Abstract
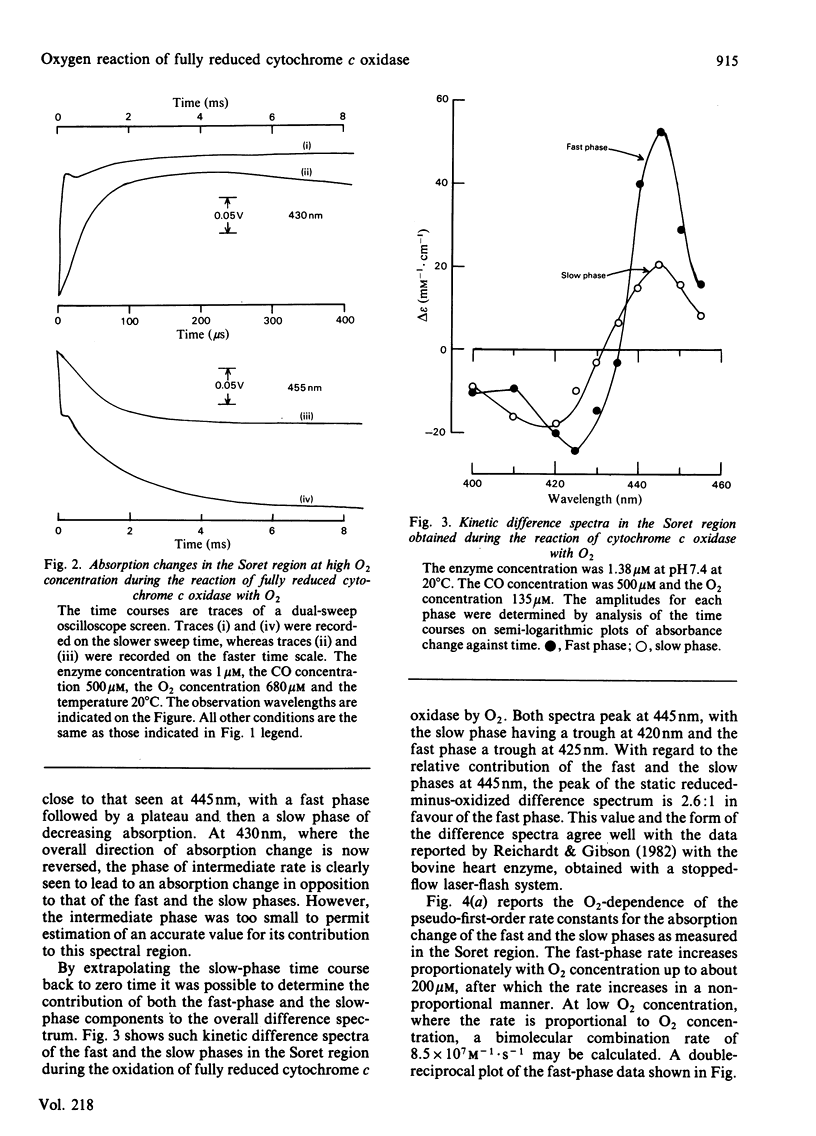
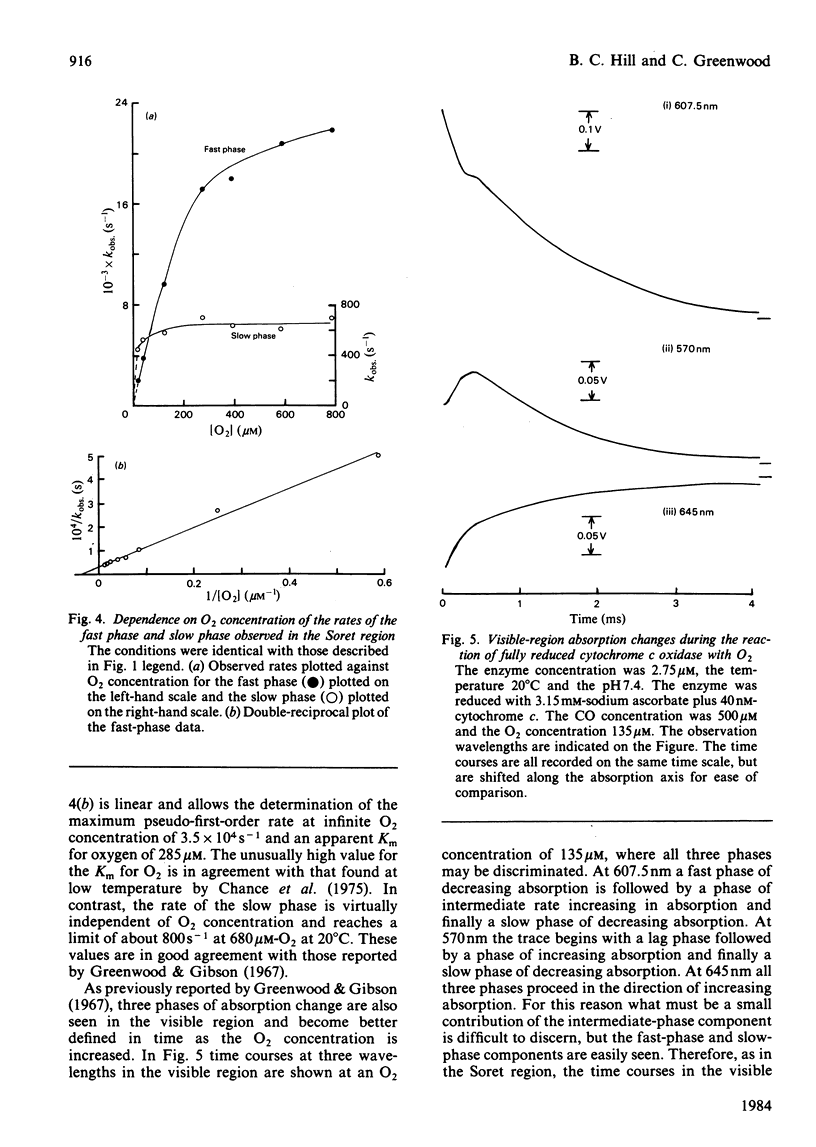
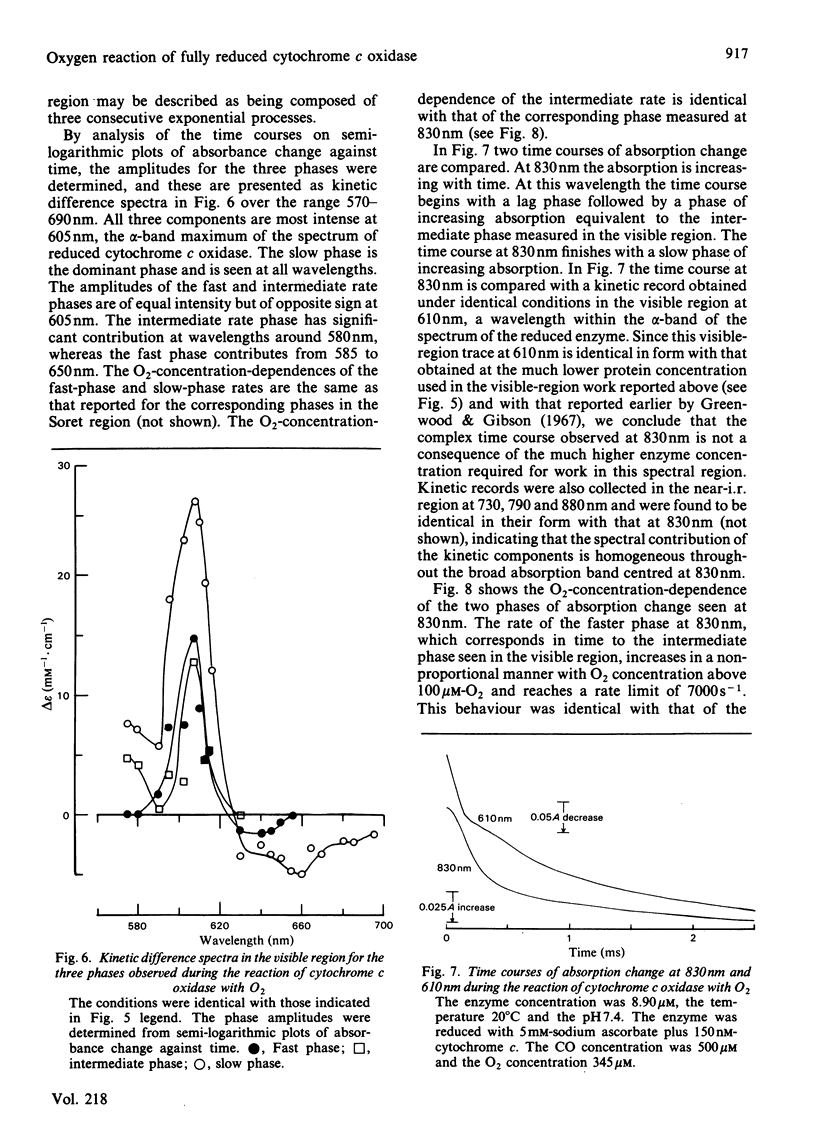
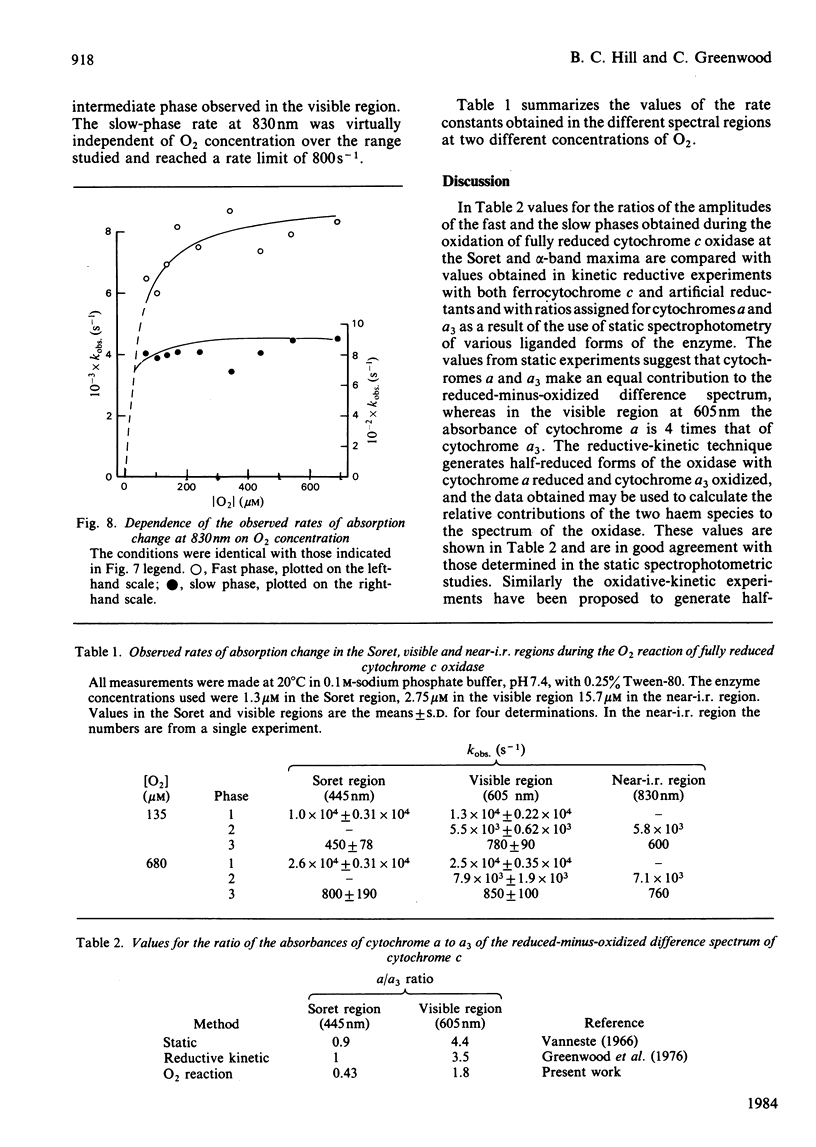
Absorption changes during the O2 reaction of reduced bovine cytochrome c oxidase were investigated by the rapid-reaction technique of flow-flash spectrophotometry in the Soret, visible and near-i.r. spectral regions. New features in the time courses of absorption change were observed relative to the earlier findings reported by Greenwood & Gibson [(1967) J. Biol. Chem. 242, 1782-1787]. These new features arise in the Soret and near-i.r. regions and allow the reaction to be described at all wavelengths as a composite of three exponential processes. There is a rapid O2-sensitive phase detectable in the Soret and visible region. The second phase has a rate that is somewhat less dependent on O2 concentration than is the fastest phase rate and is detectable in all three spectral regions. The rate of the third phase is almost independent of the O2 concentration and is also detectable in all spectral regions. Analysis of the three phases gives their rates and absorption amplitudes. The fast phase reaches a rate of 2.5 X 10(4) s-1 at the highest O2 concentration available at 20 degrees C, whereas the phase of intermediate rate is limited at a value of 7 X 10(3) s-1 and the slow phase rate is limited at 700 s-1. The ratios of the kinetic difference spectra for the fast phase and the slow phase do not correspond to the spectra of the individual haem centres. A branched mechanism is advanced that is able to reconcile the kinetic and static difference spectra. This mechanism suggests that some of the cytochrome a is oxidized along with cytochrome a3 in the initial O2-sensitive phase. In addition, the model requires that CuA is oxidized heterogeneously. This fits with the complex time course of oxidation observed at 830 nm while retaining CuA as virtually the sole contributor to absorbance at this wavelength.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. The electronic state of heme in cytochrome oxidase II. Oxidation-reduction potential interactions and heme iron spin state behavior observed in reductive titrations. J Biol Chem. 1978 Apr 10;253(7):2400–2411. [PubMed] [Google Scholar]
- Beinert H., Shaw R. W., Hansen R. E., Hartzell C. R. Studies on the origin of the near-infrared (800-900 nm) absorption of cytochrome c oxidase. Biochim Biophys Acta. 1980 Jul 8;591(2):458–470. doi: 10.1016/0005-2728(80)90176-0. [DOI] [PubMed] [Google Scholar]
- Brittain T., Greenwood C. Photolytic studies on the carbon monoxide complex of sulphaemoglobin. Biochem J. 1982 Jan 1;201(1):153–159. doi: 10.1042/bj2010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Colosimo A., Wilson M. T., Sarti P., Antonini E. Interconversion between states in cytochrome oxidase: interpretation of kinetic data on mixed-valence oxidase. FEBS Lett. 1983 Feb 7;152(1):75–78. doi: 10.1016/0014-5793(83)80485-2. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr, Ingledew W. J., King T. E. Low-temperature kinetics of the reaction of oxygen and solubilized cytochrome oxidase. Biochem J. 1978 Jun 1;171(3):787–798. doi: 10.1042/bj1710787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. The cycling of oxygen through intermediates in the cytochrome oxidase-oxygen reaction. Curr Top Cell Regul. 1981;18:343–360. doi: 10.1016/b978-0-12-152818-8.50026-8. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the low-temperature intermediates of the reaction of fully reduced soluble cytochrome oxidase with oxygen by electron-paramagnetic-resonance and optical spectroscopy. Biochem J. 1980 Jan 1;185(1):139–154. doi: 10.1042/bj1850139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Brittain T. Studies on partially reduced mammalian cytochrome oxidase reactions with ferrocytochrome c. Biochem J. 1976 Sep 1;157(3):591–598. doi: 10.1042/bj1570591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood C., Gibson Q. H. The reaction of reduced cytochrome C oxidase with oxygen. J Biol Chem. 1967 Apr 25;242(8):1782–1787. [PubMed] [Google Scholar]
- Greenwood C., Hill B. C., Barber D., Eglinton D. G., Thomson A. J. The optical properties of CuA in bovine cytochrome c oxidase determined by low-temperature magnetic-circular-dichroism spectroscopy. Biochem J. 1983 Nov 1;215(2):303–316. doi: 10.1042/bj2150303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C. Spectroscopic evidence for the participation of compound A (Fea32+-O2) in the reaction of mixed-valence cytochrome c oxidase with oxygen at room temperature. Biochem J. 1983 Dec 1;215(3):659–667. doi: 10.1042/bj2150659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. C., Nicholls P., Degn H. The effect of oxygen concentration on the steady-state kinetics of the solubilized cytochrome c oxidase. Biochim Biophys Acta. 1976 Nov 8;452(1):59–65. doi: 10.1016/0005-2744(76)90057-7. [DOI] [PubMed] [Google Scholar]
- Powers L., Chance B., Ching Y., Angiolillo P. Structural features and the reaction mechanism of cytochrome oxidase: iron and copper X-ray absorption fine structure. Biophys J. 1981 Jun;34(3):465–498. doi: 10.1016/S0006-3495(81)84863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt J. K., Gibson Q. H. Spectra of intermediates in oxidation and reduction of cytochrome c oxidase. J Biol Chem. 1982 Aug 25;257(16):9268–9270. [PubMed] [Google Scholar]
- Thomson A. J., Johnson M. K., Greenwood C., Gooding P. E. A study of the magnetic properties of haem a3 in cytochrome c oxidase by using magnetic-circular-dichroism spectroscopy. Biochem J. 1981 Mar 1;193(3):687–697. doi: 10.1042/bj1930687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Peterson J., Antonini E., Brunori M., Colosimo A., Wyman J. A plausible two-state model for cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7115–7118. doi: 10.1073/pnas.78.11.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]


