Abstract
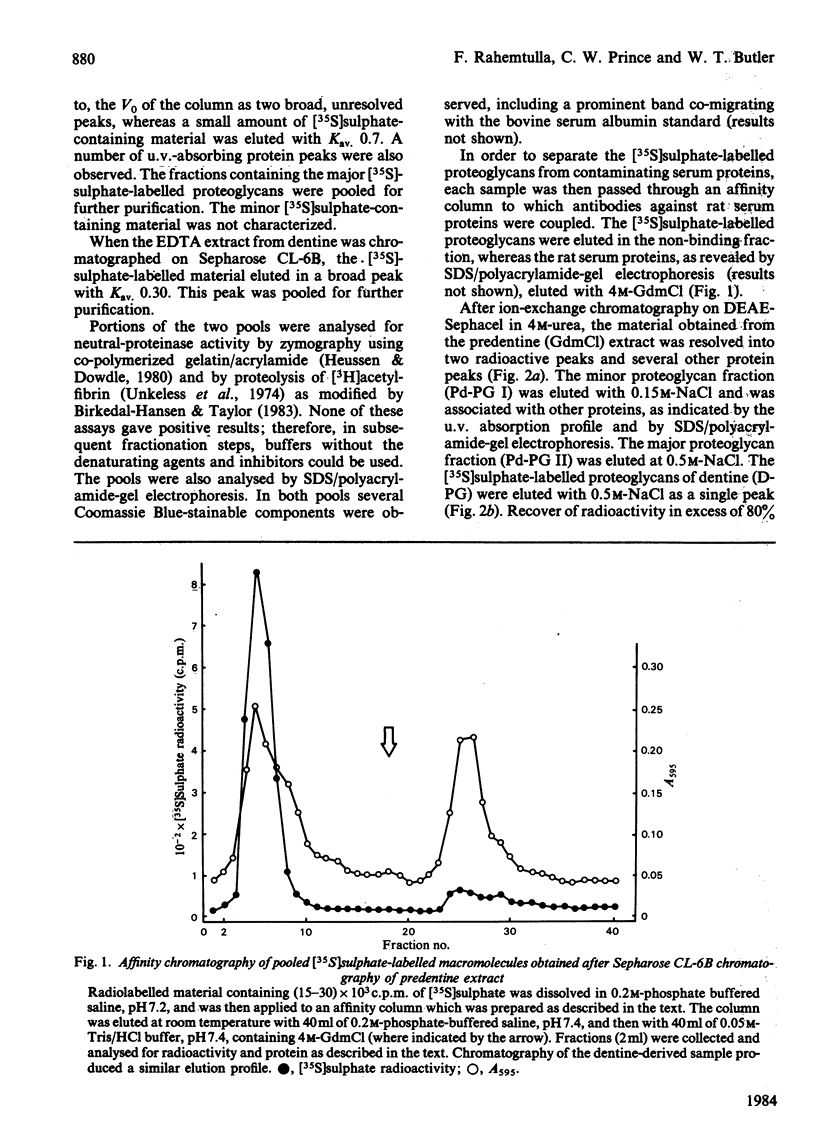
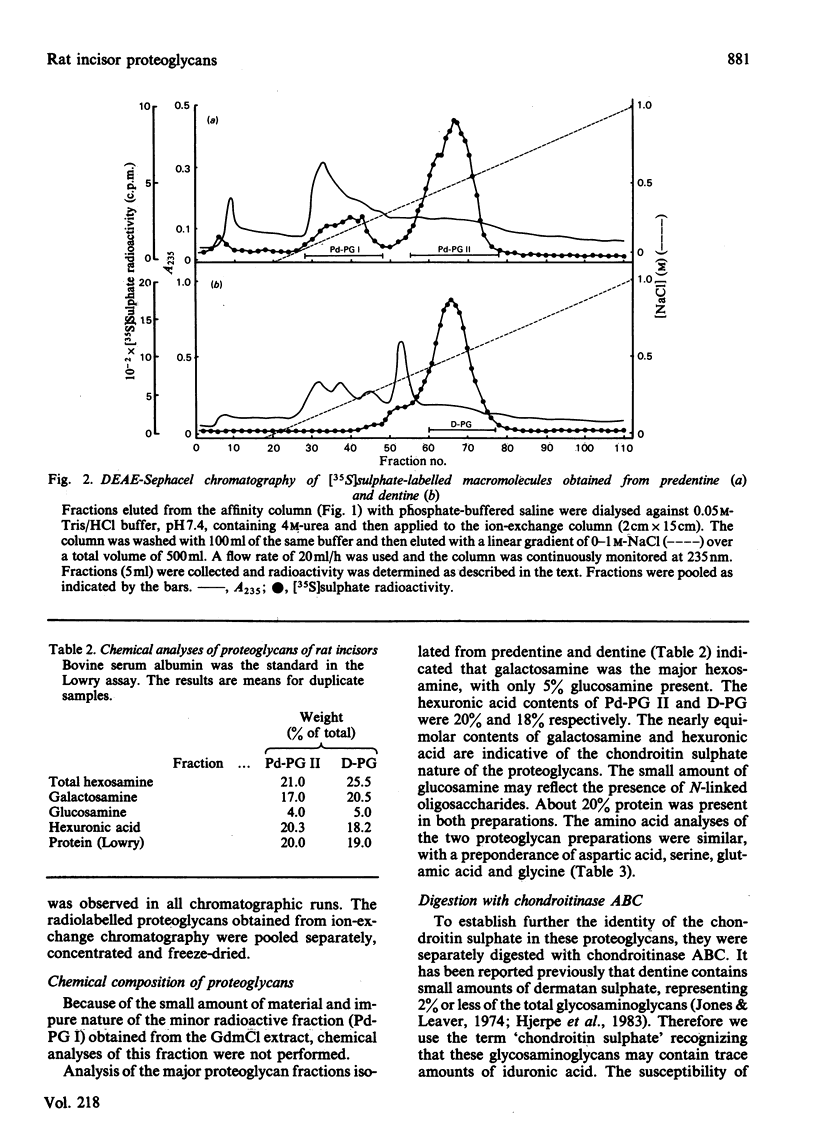
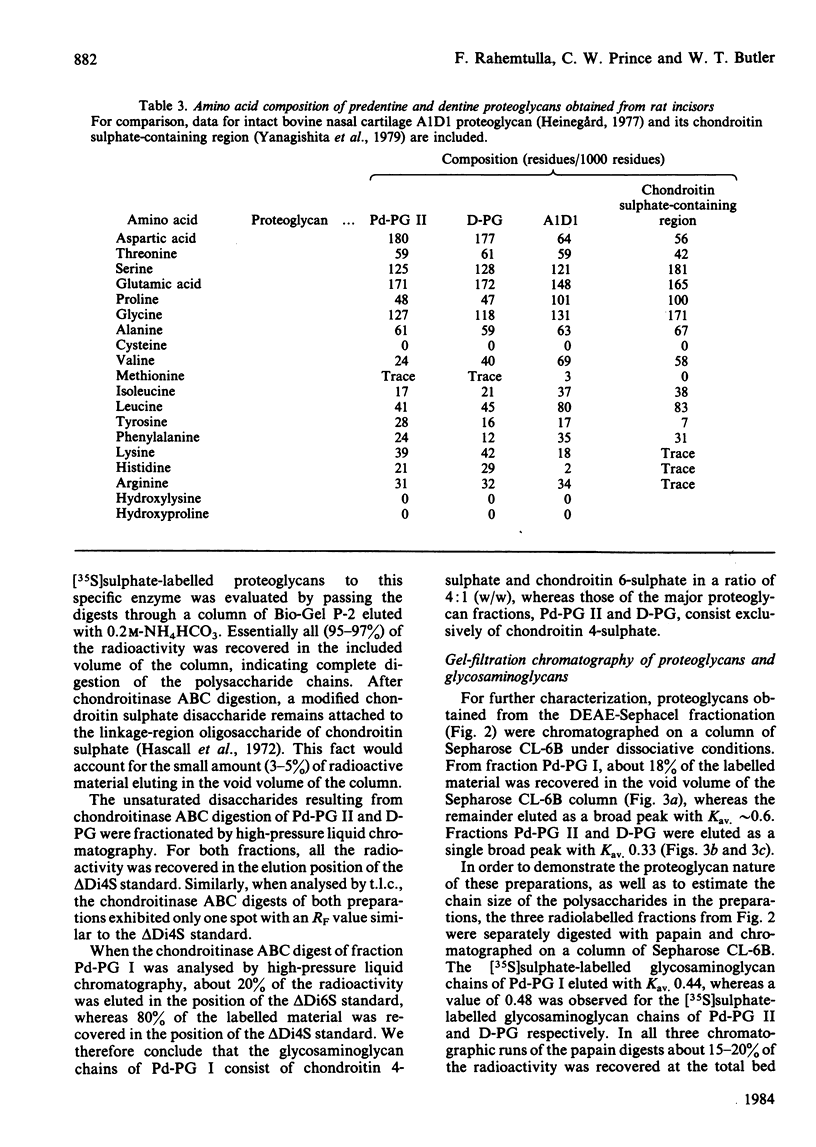
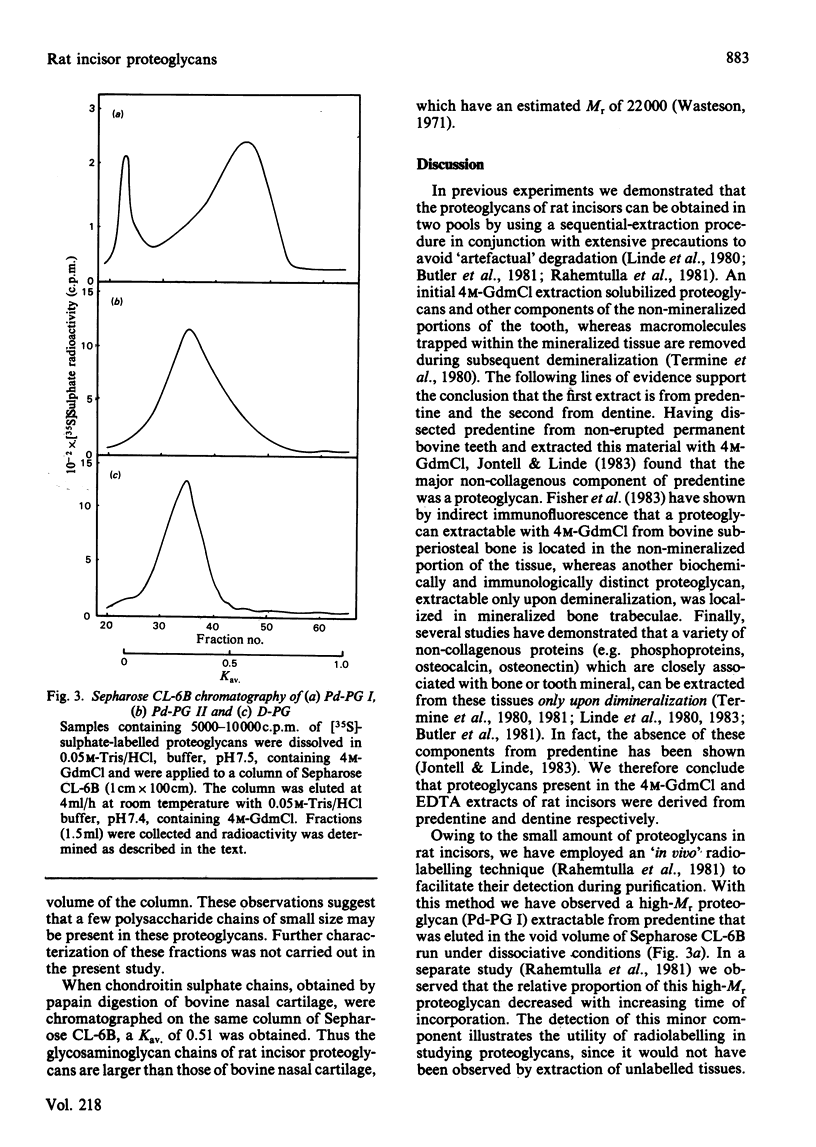
Newly synthesized proteoglycans of rat incisors were labelled in vivo for 6h with [35S]-sulphate in order to facilitate their detection during purification and characterization. Proteoglycans were extracted from non-mineralized portions (predentine) of rat incisors with 4M-guanidinium chloride and subsequently from dentine by demineralization with a 0.4M-EDTA solution containing 4M-guanidinium chloride. Both extractions were performed at 4 degrees C in the presence of proteinase inhibitors. Purification of proteoglycans was achieved with a procedure involving gel-filtration chromatography, selective precipitation of phosphoproteins, affinity chromatography and ion-exchange chromatography. Two proteoglycan populations were found in the initial extract (Pd-PG I and Pd-PG II), whereas only one fraction (D-PG) was obtained after demineralization. The minor proteoglycan fraction from the first extract, Pd-PG I, although not totally characterized, differed sharply from the other proteoglycans in that it had a larger molecular size with larger glycosaminoglycan chains composed of chondroitin 4- and 6-sulphate isomers. In contrast, the major proteoglycans Pd-PG II and D-PG had smaller hydrodynamic sizes with smaller glycosaminoglycan chains (but larger than those from bovine nasal cartilage proteoglycans) composed exclusively of chondroitin 4-sulphate. The major proteoglycans were incapable of interacting with hyaluronic acid. In general, the amino acid compositions of the major proteoglycans of rat incisors resembled that of bovine nasal cartilage proteoglycans, but the former had lower proline, valine, isoleucine, leucine, and higher aspartic acid, contents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenasi R. A new rapid method for measuring hydroxylysine and its glycosides in hydrolysates and physiological fluids. Biochim Biophys Acta. 1973 Apr 28;304(2):375–383. doi: 10.1016/0304-4165(73)90256-0. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Production of three plasminogen activators and an inhibitor in keratinocyte cultures. Biochim Biophys Acta. 1983 Apr 20;756(3):308–318. doi: 10.1016/0304-4165(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Bhown M., Dimuzio M. T., Linde A. Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981 Feb;1(2):187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Finch J. E., Jr, Miller E. J. The covalent structure of cartilage collagen. Evidence for sequence heterogeneity of bovine alpha1(II) chains. J Biol Chem. 1977 Jan 25;252(2):639–643. [PubMed] [Google Scholar]
- Clark R. D., Smith J. G., Jr, Davidson E. A. Hexosamine and acid glycosaminoglycans in human teeth. Biochim Biophys Acta. 1965 Nov 1;101(3):267–272. doi: 10.1016/0926-6534(65)90004-7. [DOI] [PubMed] [Google Scholar]
- Embery G. The isolation of chondroitin 4-(35S)sulphate from the molar teeth of young rats receiving sodium(35S)sulphate. Calcif Tissue Res. 1974;14(1):59–65. doi: 10.1007/BF02060283. [DOI] [PubMed] [Google Scholar]
- Engfeldt B., Hjerpe A. Glycosaminoglycans of dentine and predentine. Calcif Tissue Res. 1972;10(2):152–159. doi: 10.1007/BF02012545. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Dejter S. W., Jr, Whitson S. W., Yanagishita M., Kimura J. H., Hascall V. C., Kleinman H. K., Hassell J. R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983 May 25;258(10):6588–6594. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Antonopoulos C. A., Engfeldt B. Determination of sulphated disaccharides from chondroitin sulphates by high-performance liquid chromatography. J Chromatogr. 1979 Apr 1;171:339–344. doi: 10.1016/s0021-9673(01)95313-0. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Antonopoulos C. A., Engfeldt B., Wikström B. Analysis of dentine glycosaminoglycans using high-performance liquid chromatography. Calcif Tissue Int. 1983 Jul;35(4-5):496–501. doi: 10.1007/BF02405083. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Engfeldt B. Proteoglycans of dentine and predentine. Calcif Tissue Res. 1976 Dec 22;22(2):173–182. doi: 10.1007/BF02010356. [DOI] [PubMed] [Google Scholar]
- Jones I. L., Leaver A. G. Glycosaminoglycans of human dentine. Calcif Tissue Res. 1974;16(1):37–44. doi: 10.1007/BF02008211. [DOI] [PubMed] [Google Scholar]
- Jontell M., Linde A. Non-collagenous proteins of predentine from dentinogenically active bovine teeth. Biochem J. 1983 Sep 15;214(3):769–776. doi: 10.1042/bj2140769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linde A., Bhown M., Butler W. T. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980 Jun 25;255(12):5931–5942. [PubMed] [Google Scholar]
- Linde A. Glycosaminoglycans of the odontoblast-predentine layer in dentinogenically active porcine teeth. Calcif Tissue Res. 1973;12(4):281–294. doi: 10.1007/BF02013741. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Brown M., Dziewiatkowski D. D. The link protein in proteoglycan aggregates from the Swarm rat chondrosarcoma. J Biol Chem. 1977 Sep 25;252(18):6470–6477. [PubMed] [Google Scholar]
- Prince C. W., Rahemtulla F., Butler W. T. Metabolism of rat bone proteoglycans in vivo. Biochem J. 1983 Dec 15;216(3):589–596. doi: 10.1042/bj2160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Smalley J. W., Embery G. The influence of fluoride administration on the structure of proteoglycans in the developing rat incisor. Biochem J. 1980 Aug 15;190(2):263–272. doi: 10.1042/bj1900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann D. A. Studies on hyaluronic acid. I. The preparation and properties of rooster comb hyaluronic acid. Biochim Biophys Acta. 1968 Feb 1;156(1):17–30. [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Christner P. J., Conn K. M., Nylen M. U. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem. 1980 Oct 25;255(20):9760–9768. [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman L., Ber A., Allalouf D. Use of thin-layer chromatography in the separation of disaccharides resulting from digestion of chondroitin sulphates with chondroitinases. J Chromatogr. 1977 Jun 11;136(2):342–347. doi: 10.1016/s0021-9673(00)86291-3. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- White C. J. Molecular organization of heparan sulphate proteoglycan from human dentine. Arch Oral Biol. 1978;23(12):1141–1144. doi: 10.1016/0003-9969(78)90121-8. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Rodbard D., Hascall V. C. Isolation and characterization of proteoglycans from porcine ovarian follicular fluid. J Biol Chem. 1979 Feb 10;254(3):911–920. [PubMed] [Google Scholar]



