Abstract
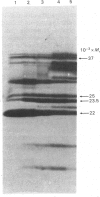
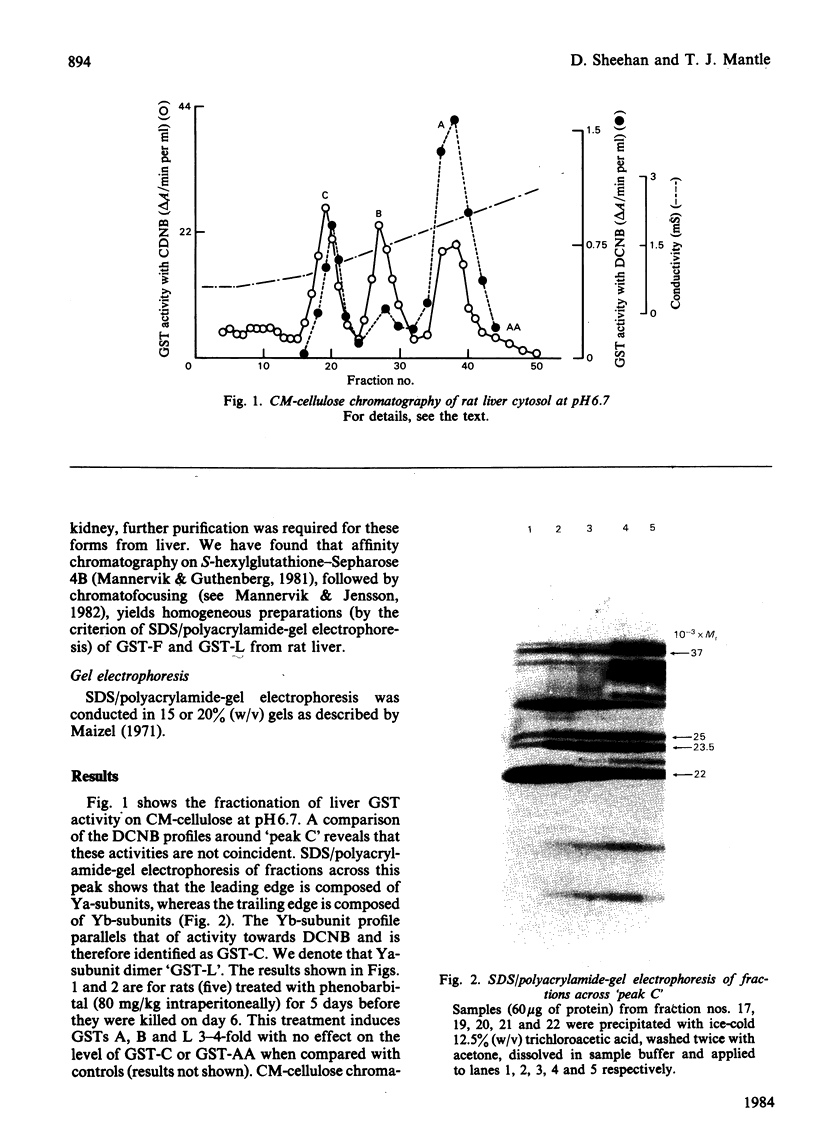
CM-cellulose chromatography of rat liver and kidney cytosol at pH6 reveals the presence of a second Ya-subunit dimer of glutathione S-transferase (GST-F) in addition to the recently described GST-YaYa (GST-L; our nomenclature) [Hayes & Clarkson (1982) Biochem. J. 207, 459-470]. The two forms are structurally similar (by the criteria of CNBr- and Staphylococcus-V8-proteinase-cleavage peptide maps), and both are sensitive to inhibition by haemin. However, their kinetic parameters with 1-chloro-2,4-dinitrobenzene are quite distinct, and they show differential inducibility by phenobarbitone. These results suggest a similar heterogeneity in Ya-subunits to that previously described for Yb-subunits of glutathione S-transferase and indicate that significant gene duplication may have occurred in these multifunctional intracellular binding proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniel V., Sarid S., Bar-Nun S., Litwack G. Rat ligandin mRNA molecular cloning and sequencing. Arch Biochem Biophys. 1983 Nov;227(1):266–271. doi: 10.1016/0003-9861(83)90370-3. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Clarkson G. H. Purification and characterization of three forms of glutathione S-transferase A. A comparative study of the major YaYa-, YbYb- and YcYc-containing glutathione S-transferases. Biochem J. 1982 Dec 1;207(3):459–470. doi: 10.1042/bj2070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Rat liver glutathione S-transferases. A study of the structure of the basic YbYb-containing enzymes. Biochem J. 1983 Sep 1;213(3):625–633. doi: 10.1042/bj2130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Cholic acid binding by glutathione S-transferases from rat liver cytosol. Biochem J. 1980 Jan 1;185(1):83–87. doi: 10.1042/bj1850083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kalinyak J. E., Taylor J. M. Rat glutathione S-transferase. Cloning of double-stranded cDNA and induction of its mRNA. J Biol Chem. 1982 Jan 10;257(1):523–530. [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Scully N. C., Mantle T. J. Studies on the nature of the multiple forms of the glutathione S-transferases. Biochem Soc Trans. 1980 Aug;8(4):451–452. doi: 10.1042/bst0080451. [DOI] [PubMed] [Google Scholar]
- Scully N. C., Mantle T. J. Tissue distribution and subunit structures of the multiple forms of glutathione S-transferase in the rat. Biochem J. 1981 Jan 1;193(1):367–370. doi: 10.1042/bj1930367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Weiss M. J., Karakawa W. W., Reddy C. C. Cloning and sequence analysis of a cDNA plasmid for one of the rat liver glutathione S-transferase subunits. Nucleic Acids Res. 1982 Sep 25;10(18):5407–5419. doi: 10.1093/nar/10.18.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Weiss M. J., Reddy C. C. Subunit composition of rat liver glutathione S-transferases. Biochem Biophys Res Commun. 1982 Sep 30;108(2):461–467. doi: 10.1016/0006-291x(82)90851-8. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Jensson H., Mannervik B. A set of inhibitors for discrimination between the basic isozymes of glutathione transferase in rat liver. Biochem Biophys Res Commun. 1983 Jul 29;114(2):829–834. doi: 10.1016/0006-291x(83)90856-2. [DOI] [PubMed] [Google Scholar]