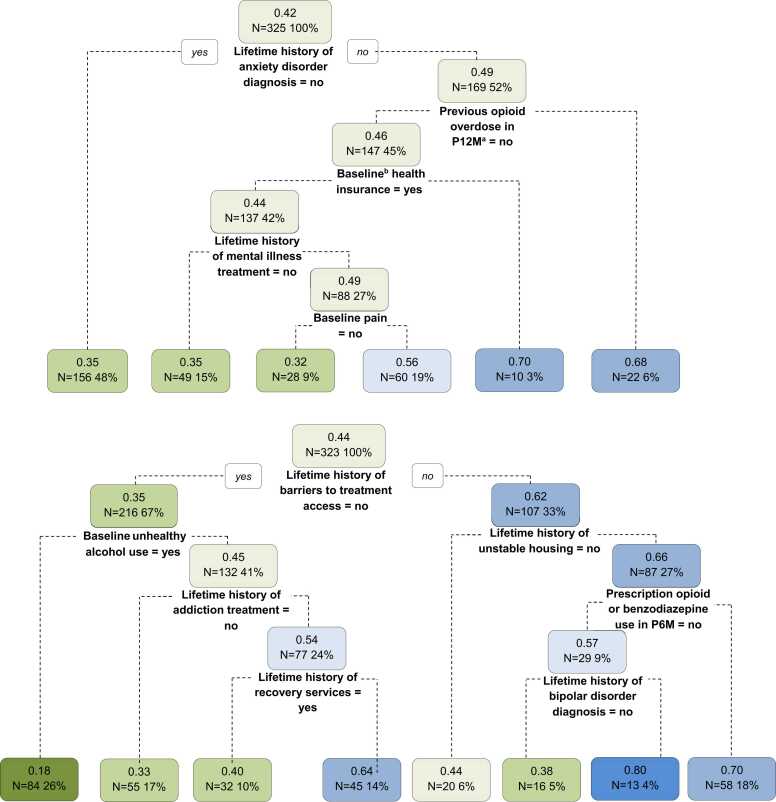
3.3. Baseline predictors of SUD treatment engagement
In bivariate analyses, 16 baseline characteristics met our pre-specified criteria for inclusion in prediction analyses as potential predictors (Table 3). For participants who received CPRS services, of these 16 variables, the CART analyses selected no lifetime history of bipolar disorder, having unhealthy alcohol use at baseline, not using prescription opioid or benzodiazepine use in the P6M, no lifetime history of unstable housing, no lifetime history of barriers to treatment access, no lifetime history of addiction treatment, and lifetime history of recovery services as predictors of 90-day SUD treatment engagement (Table 3 and Fig. 2; bottom panel). However, the predictive performance of the optimal CART decision tree was poor, as it accurately predicted 90-day treatment engagement for only 60.9 % of participants who received CPRS services, and its sensitivity and specificity were 65.7 % and 55.2 %, respectively (Supplemental Table 3). For participants who received LCSW services, CART analysis identified the following predictors of 90-day SUD treatment engagement: having health insurance at baseline, no lifetime history of anxiety disorder, no pain at baseline, no previous opioid overdose in the P12M, and no lifetime history of mental illness treatment (Table 3 and Fig. 2; top panel). The performance of the optimal CART decision tree for the LCSW group was also poor; it had low predictive accuracy (54.8 %), sensitivity (58.3 %), and specificity (42.9 %).
Table 3.
Predictors selected using CART, LASSO, and stepwise regression algorithms by behavioral service among Navigator trial participants (n = 648)a.
| Predictors | CART | Adaptive LASSO | Stepwise Regression | |||
|---|---|---|---|---|---|---|
| LCSW | CPRS | LCSW | CPRS | LCSW | CPRS | |
| Enabling factors | ||||||
| Baseline health insurance | O | X | ||||
| Baseline monthly income | X | |||||
| Baseline employment | X | X | ||||
| Lifetime history of unstable housing | X | O | ||||
| Lifetime history of barriers to treatment access | X | X | X | |||
| Need factors | ||||||
| Baseline unhealthy alcohol use | X | X | X | |||
| Baseline pain | O | |||||
| Prescription opioid or benzodiazepine use in P6Mc | X | X | X | |||
| Previous opioid overdose in P12Mb | O | |||||
| Lifetime history of anxiety disorder diagnosis | O | O | ||||
| Lifetime history of bipolar disorder diagnosis | X | |||||
| Lifetime history of injecting drugs | X | |||||
| Health behavior factors | ||||||
| Lifetime history of mental illness treatment | O | X | X | |||
| Lifetime history of addiction treatment | X | X | X | |||
| Lifetime history of recovery services | X | O | ||||
The table only lists 15 of the 16 baseline characteristics that met the pre-specified criteria for inclusion in prediction analyses from bivariate analyses; lifetime history of depressive disorder was omitted from the table as it was not selected by any of the three prediction methods.
P12M: past 12 months.
P6M: past 6months.
Fig. 2.
Classification and regression trees predicting engagement in SUD treatment 90 days post ED discharge for Navigator trial participants randomized to LCSW (top; n = 325) or CPRS (bottom; n = 323). A round square box represents a node. Within each node, the number on the first line is the probability of engagement in SUD treatment within 90 days post-discharge. Green nodes indicate probabilities of engagement in SUD treatment <50 % (the lower the probability, the greener the node), and blue nodes indicate probabilities of engagement in SUD treatment ≥50 % (the higher the probability, the bluer the node). The text on the second line shows the sample size in that node. aP12M = past 12 months. bBaseline = ED visit.
In sensitivity analyses, the LASSO and stepwise regression identified nine and six predictors, respectively, for 90-day SUD treatment engagement among participants who received CPRS services (Table 3). Four predictors from these sensitivity analyses overlapped with the predictors identified by CART analyses. However, the performance of these two predictive methods was also poor (Supplemental Table 3). For participants who received LCSW services, the LASSO model did not identify any predictors of 90-day SUD treatment engagement, while stepwise regression identified three predictors, only one of which overlapped with the predictors identified by CART.
4. Discussion
Among ED patients at high risk of opioid overdose who were randomized to receive ED-based behavioral services from either a CPRS or LCSW, a similar percentage engaged in SUD treatment within 90 days post-discharge (42–44 %). Time to SUD treatment engagement was also similar across the two groups (median=30 days). Prediction analysis using a non-parametric algorithm identified that, for patients who received LCSW services, not having social/structural barriers to care access (e.g., having health insurance), no diagnosed/perceived health conditions (e.g., no lifetime history of anxiety disorder), and not having co-morbid conditions (e.g., no lifetime history of mental illness treatment) predicted 90-day SUD treatment engagement. Similarly, for participants who received CPRS services, not having social/structural barriers to care access (e.g., no lifetime history unstable housing) and no diagnosed/perceived health conditions (e.g., no lifetime history of bipolar disorder) predicted treatment engagement. However, given the low predictive accuracy and the variation in predictors identified in sensitivity analyses, the current analysis cannot draw firm conclusions about the ability of these predictors to distinguish between patients more likely to benefit from ED-based behavioral services provided by a CPRS versus LCSW. Nonetheless, the predictors clustered within common overarching themes of the Behavioral Model of Healthcare Utilization Framework and may inform future efforts to identify barriers to treatment access.
Social/structural barriers (enabling factors) such as lack of health insurance and unstable housing are markers of economic instability that greatly influence SUD treatment engagement and treatment of other co-morbid conditions (Bell et al., 2023). Individuals experiencing economic instability may not be able to prioritize SUD treatment due to more critical needs of housing or due to limited insurance coverage and high deductibles (Acevedo et al., 2020). Medicaid expansion has significantly enhanced coverage of SUD care and decreased the uninsured rates of individuals with opioid-related hospital visits (Bailey et al., 2024). RI is among the few states that offer the full continuum of SUD care in the state’s Medicaid program; however, the present study suggests that further provisions in housing support services may be needed (Executive Office of Health and Human Services State of Rhode Island, 2024a, Executive Office of Health and Human Services State of Rhode Island, 2024b, Executive Office of Health and Human Services State of Rhode Island, 2024c, Executive Office of Health and Human Services State of Rhode Island, 2024d). For example, resources for and linkage to residential aftercare programs for patients receiving ED-based behavioral services may increase SUD treatment engagement for those who are homeless (Jason and Harvey, 2022).
The presence (need factors) and management (health behavior factors) of comorbidities, especially mental health conditions, are integral to successful linkage to SUD care (National Institute on Drug Abuse NIDA, 2024a). Mental health conditions and substance use often co-occur due to shared risk factors (e.g., environmental stressors, genetic vulnerabilities, trauma/stress) (Nestler, 2014, Cerdá et al., 2010, Pelayo-Teran et al., 2012, Kelly and Daley, 2013). In the current study, 73 % of ED patients at high risk of opioid overdose also reported a prior mental health diagnosis. Individuals with co-occurring mental health conditions and SUD experience significant barriers to treatment for both conditions, due to a fragmented healthcare system that is unable to provide comprehensive care suitable to their complex needs (Jones and McCance-Katz, 2019, Novak et al., 2019, Iturralde et al., 2021). The absence of prior mental illness treatment and mental health diagnoses (e.g., anxiety, bipolar disorder) predicted SUD treatment engagement and may indicate that those without these co-morbid conditions experienced fewer barriers to treatment. However, a prior study among the current study population identified that prior hospitalization for mental illness was associated with increased treatment access (Rosenfield et al., 2023). Pain and prior overdose have been identified as health conditions associated with decreased treatment engagement (Mutter et al., 2022, Naeger et al., 2016). Others have suggested that patients may be hesitant to seek SUD treatment for pain relief as they perceive it as a pathway to opioid dependence, and patients who experienced a recent opioid overdose may have complex and distinctive treatment needs post-discharge compared those without a recent overdose (Naeger et al., 2016, Stumbo et al., 2017). While individuals who experience life-threatening events may exhibit behavioral changes towards an increased willingness of recovery, it is possible that the behavioral interventions in the current study are not sufficiently engaging ED patients with a prior overdose to access treatment (Langabeer et al., 2020). Prescriptions for opioids or benzodiazepines was also associated with decreased treatment engagement. Due to the considerable health risks associated with co-prescribing benzodiazepines and opioids, patients receiving SUD treatment, which includes opioid agonist treatment, are less likely to use benzodiazepines (National Institute on Drug Abuse NIDA, 2024b). In contrast, unhealthy alcohol use predicted SUD treatment engagement, possibly owing to ease of identifying symptoms of alcohol misuse within the ED context. Lastly, prior experience with recovery services unsurprisingly increased SUD engagement.
Compared to prior studies of ED behavioral interventions, the percentage of patients who engaged in SUD treatment differed in the current study. Chambers et al. found that 60 % of ED patients treated for opioid overdose and who received ED behavioral counseling (including psychiatry, social work, and/or peer support consultations) engaged in OUD treatment within 30 days (Chambers et al., 2023). Similarly, among ED patients with SUD, 50 % engaged in outpatient addiction treatment within 30 days following an ED behavioral intervention administered by a community health worker (Anderson et al., 2023). However, the latter study may have included some lower risk ED patients with SUD, and the ED behavioral interventions differed across studies. Lower treatment engagement in the current study may also reflect the additional barriers that influence SUD treatment engagement among ED patients at high risk of overdose, (Collins et al., 2023) including housing needs or lack of employment (Hawk et al., 2021). Of note, 35 % of participants in the current study had previously experienced barriers to treatment access. Finally, experiences of poor provider communication, stigma, and negative perceived attitudes on drug use in the ED may have also reduced treatment engagement (Hawk et al., 2021, Carusone et al., 2019).
Although accurate prediction models of SUD treatment engagement following an ED visit with LCSW and CPRS services were not identified, these ED services nonetheless have an important role. LCSWs are the largest group of behavioral and mental health providers trained in recovery-oriented practices (Serrano and Conley, 2021, Lombardi et al., 2019). Although they cannot prescribe medications for OUD, they partake in clinical decisions on SUD treatment programs and have a considerable understanding of the community setting that promotes engagement and retention in care (Lundgren and Krull, 2018, Bride et al., 2013). On the other hand, CPRS services are unique non-clinical mentoring and coaching services provided by individuals with lived experiences navigating addiction and recovery, including ongoing support following ED discharge (Liebling et al., 2021, Executive Office of Health and Human Services State of Rhode Island, 2024b). Both LCSW and CPRS operate within the acute-crisis setting of the ED to assess, engage, and link patients to SUD treatment services at a critical point in time, thus, preventing future ED visits and hospitalizations (Ashford et al., 2019). Studies have found that these behavioral services are effective in improving the health and recovery of patients with SUD within both inpatient and outpatient settings (Anderson et al., 2023, Lintzeris et al., 2020, Magidson et al., 2021, Shumway et al., 2008). However, the current study is unique in its evaluation of predictors of treatment engagement following both LCSW and CPRS services in the ED using a non-parametric algorithm. Of note, prior work has found that interventions to improve linkage to SUD treatments in the ED were most successful when a multidisciplinary team of LCSW, CPRS, psychiatrists, addiction specialists, and pharmacists coordinated to provide patients with a customized treatment plan of psychosocial support as well as inpatient, outpatient, and community-based recovery services (Lintzeris et al., 2020, Wakeman et al., 2017). The effectiveness of care coordination efforts started in the ED likely depends on continued connections with both clinical and community resources capable of providing inclusive, person-centered, relationship-based, tailored care.
4.1. Strengths and limitations
This study has some limitations. First, the analysis included a high-risk ED patient population receiving treatment within RI, which may limit generalizability to other clinical and geographic settings. Second, the study was conducted in a relatively small sample size with limited power to detect small differences in SUD treatment engagement by LCSW and CPRS services. Third, most of the covariates from the baseline questionnaire were self-reported and subject to recall and social desirability bias. While missingness was <10 % overall, there is still a chance of misclassification bias as categorical variables were collapsed. Fourth, there may have been other important predictors of SUD treatment engagement that were not available in the baseline questionnaire. Fifth, participants currently or recently engaged in SUD treatment at the time of the ED visit were not excluded. Preliminary analyses showed this variable to be a near-perfect predictor of the outcome, and it was therefore not included as a potential predictor. Findings might therefore differ if the sample was limited to those without prior SUD treatment. Lastly, the current study did not aim to differentiate the effectiveness of ED-based behavioral interventions delivered by CPRS compared to LCSWs among subgroups of ED patients at high risk of opioid overdose. Instead, it sought to identify whether the patient subgroups engaging in SUD treatment following each service type were similar or distinctive. However, the study was strengthened by its use of multiple prediction methods, with the primary method using a non-parametric strategy. CART is a useful method for identifying complex relationships in the data and displaying those associations in easy-to-interpret visualizations (Morgan, 2014). The Behavioral Model of Healthcare Utilization Framework provided a structured approach to identifying relevant predictors informed by clinical expertise. Finally, the exposure was well-defined, and the outcome of SUD treatment engagement was obtained using statewide administrative data which circumvented loss to follow-up.
5. Conclusion
While engagement in SUD treatment was observed for about 43 % of ED patients at high risk of opioid overdose following an ED behavioral intervention with either a LCSW or CPRS, there were no strong predictors of treatment engagement in either group. These findings underscore the importance of providing behavioral counseling services for all patients in the ED at high risk of opioid overdose.
Role of funding source
The Navigator trial was funded by Arnold Ventures and the Cigna Foundation through investigator-initiated trial programs. Dr. Marshall was partially supported by the National Institute of General Medical Sciences of the NIH [grant number: P20GM125507] and the National Institute on Allergy and Infectious Disease of the NIH [grant number: 3P30AI042853]. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
CRediT authorship contribution statement
Brandon David Lewis Marshall: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Francesca Beaudoin: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Laura Chambers: Writing – review & editing, Validation, Supervision, Resources, Project administration, Data curation. Mackenzie Daly: Writing – review & editing, Resources, Data curation. Jamieson Goulet: Writing – review & editing, Resources, Data curation. Benjamin Hallowell: Writing – review & editing, Resources, Data curation. Linda Mahoney: Writing – review & editing, Resources, Data curation. Fiona Bhondoekhan: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Formal analysis, Conceptualization. Yu Li: Writing – review & editing, Software, Resources, Methodology, Formal analysis, Data curation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brandon Marshall reports financial support was provided by Arnold Ventures LLC. Brandon Marshall reports financial support was provided by Cigna Foundation. Dr. Brandon Marshall was partially supported by the National Institute of General Medical Sciences of the NIH [grant number: P20GM125507] and the National Institute on Allergy and Infectious Disease of the NIH [grant number: 3P30AI042853]. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the Navigator Trial participants for their contributions to the study, as well as Dr. Brendan Jacka for assisting with the design and concept of this analysis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dadr.2024.100287.
Appendix A. Supplementary material
Supplementary material
References
- Abbott M., Landers P., Pratt E. Office of the Assistant Secretary for Planning and Evaluation. U.S. Department of Health and Human Services; 2019. Peer-to-Peer Supports: Promoting Employment and Well-Being; p. 11.〈https://aspe.hhs.gov/sites/default/files/private/aspe-files/261791/promotingemploymentwellbeing.pdf〉 [Google Scholar]
- Acevedo A., Harvey N., Kamanu M., Tendulkar S., Fleary S. Barriers, facilitators, and disparities in retention for adolescents in treatment for substance use disorders: a qualitative study with treatment providers. Subst. Abus. Treat. Prev. Policy. 2020;15(1):1–13. doi: 10.1186/S13011-020-00284-4/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.S., Rusoja E., Luftig J., et al. Effectiveness of substance use navigation for emergency department patients with substance use disorders: an implementation study. Ann. Emerg. Med. 2023;81(3):297–308. doi: 10.1016/J.ANNEMERGMED.2022.09.025. [DOI] [PubMed] [Google Scholar]
- Ashford R.D., Meeks M., Curtis B., Brown A.M. Utilization of peer-based substance use disorder and recovery interventions in rural emergency departments: patient characteristics and exploratory analysis. J. Rural Ment. Health. 2019;43(1):17–29. doi: 10.1037/RMH0000106. [DOI] [Google Scholar]
- Auerbach C., Mason S.E. The value of the presence of social work in emergency departments. Soc. Work Health Care. 2010;49(4):314–326. doi: 10.1080/00981380903426772. [DOI] [PubMed] [Google Scholar]
- Bailey A., Hayes K., Katch H., Solomon. Medicaid Is Key to Building a System of Comprehensive Substance Use Care for Low-Income People. Center on Budget and Policy Priorities. Accessed March 28, 2024. 〈https://www.cbpp.org/research/health/medicaid-is-key-to-building-a-system-of-comprehensive-substance-use-care-for-low〉
- Beaudoin F.L., Jacka B.P., Li Y., et al. Effect of a peer-led behavioral intervention for emergency department patients at high risk of fatal opioid overdose. JAMA Netw. Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J.S., Kang A., Benner S., Bhatia S., Jason L.A. J Soc Work Pract Addict. Published online; 2023. Predictors of health in substance use disorder recovery: economic stability in residential aftercare environments. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsimas D., Dunn J. Optimal classification trees. Mach. Learn. 2017;106(7):1039–1082. doi: 10.1007/S10994-017-5633-9/TABLES/19. [DOI] [Google Scholar]
- Blakeman T., Macdonald W., Bower P., Gately C., Chew-Graham C. A qualitative study of GPs’ attitudes to self-management of chronic disease. Br. J. Gen. Pr. 2006;56(527):407–414. [PMC free article] [PubMed] [Google Scholar]
- Boisvert R.A., Martin L.M., Grosek M., Clarie A.J. Effectiveness of a peer-support community in addiction recovery: participation as intervention. Occup. Ther. Int. 2008;15(4):205–220. doi: 10.1002/oti.257. [DOI] [PubMed] [Google Scholar]
- Bride B.E., Abraham A.J., Kintzle S., Roman P.M. Social Workers’ knowledge and perceptions of effectiveness and acceptability of medication assisted treatment of substance use disorders. Soc. Work Health Care. 2013;52(1):43. doi: 10.1080/00981389.2012.725457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusone S.C., Guta A., Robinson S., et al. Maybe if I stop the drugs, then maybe they’d care?-hospital care experiences of people who use drugs. Harm Reduct. J. 2019;16(1) doi: 10.1186/S12954-019-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M., Sagdeo A., Johnson J., Galea S. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J. Affect Disord. 2010;126(1-2):14–38. doi: 10.1016/J.JAD.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers L.C., Hallowell B.D., Samuels E.A., Daly M., Baird J., Beaudoin F.L. An evaluation of the association between specific post-overdose care services in emergency departments and subsequent treatment engagement. J. Am. Coll. Emerg. Physicians Open. 2023;4(1) doi: 10.1002/EMP2.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers L.C., Manhart L.E., Katz D.A., Golden M.R., Barbee L.A., Dombrowski J.C. Comparison of algorithms to triage patients to express care in a sexually transmitted disease clinic. Sex. Transm. Dis. 2018;45(10):696–702. doi: 10.1097/OLQ.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinman M., George P., Dougherty R., et al. Peer support services for individuals with serious mental illnesses: assessing the evidence. Psychiatr. Serv. 2024;65(4) doi: 10.1176/appi.ps.201300244. Accessed August 19. Accessed August 19. [DOI] [PubMed] [Google Scholar]
- Collins A.B., Baird J., Nimaja E., Ashenafi Y., Clark M.A., Beaudoin F.L. Experiences of patients at high risk of opioid overdose accessing emergency department and behavioral health interventions: a qualitative analysis in an urban emergency department. BMC Health Serv. Res. 2023;23(1):1–11. doi: 10.1186/S12913-023-09387-7/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbia University Mailman School of Public Health. Least Absolute Shrinkage and Selection Operator (LASSO). Accessed March 28, 2024. 〈https://www.publichealth.columbia.edu/research/population-health-methods/least-absolute-shrinkage-and-selection-operator-lasso〉
- Cos T.A., LaPollo A.B., Aussendorf M., Williams J.M., Malayter K., Festinger D.S. Do peer recovery specialists improve outcomes for individuals with substance use disorder in an integrative primary care setting? A program evaluation. J. Clin. Psychol. Med Settings. 2020;27(4):704–715. doi: 10.1007/s10880-019-09661-z. [DOI] [PubMed] [Google Scholar]
- Courtois É., Tubert-Bitter P., Ahmed I. New adaptive lasso approaches for variable selection in automated pharmacovigilance signal detection. BMC Med Res Method. 2021;21(1) doi: 10.1186/s12874-021-01450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L., amy C.B., Guy K., er R a M. Peer support among persons with severe mental illnesses: a review of evidence and experience. World Psychiatry. 2012;11(2):123–128. doi: 10.1016/j.wpsyc.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D., Hoffman L., Vilsaint C., et al. Lived experience in new models of care for substance use disorder: a systematic review of peer recovery support services and recovery coaching. Front Psychol. 2019;10(JUN) doi: 10.3389/fpsyg.2019.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive Office of Health and Human Services State of Rhode Island. Rehabilitative Services Policy: Substance Abuse Treatment Services. Accessed March 28, 2024a. 〈https://eohhs.ri.gov/ProvidersPartners/ProviderManualsGuidelines/MedicaidProviderManual/RehabilitativeService/SubstanceAbuseTreatmentServices.aspx〉
- Executive Office of Health and Human Services State of Rhode Island. Peer Recovery Services. March 28, 2024b. 〈https://eohhs.ri.gov/providers-partners/provider-manuals-guidelines/medicaid-provider-manual/peer-recovery-services〉
- Executive Office of Health and Human Services State of Rhode Island. Recovery Navigation Program |. Accessed March 28, 2024c. 〈https://eohhs.ri.gov/providers-partners/provider-manuals-guidelines/medicaid-provider-manual/recovery-navigation-program〉
- Executive Office of Health and Human Services State of Rhode Island. Rehabilitative Services: Medical Coverage Guidelines. March 28, 2024d. 〈https://eohhs.ri.gov/providers-partners/provider-manuals-guidelines/medicaid-provider-manual/rehabilitative-services〉
- García-Portugués E. Lab notes for Statistics for Social Sciences II: Multivariate Techniques - 4.7 Deviance and model fit. Accessed March 28, 2024. 〈https://bookdown.org/egarpor/SSS2-UC3M/logreg-deviance.html〉
- Gideon C. Predictors of Short-term Residential Treatment Completion Predictors of Short-term Residential Treatment Completion Preceded by Detoxification for Opioid Use Disorder Preceded by Detoxification for Opioid Use Disorder. 〈https://digitalcommons.library.tmc.edu/uthson_etd/41〉
- Goedel W.C., Marshall B.D.L., Samuels E.A., et al. Randomised clinical trial of an emergency department-based peer recovery support intervention to increase treatment uptake and reduce recurrent overdose among individuals at high risk for opioid overdose: Study protocol for the navigator trial. BMJ Open. 2019;9(11) doi: 10.1136/bmjopen-2019-032052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk K., Grau L.E., Fiellin D.A., et al. A qualitative study of emergency department patients who survived an opioid overdose: perspectives on treatment and unmet needs. Acad. Emerg. Med. 2021;28(5):542–552. doi: 10.1111/ACEM.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari O., Genberg B.L., Perrin N., et al. Multimorbidity classes indicate differential patterns of health care engagement among people who inject drugs. J. Subst. Abus. Treat. May 2022 doi: 10.1016/j.jsat.2022.108806. (Published online) (Published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturralde E., Slama N., Kline-Simon A.H., Young-Wolff K.C., Mordecai D., Sterling S.A. Premature mortality associated with severe mental illness or substance use disorder in an integrated health care system. Gen. Hosp. Psychiatry. 2021;68:1–6. doi: 10.1016/J.GENHOSPPSYCH.2020.11.002. [DOI] [PubMed] [Google Scholar]
- James G., Witten D., Hastie T., Tibshirani R. An Introduction to Statistical Learning: With Applications in R. 2nd ed. Springer Texts in Statistics; 2021.
- Jason L.A., Harvey R. Recovery homes provide inexpensive and accessible community-based support. J. Prev. Inter. Community. 2022;50(2):117–123. doi: 10.1080/10852352.2021.1934949. [DOI] [PubMed] [Google Scholar]
- Jones C.M., McCance-Katz E.F. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78–82. doi: 10.1016/J.DRUGALCDEP.2018.12.030. [DOI] [PubMed] [Google Scholar]
- Kelly T.M., Daley D.C. Integrated treatment of substance use and psychiatric disorders. Soc. Work Public Health. 2013;28(0):388. doi: 10.1080/19371918.2013.774673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langabeer J., Champagne-Langabeer T., Luber S.D., et al. Outreach to people who survive opioid overdose: Linkage and retention in treatment. J. Subst. Abus. Treat. 2020;111:11–15. doi: 10.1016/J.JSAT.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Langabeer J.R., Stotts A.L., Bobrow B.J., et al. Prevalence and charges of opioid-related visits to U.S. emergency departments. Drug Alcohol Depend. 2021;221 doi: 10.1016/J.DRUGALCDEP.2021.108568. [DOI] [PubMed] [Google Scholar]
- Laudet A.B., Humphreys K. Promoting recovery in an evolving policy context: what do we know and what do we need to know about recovery support services? J. Subst. Abus. Treat. 2013;45(1):126–133. doi: 10.1016/j.jsat.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebling E.J., Perez J.J.S., Litterer M.M., Greene C. Implementing hospital-based peer recovery support services for substance use disorder. Am. J. Drug Alcohol Abus. 2021;47(2):229–237. doi: 10.1080/00952990.2020.1841218. [DOI] [PubMed] [Google Scholar]
- Lintzeris N., Deacon R.M., Shanahan M., et al. Evaluation of an assertive management and integrated care service for frequent emergency department attenders with substance use disorders: the impact project: evaluating an assertive management service for frequent ED attenders with substance use disorders. Int J. Integr. Care. 2020;20(2) doi: 10.5334/IJIC.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi B.M., Zerden L. de S., Guan T., Prentice A. The role of social work in the opioid epidemic: office-based opioid treatment programs. Soc. Work Health Care. 2019;58(3):339–344. doi: 10.1080/00981389.2018.1564109. [DOI] [PubMed] [Google Scholar]
- Lundgren L., Krull I. Oxford University Press; 2018. Screening, assessment, and treatment of substance use disorders: Evidence-based practices, community and organizational setting in the era of integrated care. [Google Scholar]
- Macmadu A., Paull K., Youssef R., et al. Predictors of enrollment in opioid agonist therapy after opioid overdose or diagnosis with opioid use disorder: a cohort study. Drug Alcohol Depend. 2021;226 doi: 10.1016/j.drugalcdep.2021.108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras B.K., Sharfstein J., Hopkins J., School B. Improving Access to Evidence-Based Medical Treatment for Opioid Use Disorder: Strategies to Address Key Barriers within the Treatment System. Published online 2020. [DOI] [PMC free article] [PubMed]
- Magidson J.F., Regan S., Powell E., et al. Peer recovery coaches in general medical settings: changes in utilization, treatment engagement, and opioid use. J. Subst. Abus. Treat. 2021;122 doi: 10.1016/J.JSAT.2020.108248. [DOI] [PubMed] [Google Scholar]
- Mauro P.M., Gutkind S., Annunziato E.M., Samples H. Use of medication for opioid use disorder among us adolescents and adults with need for opioid treatment, 2019. JAMA Netw. Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire A.B., Powell K.G., Treitler P.C., et al. Emergency department-based peer support for opioid use disorder: emergent functions and forms. J. Subst. Abus. Treat. 2020;108:82–87. doi: 10.1016/j.jsat.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Whiteside L.K., Dotolo D., et al. The role of social work in providing mental health services and care coordination in an urban trauma center emergency department. Psychiatr. Serv. 2016;67(12):1348–1354. doi: 10.1176/appi.ps.201500469. [DOI] [PubMed] [Google Scholar]
- Morgan J. Classification and regression tree analysis. Boston Univ Sch Public Health. Published online 2014. 〈https://www.bu.edu/sph/files/2014/05/MorganCART.pdf〉
- Mutter R., Spencer D., McPheeters J. Factors associated with initial treatment choice, engagement, and discontinuation for patients with opioid use disorder. Psychiatr. Serv. Wash. DC. 2022;73(6):604–612. doi: 10.1176/APPI.PS.202100239/SUPPL_FILE/APPI.PS.202100239.DS001.PDF. [DOI] [PubMed] [Google Scholar]
- Naeger S., Mutter R., Ali M.M., Mark T., Hughey L. Post-discharge treatment engagement among patients with an opioid-use disorder. J. Subst. Abus. Treat. 2016;69:64–71. doi: 10.1016/J.JSAT.2016.07.004. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA). Part 1: The Connection Between Substance Use Disorders and Mental Illness. Common Comorbidities with Substance Use Disorders Research Report. Accessed March 28, 2024a. 〈https://nida.nih.gov/publications/research-reports/common-comorbidities-substance-use-disorders/part-1-connection-between-substance-use-disorders-mental-illness〉
- National Institute on Drug Abuse (NIDA). Benzodiazepines and Opioids. Accessed March 28, 2024b. 〈https://nida.nih.gov/research-topics/opioids/benzodiazepines-opioids〉
- Nestler E.J. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(0 0):259–268. doi: 10.1016/J.NEUROPHARM.2013.04.004. (Pt B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Feder K.A., Ali M.M., Chen J. Behavioral health treatment utilization among individuals with co-occurring opioid use disorder and mental illness: evidence from a national survey. J. Subst. Abus. Treat. 2019;98:47–52. doi: 10.1016/J.JSAT.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober A.J., Watkins K.E., McCullough C.M., Setodji C.M., Osilla K., Hunter S.B. Patient predictors of substance use disorder treatment initiation in primary care. J. Subst. Abus. Treat. 2018;90:64–72. doi: 10.1016/j.jsat.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo-Teran J.M., Suarez-Pinilla P., Chadi N., Crespo-Facorro B. Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr. Pharm. Des. 2012;18(32):5024–5035. doi: 10.2174/138161212802884609. [DOI] [PubMed] [Google Scholar]
- Reblin M., Uchino B.N. Social and emotional support and its implication for health. Curr. Opin. Psychiatry. 2008;21(2):201–205. doi: 10.1097/YCO.0b013e3282f3ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda L.N., West K.L., LeSaint K.T. Opioid overdose–related emergency department visits and accidental deaths during the COVID-19 pandemic. J. Urban Health. 2020;97(6):808–813. doi: 10.1007/S11524-020-00486-Y/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield M.N., Beaudoin F.L., Gaither R., et al. Association between comorbid chronic pain or prior hospitalization for mental illness and substance use treatment among a cohort at high risk of opioid overdose. J. Subst. Use Addict. Treat. 2023;159 doi: 10.1016/J.JOSAT.2023.209273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby S., Wang D., Murray E., Lang E. (n.d.)Emergency Departments as the Health Safety Nets of Society: A Descriptive and Multicenter Analysis of Social Worker Support in the Emergency Room. Cureus. 10 (9):e3247. doi:10.7759/cureus.3247 [DOI] [PMC free article] [PubMed]
- Sells D., Curtis A., Abdur-Raheem J., et al. Peer-mentored community reentry reduces recidivism. Crim. Justice Behav. 2020;47(4):437–456. doi: 10.1177/0093854820901562. [DOI] [Google Scholar]
- Serrano M.D., Conley T.B. The role of social work and peer support workers in addressing the opioid crisis. Soc. Work Ment. Health. 2021;19(6):517–525. doi: 10.1080/15332985.2021.1929661. [DOI] [Google Scholar]
- Shumway M., Boccellari A., O’Brien K., Okin R.L. Cost-effectiveness of clinical case management for ED frequent users: results of a randomized trial⋆. Am. J. Emerg. Med. 2008;26(2):155–164. doi: 10.1016/J.AJEM.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Soares W.E., Melnick E.R., Nath B., et al. Emergency department visits for nonfatal opioid overdose during the COVID-19 pandemic across six US health care systems. Ann. Emerg. Med. 2022;79(2):158–167. doi: 10.1016/J.ANNEMERGMED.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata 16. Lasso for prediction and model selection. Accessed March 28, 2024. 〈https://www.stata.com/features/overview/lasso-model-selection-prediction/〉
- StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.
- StataCorp. STATA LASSO reference manual release 18. TX: College Station. Published online 2023.
- State of Rhode Island Department of Health. Prescription Drug Monitoring Program. Accessed April 4, 2024. 〈https://health.ri.gov/healthcare/medicine/about/prescriptiondrugmonitoringprogram/〉
- Statistical tools for high-throughput data analysis (STHDA). CART Model: Decision Tree Essentials. March 11, 2018. Accessed August 11, 2024. 〈http://www.sthda.com/english/articles/35-statistical-machine-learning-essentials/141-cart-model-decision-tree-essentials/〉
- Strobl C., Malley J., Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods. 2009;14(4):323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumbo S.P., Yarborough B.J.H., McCarty D., Weisner C., Green C.A. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J. Subst. Abus. Treat. 2017;73:47–54. doi: 10.1016/J.JSAT.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). The evidence: Consumer operated services. (HHS Publication No. SMA-11-4633). Rockville, MD: Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services. Accessed August 9, 2024. 〈https://store.samhsa.gov/system/files/theevidence-cosp.pdf〉
- U.S Department of Veterans Affairs. Alcohol Use Disorders Identification Test. Viral Hepatitis and Liver Disease. Accessed April 4, 2024. 〈https://www.hepatitis.va.gov/alcohol/treatment/audit-c.asp〉
- Volkow N.D., Blanco C. Interventions to address the opioid crisis-modeling predictions and consequences of inaction. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37385. [DOI] [PubMed] [Google Scholar]
- Wakeman S.E., Metlay J.P., Chang Y., Herman G.E., Rigotti N.A. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J. Gen. Intern Med. 2017;32(8):909–916. doi: 10.1007/S11606-017-4077-Z/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse J.J., McGinnis K.A., Edelman E.J., et al. Twelve-month retention in opioid agonist treatment for opioid use disorder among patients with and without HIV. AIDS Behav. 2022;26(3):975–985. doi: 10.1007/s10461-021-03452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H. The adaptive lasso and its oracle properties. J. Am. Stat. Assoc. 2006;101(476):1418–1429. doi: 10.1198/016214506000000735. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material