Abstract
Background
Gestational diabetes mellitus (GDM) affects a significant number of women each year. GDM is associated with a wide range of adverse outcomes for women and their babies. Recent observational studies have found physical activity during normal pregnancy decreases insulin resistance and therefore might help to decrease the risk of developing GDM.
Objectives
To assess the effects of physical exercise for pregnant women for preventing glucose intolerance or GDM.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (2 April 2012), ClinicalTrials.gov (2 April 2012) and the WOMBAT Perinatal Trials Registry (2 April 2012).
Selection criteria
Randomised and cluster‐randomised trials assessing the effects of exercise for preventing pregnancy glucose intolerance or GDM.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of included studies.
Main results
We included five trials with a total of 1115 women and their babies (922 women and their babies contributed outcome data). Four of the five included trials had small sample sizes with one large trial that recruited 855 women and babies. All five included trials had a moderate risk of bias. When comparing women receiving additional exercise interventions with those having routine antenatal care, there was no significant difference in GDM incidence (three trials, 826 women, risk ratio (RR) 1.10, 95% confidence interval (CI) 0.66 to 1.84), caesarean section (two trials, 934 women, RR 1.33, 95% CI 0.97 to 1.84) or operative vaginal birth (two trials, 934 women, RR 0.83, 95% CI 0.58 to 1.17). No trial reported the infant primary outcomes prespecified in the review.
None of the five included trials found significant differences in insulin sensitivity. Evidence from one single large trial suggested no significant difference in the incidence of developing pregnancy hyperglycaemia not meeting GDM diagnostic criteria, pre‐eclampsia or admission to neonatal ward between the two study groups. Babies born to women receiving exercise interventions had a non‐significant trend to a lower ponderal index (mean difference (MD) ‐0.08 gram x 100 m3, 95% CI ‐0.18 to 0.02, one trial, 84 infants). No significant differences were seen between the two study groups for the outcomes of birthweight (two trials, 167 infants, MD ‐102.87 grams, 95% CI ‐235.34 to 29.60), macrosomia (two trials, 934 infants, RR 0.91, 95% CI 0.68 to 1.22), or small‐for‐gestational age (one trial, 84 infants, RR 1.05, 95% CI 0.25 to 4.40) or gestational age at birth (two trials, 167 infants, MD ‐0.04 weeks, 95% CI ‐0.37 to 0.29) or Apgar score less than seven at five minutes (two trials, 919 infants, RR 1.00, 95% CI 0.27 to 3.65). None of the trials reported long‐term outcomes for women and their babies. No information was available on health services costs.
Authors' conclusions
There is limited randomised controlled trial evidence available on the effect of exercise during pregnancy for preventing pregnancy glucose intolerance or GDM. Results from three randomised trials with moderate risk of bias suggested no significant difference in GDM incidence between women receiving an additional exercise intervention and routine care.
Based on the limited data currently available, conclusive evidence is not available to guide practice. Larger, well‐designed randomised trials, with standardised behavioural interventions are needed to assess the effects of exercise on preventing GDM and other adverse pregnancy outcomes including large‐for‐gestational age and perinatal mortality. Longer‐term health outcomes for both women and their babies and health service costs should be included. Several such trials are in progress. We identified another seven trials which are ongoing and we will consider these for inclusion in the next update of this review.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Birth Weight; Cesarean Section; Cesarean Section/statistics & numerical data; Diabetes, Gestational; Diabetes, Gestational/epidemiology; Diabetes, Gestational/prevention & control; Exercise; Exercise/physiology; Hyperglycemia; Hyperglycemia/epidemiology; Incidence; Insulin Resistance; Insulin Resistance/physiology; Pre‐Eclampsia; Pre‐Eclampsia/epidemiology; Prenatal Care; Randomized Controlled Trials as Topic
Plain language summary
Exercise for pregnant women for preventing gestational diabetes mellitus
Each year, a significant number of pregnant women around the world develop gestational diabetes mellitus (GDM), defined as glucose intolerance or high blood glucose concentration (hyperglycaemia) with onset or first recognition during pregnancy. During normal pregnancy, insulin becomes less effective in transferring glucose from the blood stream to the mother’s tissues to ensure an adequate nutrient supply to the baby. This insulin resistance increases as the pregnancy advances and GDM occurs when a mother does not secrete enough insulin to be able to meet this resistance. Women with GDM are at risk of future type 2 diabetes and their babies are at increased risk of adverse outcomes including being large‐for‐gestational age, having birthweight of at least 4000 grams and birth trauma. The modifiable risk factors for GDM include being overweight or obese; physical inactivity or sedentary lifestyle; low fibre and high glycaemic load diet and polycystic ovarian syndrome. This review aimed to assess the effects of physical exercise for pregnant women in preventing glucose intolerance or GDM and was based on limited evidence from five randomised controlled trials. Two trials involved obese women. The trials provided data from 922 women and their babies and were of moderate risk of bias. The exercise programs including individualised exercise with regular advice, weekly supervised group exercise session or home‐based stationary cycling, either supervised or unsupervised, had no clear effect on preventing GDM (three trials with 826 women screened at 18 to 36 weeks' gestation), or improving insulin sensitivity (five trials) compared with standard antenatal care with normal daily activities. Based on these limited data, conclusive evidence is not available to guide practice. Larger, well‐designed randomised trials are needed. Several such trials are in progress. We identified another seven trials which are ongoing and we will consider these for inclusion in the next update.
Background
Description of the condition
Introduction and definition of gestational diabetes mellitus
Although there is no universally accepted diagnostic criteria (Coustan 2010), gestational diabetes mellitus (GDM) can be defined as 'glucose intolerance or hyperglycaemia (high blood glucose concentration) with onset or first recognition during pregnancy' (ACOG 2001; Hoffman 1998; Metzger 1998; NICE 2008). GDM affects about 1% to 14% of pregnancies around the world and the prevalence is increasing in line with increasing rates of maternal obesity and type 2 diabetes mellitus (Bottalico 2007; Dabelea 2005; Mulla 2010).
Pathophysiology of gestational diabetes mellitus
In pregnancy, insulin resistance increases with advancing gestation (Clapp 2006). Hormones secreted from the placenta, including tumour necrosis factor‐alpha (TNF‐α), placental lactogen, placental growth hormone, cortisol and progesterone are thought to be the likely triggers of these physiological changes (Clapp 2006; Devlieger 2008). Increasing insulin resistance in normal pregnancy, especially during the third trimester, helps to meet the increased nutrient requirement for fetal development and promotes fetal growth by increasing maternal glucose supply (Devlieger 2008). GDM results when the insulin secretion is inadequate for the degree of insulin resistance (Clapp 2006).
Health risks for gestational diabetes mellitus
GDM is associated with a range of adverse pregnancy outcomes (Crowther 2005; HAPO Study Cooperative Research Group 2008; Landon 2009; Reece 2009).
Women with GDM are at increased risk of developing pre‐eclampsia and increased need for induction of labour (Crowther 2005;Landon 2009) and caesarean section (Landon 2009). As women with GDM are more likely to have a large‐for‐gestational age (LGA) or macrosomic (birthweight of 4000 grams or more) infant, they are at higher risk of cephalopelvic disproportion, uterine rupture and perineal lacerations (Jastrow 2010). In the longer term, women with GDM have seven to eight times the risk of developing type 2 diabetes (T2DM) when compared with those who have had a normoglycaemic pregnancy (Bellamy 2009; Chodick 2010). A comprehensive systematic review found that the cumulative incidence of T2DM in women with GDM ranged from 2.6% to over 70% with a follow‐up of six weeks to 28 years postpartum (Kim 2002). Therefore, GDM is usually considered a significant initiating factor in T2DM, and GDM prevention may lead to a decreased rate of T2DM in successive generations (Mottola 2008).
As mentioned above, babies born to mothers with GDM are more likely to be LGA or macrosomic (HAPO Study Cooperative Research Group 2008; Ju 2008; Reece 2009). LGA or macrosomic infants are at increased risk of injury during birth, such as shoulder dystocia, perinatal asphyxia, bone fractures and nerve palsies (HAPO Study Cooperative Research Group 2008; Henriksen 2008; Langer 2005). Babies of women with GDM are also at higher risk of having other neonatal complications such as respiratory distress syndrome, hypoglycaemia, hyperbilirubinaemia (increased levels of bilirubin in the blood), cardiomyopathy (the deterioration of the function of the heart muscle layer), hypocalcaemia, hypomagnesaemia, polycythaemia, hyperviscosity and need admission to neonatal nursery (HAPO Study Cooperative Research Group 2008; Ju 2008; Reece 2009; Soler 1978). In the longer term, children born to mothers with GDM are at increased risk of becoming overweight or obese, developing type 1 and T2DM and having impaired intellectual achievement (Harder 2009; Mulla 2010; Rizzo 1997; Whincup 2008; Yogev 2009). Infants born LGA have a higher risk of developing metabolic syndrome (a cluster of risk factors defined by the occurrence of three of the following: obesity, hypertension, hypertriglyceridaemia and low HDL cholesterol concentration) in childhood, adolescence and adulthood (Barker 1994; Guerrero‐Romero 2010; Harder 2009). Development of the metabolic syndrome during childhood predicts adulthood T2DM at 25 to 30 years of age (Morrison 2008). These health problems may repeat across generations (Mulla 2010; Petitt 1985).
Risk factors for GDM
There are a range of established risk factors for GDM; some are modifiable and some are non‐modifiable (Morisset 2010). The modifiable risk factors include being overweight or obese (body mass index (BMI) at least 25 kg/m² or at least 30 kg/m²); physical inactivity or sedentary lifestyle; excessive weight gain during pregnancy; low fibre and high glycaemic load diet and polycystic ovarian syndrome (Chasan‐Taber 2008; Hedderson 2010; Lo 2006; Mottola 2008; Petry 2010; Zhang 2006). Non‐modifiable risk factors include advanced maternal age, nonwhite race/ethnicity, history of having a macrosomic (birthweight at least 4000 gram) infant, history of GDM, family history of diabetes mellitus, maternal high or low birthweight and high parity (Cypryk 2008; Petry 2010; Solomon 1997).
Management of GDM
The primary aims of treatment for GDM are to optimise glycaemic control and improve pregnancy outcomes (Alwan 2009). Management for women with GDM is effective in improving pregnancy outcomes, which includes any or all of: diet and lifestyle advice, use of oral glucose‐lowering agents (e.g. metformin, glyburide), administration of insulin, fetal surveillance (e.g. doppler umbilical blood flow measurement, cardiotocograph and ultrasonography) and maternal glucose monitoring (Crowther 2005; Hoffman 1998; Landon 2009; Metzger 2007; NICE 2008).
Dietary and lifestyle advice is effective (Crowther 2005; Landon 2009) and is usually recommended as the primary therapeutic strategy for women with GDM to achieve acceptable glycaemic control (ACOG 2001; Hoffman 1998; NICE 2008). As part of the treatment for GDM, women are encouraged to start or continue moderate intensity exercise as long as they have no medical or obstetrical contraindications (ADA 2003; Hoffman 1998; NICE 2008). If these interventions alone are not enough to achieve good maternal glycaemia control, insulin therapy may be indicated (ACOG 2001; Hoffman 1998; NICE 2008). Oral hypoglycaemics such as glyburide and metformin have been used as alternatives to insulin therapy (Silva 2010; Simmons 2004). As part of management of GDM, maternal glucose monitoring and ultrasonography are advised to monitor treatment and guide care for birth (ACOG 2001; Hoffman 1998; NICE 2008).
Description of the intervention
Physical activity and pregnancy
Until several decades ago, physical activity had been discouraged in pregnancy due to theoretical concerns of exercise‐induced injury leading to adverse fetal and maternal outcomes (Dempsey 2005; Schlüssel 2008).
Since early this century, the benefits of exercise during pregnancy have been realised and pregnant women have been encouraged to have regular physical activity in the absence of medical or obstetric complications (Dempsey 2005; Schlüssel 2008). Light to moderate physical activity during a normal pregnancy provides various benefits for the mother and her fetus (Melzer 2010). For mothers, it helps reduce and prevent lower back pain, decreases fluid retention, reduces cardiovascular stress, increases oxygenation capacity and decreases blood pressure (Melzer 2010; Schlüssel 2008). Fetal benefits include decreased fat mass, reduced risk of being a LGA fetus, improved stress tolerance, and advanced neurobehavioural maturation (Melzer 2010; Snapp 2008). Recent observational studies have found physical activity during normal pregnancy decreased insulin resistance and therefore, might help to decrease the risk of GDM (Redden 2010; Reece 2009). Some evidence from observational studies has suggested that the risk of GDM was decreased by 20% to 55% among women with physical exercise of various duration and intensity before or during pregnancy (Dempsey 2004a; Dempsey 2004b; Oken 2006; Zhang 2006).
How the intervention might work
Undertaking a period of exercise and regular weight‐bearing exercise have both been found to decrease circulating glucose and insulin concentrations during, and for a period of time after, exercise sessions (Clapp 1991; Clapp 1998). The effect was greatest with low‐intensity prolonged exercise that utilises a large muscle mass in late pregnancy shortly (less than two hours) after food intake (Clapp 2006). Investigators have shown that physical exercise was effective in preventing and managing T2DM by reducing insulin resistance in men and non‐pregnant women (Clapp 2006; Knowler 2002; Oken 2006; Redden 2010). Regular exercise during pregnancy is associated with decreased circulating TNF‐α levels in a dose‐ and time‐dependent manner (Clapp 2000). These research findings suggest that physical exercise during normal pregnancy may be effective in preventing GDM.
In addition, being overweight or obese, or gaining excessive weight during pregnancy are significant risk factors for developing GDM (Hedderson 2010; Kim 2010b). A recent systematic review suggested physical activity during pregnancy might be successful in restricting gestational weight gain (Streuling 2011), thereby reducing the risk of developing GDM. However, such benefit was not found in another Cochrane review entitled 'Aerobic exercise for women during pregnancy', aimed at assessing the effects of exercise for healthy pregnant women (Kramer 2006).
Why it is important to do this review
GDM affects a significant proportion of pregnant women each year and the prevalence is increasing worldwide (Bottalico 2007; Dabelea 2005; Mulla 2010). GDM is associated with a range of negative pregnancy outcomes and these adverse health outcomes can repeat across generations (HAPO Study Cooperative Research Group 2008; Mulla 2010). Therefore, identifying ways that might help prevent GDM is of urgent public health importance.
Although the risk factors and health outcomes of GDM have been well recognised, there is little known about ways to prevent GDM in high‐risk populations (Mottola 2008; Petry 2010; Pivarnik 2006). Physical exercise, as one of the modifiable risk factors for GDM, has attracted great attention in recent years (Melzer 2010). There has been a suggestion that physical exercise before and during pregnancy may be effective in preventing GDM; however, little robust evidence from randomised controlled trials is available (Petry 2010). This review will help to provide reliable evidence for pregnant women on the effects of physical exercise on GDM prevention. Three other Cochrane reviews have addressed the effects of exercise for diabetic pregnant women (Ceysens 2006), the role of aerobic exercise for healthy pregnant women (Kramer 2006) and the effects of diet and exercise on postpartum weight retention (Amorim 2007). Another Cochrane review to assess the effects of combined diet and exercise for preventing GDM is being planned.
Objectives
To assess the effects of physical exercise for pregnant women for preventing glucose intolerance or gestational diabetes.
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials assessing the effects of physical exercise in preventing pregnancy glucose intolerance or GDM. We planned to include cluster‐randomised trials. We also planned to include published abstracts for randomised controlled trials and cluster‐randomised controlled trials when relevant outcome data were available. We excluded quasi‐randomised controlled trials and cross‐over trials.
We planned to include trials assessing the effects of lifestyle interventions (e.g. include both nutrition and physical exercise interventions) in preventing pregnancy glucose intolerance or GDM if we are able to extract data for the effects of physical exercise separately.
Types of participants
Pregnant women regardless of age, gestation, parity or plurality. We excluded women with pre‐existing type 1 and type 2 diabetes.
Types of interventions
Interventions included any types of exercise and lifestyle management (i.e. exercise advice, providing exercise sessions) for pregnant women for preventing GDM before screening tests.
One type of intervention would be compared to standard antenatal care, i.e. any type of exercise advice (standard advice or individualised advice) compared with standard antenatal care; providing exercise sessions (group exercise or individual exercise session) compared with standard care. Multiple form of interventions would be compared with standard care, i.e. providing exercise advice and exercise sessions compared with standard care. Two forms of interventions would be compared with each other, i.e. providing exercise advice compared with providing exercise session. Two or more types of the same form of management would be compared against each other, i.e. standard exercise advice compared with individualised exercise advice; group exercise session compared with individual exercise session; different intensities of exercise sessions compared with each other; exercise interventions only compared with exercise interventions plus other forms of intervention (e.g. providing dietary advice).
Types of outcome measures
Primary outcomes
Maternal outcomes
Incidence of GDM (diagnostic criteria as defined in individual trials)
Mode of birth (normal vaginal birth, operative vaginal birth, caesarean section)
Fetal/neonatal outcomes
LGA
Perinatal mortality (fetal and neonatal mortality)
Secondary outcomes
Maternal outcomes
Perinatal
Incidence of pregnancy hyperglycaemia not meeting GDM diagnostic criteria (diagnostic criteria as defined in individual trials)
Induction of labour
Perineal trauma
Pre‐eclampsia
Weight gain during pregnancy
Maternal body mass index (BMI) at late pregnancy (third trimester)
Gestational age at screening for gestational diabetes mellitus
Postpartum haemorrhage
Postpartum infection
Placental abruption
Adherence to exercise intervention
Women’s sense of well‐being and quality of life (defined by author(s))
Women’s view of intervention
Long term
Postnatal weight retention
BMI
Gestational diabetes in subsequent pregnancy
Development of type 2 diabetes mellitus
Development of type 1 diabetes mellitus
Impaired glucose tolerance (defined by author(s))
Insulin sensitivity (defined by author(s))
Fetal/neonatal outcomes
Macrosomia (birthweight at least 4000 g)
Birthweight
Small‐for‐gestational age
Neonatal hypoglycaemia requiring treatment (variously defined by authors of individual trials)
Gestational age at birth
Preterm birth (less than 37 weeks' gestation)
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hyperbilirubinaemia requiring treatment (variously defined by authors of individual trials)
Apgar scores (less than seven at five minutes)
Ponderal index
Skinfold thickness measurements
Childhood outcomes
Weight
Height
BMI
Fat mass/fat‐free mass
Skinfold thickness measurements
Blood pressure
Impaired glucose tolerance (as defined by author(s))
Development of type 1 diabetes
Development of type 2 diabetes
Insulin sensitivity (as defined by author(s))
Dyslipidaemia or metabolic syndrome
Neurodisability
Educational achievement
Adulthood outcomes
Weight
Height
BMI
Fat mass/fat‐free mass
Skinfold thickness measurements
Blood pressure
Impaired glucose tolerance (defined by author(s))
Development of type 1 diabetes
Development of type 2 diabetes
Insulin sensitivity (defined by author(s))
Dyslipidaemia or metabolic syndrome
Educational achievement
Health services cost
Number of hospital visits or health professional visits (e.g. physiotherapist) or antenatal visits for mother
Medical physician visits
Costs to families in relation to the management provided
Length of postnatal stay (mother)
Admission to neonatal ward
Length of postnatal stay (baby)
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (2 April 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searched the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved articles and reviewed the list of perinatal trials included in the Women and Babies Health and Wellbeing: Action through Trials (WOMBAT) Perinatal Trials Registry (WOMBAT) (2 April 2012). We also searched the ClinicalTrials.gov trial registry (2 April 2012) to identify potential relevant trials. The search strategy used is included in Appendix 1.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook) (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number)
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference when outcomes were measured in the same way between trials. If necessary, we would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion. In future updates of this review, if we identify cluster‐randomised trials, we plan to include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We also plan to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If we detect asymmetry in any of these tests or by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there had been clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity had been detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would have been treated as the average range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not have combined trials.
If we had used random‐effects analyses, we would have presented the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we would have investigated it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis.
Maternal characteristics, and characteristics of exercise interventions might affect health outcomes. We planned to carry out the following subgroup analyses, but there were not enough trials providing relevant data to conduct these subgroup analyses.
1. Maternal characteristics
Maternal age:
we planned to compare women of 35 years of age or more with women less than 35 years of age.
Maternal body mass index (BMI) (at or before trial entry):
we planned to compare women with BMI ranges of 18.5 to 24.9 kg/m² with those with less than 18.5 kg/m²;
BMI ranges of 18.5 to 24.9 kg/m² with those of 25 to 29.9 kg/m²;
BMI ranges of 18.5 to 24.9 kg/m² with those of 30 kg/m² to 39.9 kg/m²;
BMI ranges of 18.5 to 24.9 kg/m² with those of 40 kg/m² or more.
-
Ethnicity:
we planned to compare high‐risk ethnic groups with low‐risk ethnic groups.
Parity:
we planned to compare parity of zero with one to two;
parity of zero with three or more.
2. Nature of exercise interventions
Exercise intervention only compared with exercise intervention plus other forms of intervention (e.g. dietary advice).
Frequency of the intervention:
we compared frequencies of one to four times/week with five or more times/week.
Duration of the intervention:
we planned to compare less than 20 minutes per session with 20 minutes or more per session.
-
Intensity of the exercise sessions:*
we planned to compare light intensity exercise with moderate intensity exercise;
we planned to compare light intensity exercise with high intensity exercise.
*intensity of exercise was defined by individual trials.
3. Ways of delivering intervention
We planned to compare:
exercise advice only with providing exercise sessions;
face‐to‐face intervention with non‐face‐to‐face intervention (e.g. phone counselling, information package, etc.);
group intervention with individual intervention.
We planned to use primary outcomes in subgroup analyses.
We planned to assess differences between subgroups by interaction tests where possible.
Sensitivity analysis
In future updates of this review, we plan to carry out sensitivity analysis to explore the effects of trial quality assessed by allocation concealment and other risk of bias components, by omitting studies rated as 'high risk of bias' for these components. Sensitivity analysis will be restricted to the primary outcomes.
Results
Description of studies
Results of the search
A total of 22 trials were identified for consideration of inclusion in this review. Nineteen trials were identified through the search conducted by the Cochrane Pregnancy and Childbirth Group and three additional trials were identified through searching ClinicalTrials.gov (ClinicalTrials 2011) and the WOMBAT (Women and Babies health and wellbeing: Action through Trials) Perinatal Trials Registry (WOMBAT 2011).
Following application of the eligibility criteria for the review, we included five trials (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). Ten trials did not meet the inclusion criteria for this review and were excluded (Chen 1997; Clapp 1997; Clapp 2002; Clapp 2002a; Gaston 2009; Haakstad 2011; Hui 2006; Kim 2010a; Luoto 2010; Quinlivan 2007). Another seven trials (Chasan‐Taber 2009; Ko 2008; Melo 2008; Newnham 2011; Oostdam 2009; Ramirez‐Velez 2009; Shen 2008) are ongoing and will be considered for inclusion in the next update of this review (seeCharacteristics of ongoing studies).
Included studies
For full details, seeCharacteristics of included studies.
Two of the five included trials (Callaway 2010; Ong 2009) were conducted in Australia, One trial each was conducted in New Zealand (Hopkins 2010), Spain (Barakat 2011) and Norway (Stafne 2012).
Participants
A total of 1115 women and their babies were recruited in the five included trials and 922 women and their babies were involved in the data analysis. Four included trials had small sample sizes (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009) and one trial (Stafne 2012) randomised 855 women.
In Callaway 2010 and Ong 2009 only obese (body mass index (BMI) greater or equal to 30 kg/m²) women were included. In Barakat 2011, women had a mean [SD] pre‐pregnancy BMI of 22.7 [2.8] kg/m² in the intervention group and 23 [2.9] kg/m² in the control group. Hopkins 2010 included healthy nulliparous women with a mean BMI [SD] of 25 [4] kg/m² at trial entry (mean trial entry gestational age [SD]: 19 [1.1] weeks). In Stafne 2012, women in the intervention group had a trial entry mean BMI [SD] of 24.7 [3.0] kg/m² and 25.0 [3.4] kg/m² for women in the control group (women at trial entry were at around 18 weeks' gestation).
Intervention and comparison
In Barakat 2011, women in the intervention group received 35–45‐minutes supervised sessions three times a week with two land aerobic sessions and one aquatic activities session. In Callaway 2010, an individualised exercise plan with an energy expenditure goal of 900 kcal per week was provided to women in the intervention group. Other interventions in this trial included four‐weekly follow‐up for assessment of adherence and diaries for self‐monitoring (Callaway 2010). In Hopkins 2010, interventions were home‐based stationary cycling for a maximum of five sessions of 40 minutes aerobic exercise per week, for the remaining weeks of pregnancy. This aimed for a moderate intensity of 65% of predicted aerobic capacity (VO2max). In addition, women had fortnightly supervised sessions, reviewing their exercise plan and checking for adherence (Hopkins 2010). In Ong 2009, women had home‐based supervised stationary cycling for three sessions a week over a 10‐week period (between 18 weeks of gestation and 28 weeks of gestation). During each session, women had a 10‐minute warm‐up followed by one or two 15‐minute bouts of cycling at an intensity of 50 to 60% HRmax. and the exercise intensity was increased to 60 to 70% HRmax as pregnancy progressed (Ong 2009). In Stafne 2012, women had a weekly supervised 60‐minute group exercise session at moderate to high intensities for a period of 12 weeks. Women were also encouraged to follow a written 45‐minute home exercise program at least twice per week (Stafne 2012).
In four included trials , women in the control group had standard antenatal care with normal daily activities (Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). One included trial did not provide information on what type of care was provided for women in the control group (Barakat 2011).
SeeCharacteristics of included studies table for more details.
Outcome
All the five included trials focused on perinatal outcomes for women and their babies (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). None of the included studies have reported any longer‐term outcomes as yet for mothers or their children.
SeeCharacteristics of included studies for more details.
Excluded studies
Of the 10 excluded trials, three trials were excluded due to their interventions not meeting the inclusion criteria specified for the review (Clapp 2002; Gaston 2009; Quinlivan 2007). In Clapp 2002, all participants received the same exercise intervention but different dietary interventions; Gaston 2009 aimed to assess whether maternal‐fetal disease information was a good source of exercise motivation during pregnancy with no clinically relevant outcomes reported in their paper; and in Quinlivan 2007, exercise was not a part of the study intervention. Another two trials (Hui 2006; Luoto 2010) were excluded as interventions in these two trials included both exercise and diet, and data on the effect of exercise were not able to be abstracted separately. Clapp 2002a and Haakstad 2011 were excluded due to no relevant data reported on pregnancy glucose tolerance. Chen 1997 and Kim 2010a were excluded due to participants not meeting the inclusion criteria of this review and Clapp 1997 was a literature review, not a report of a trial.
SeeCharacteristics of excluded studies for more details.
Risk of bias in included studies
We judged the five included trials to have a moderate risk of bias overall. SeeFigure 1 and Figure 2.
1.
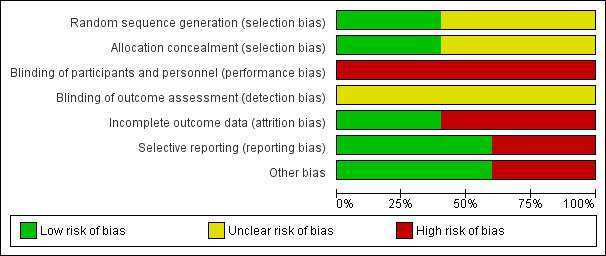
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
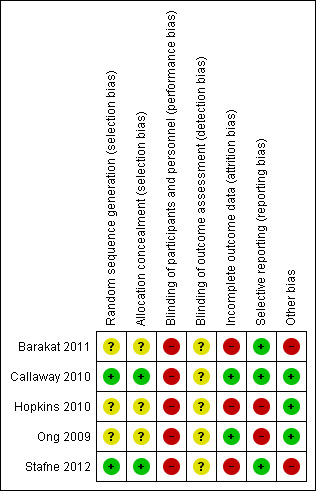
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
Three of the five included trials reported women were randomly allocated to study groups without further details being provided (Barakat 2011; Hopkins 2010; Ong 2009). In Callaway 2010, a random number allocation technique was applied for randomisation and Stafne 2012 performed by a web‐based computerised procedure in blocks of 30.
Allocation
In Callaway 2010 and Stafne 2012, randomisation was conducted by a third party at another location outside the hospital. The other three included trials had no information on allocation concealment (Barakat 2011; Hopkins 2010; Ong 2009).
Blinding
It was unclear whether outcome assessors were blinded to group allocation in all of the five included trials (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). Participants in the five trials were not blinded.
Incomplete outcome data
In Barakat 2011, 10/50 (20%) women from the exercise group and 7/50 (14%) women from the control group did not complete the study and were excluded from the analysis. Reasons for exclusion included developing medical complications and personal reasons such as change of residence (Barakat 2011).
In Callaway 2010, five (10%) women in the intervention group and nine (18%) women in the control group were lost to follow‐up by six weeks postpartum. Of the 14 women lost to follow‐up in this study, two (4%) in the intervention group and three (6%) in the control were not able to be contacted; three (6%) in the intervention group and six (12%) women in the control group were lost to follow‐up as they met the prespecified criteria to terminate the intervention (Callaway 2010). These criteria included persistent second and third trimester bleeding, placenta praevia after 26 weeks' gestation, preterm labour, ruptured membranes, and pre‐eclampsia (Callaway 2010).
For Hopkins 2010, a total of 14 (14.3%) women were lost to follow‐up. Eleven women withdrew from study two (2.4%) in the intervention group and nine (10.7%) women in the control group. Another three (3.6%) women in the control group were lost to follow‐up due to development of contraindications to exercise.
In Stafne 2012, 7.7% (33/429) women in the exercise group and 14.3% (61/426) women in the control group were lost to follow‐up during pregnancy and another 5.3% (21/396) women in the intervention group and 10.4% (38/365) women in the control group were excluded at 32 to 36 weeks assessment. Reported reasons for exclusion included developing medical complications, change of residence, did not attended oral glucose tolerance test (OGTT) or did not attend hospital interview (Stafne 2012).
No losses to follow‐up or post randomisation exclusions were reported in Ong 2009.
Selective reporting
Two included trials did not report any of the prespecified primary outcomes of this review including GDM incidence (Hopkins 2010; Ong 2009). There was no obvious risk of selective reporting in Barakat 2011, Callaway 2010 and Stafne 2012.
Other potential sources of bias
In Barakat 2011, baseline characteristics were only reported for women who completed the study. Among women who completed the study, there was baseline imbalance in maternal education level, parity and exercise habits before gestation between the two study groups (Barakat 2011).
In Stafne 2012, baseline imbalance existed in insulin resistance between women in the exercise group and the control group. Women in the intervention group had lower insulin resistance at baseline when compared with women in the control group. It was also reported that among women who completed the study, those in the intervention group had lower fasting insulin and insulin resistance than women in the control group. Women lost to follow‐up in Stafne 2012 performed exercise less often before pregnancy than those who completed the study.
There was no obvious risk of other potential sources of bias in the other four included trials (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009).
Effects of interventions
Any additional exercise intervention versus routine antenatal care only
Primary outcomes
Three trials reported GDM incidence (Barakat 2011; Callaway 2010; Stafne 2012). In Barakat 2011 and Callaway 2010, GDM was screened at 24 to 28 weeks' gestation and women in Stafne 2012 were screened for GDM at both baseline (between 18 to 22 weeks' gestation) and the end of the intervention period (between 32 to 36 weeks' gestation). Three different GDM diagnostic criteria, including American Diabetes Association (ADA) criteria, Australasian Diabetes in Pregnancy Society (ADIPS) criteria and the WHO criteria were used in the three trials (Barakat 2011; Callaway 2010; Stafne 2012).
No significant difference was seen in the GDM incidence between women receiving additional exercise intervention and those having routine antenatal care (three trials, 826 women, risk ratio (RR) 1.10, 95% confidence interval (CI) 0.66 to 1.84) (Analysis 1.1).
1.1. Analysis.
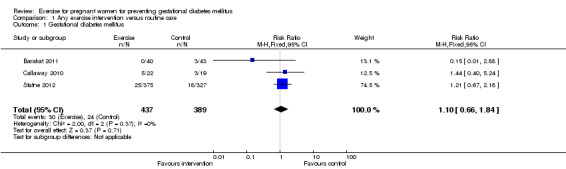
Comparison 1 Any exercise intervention versus routine care, Outcome 1 Gestational diabetes mellitus.
Women receiving exercise interventions had a slightly increased caesarean section rate, however, the difference was only of borderline significance (two trials, 934 women, RR 1.33, 95% CI 0.97 to 1.84) (Analysis 1.2). No significant difference was seen in the rate of operative vaginal birth between women in the exercise group and control group (two trials, 934 women, RR 0.83, 95% CI 0.58 to 1.17) (Analysis 1.3).
1.2. Analysis.
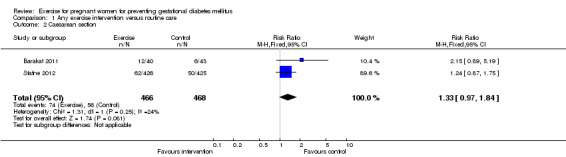
Comparison 1 Any exercise intervention versus routine care, Outcome 2 Caesarean section.
1.3. Analysis.
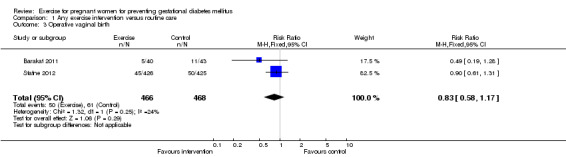
Comparison 1 Any exercise intervention versus routine care, Outcome 3 Operative vaginal birth.
No trial reported large‐for‐gestational age and perinatal mortality.
Secondary outcomes
Maternal outcomes
There was no significant difference in the incidence of women developing pregnancy hyperglycaemia not meeting GDM diagnostic criteria (one trial, 83 women, RR1.07, 95% CI 0.16 to 7.27) (Analysis 1.4). Women receiving the additional exercise intervention had no significant differences in weight in late pregnancy (one trial, 84 women, mean difference (MD) ‐ 1.10 kg, 95% CI ‐ 6.11 to 3.91), weight gain during the intervention period (exercise interventions lasted for less than one trimester) (one trial, 12 women, MD ‐ 1.50 kg, 95% CI ‐ 4.41 to 1.41) or weight gain during the intervention period (exercise interventions lasted for one trimester or more) (one trial, 83 women, MD ‐1.30 kg, 95% CI ‐2.66 to 0.06) when compared with women having routine care (Analysis 1.5). For maternal BMI in late pregnancy, data from one trial (Hopkins 2010) suggested no significant difference between the two study groups (84 women, MD 0.10 kg/m², 95% CI ‐ 1.39 to 1.59) (Analysis 1.6). No significant difference was seen in the incidence of pre‐eclampsia between women in the exercise intervention group and the control group (one trial, 852 women, RR 1.00, 95% CI 0.51to 1.97) (Analysis 1.7).
1.4. Analysis.
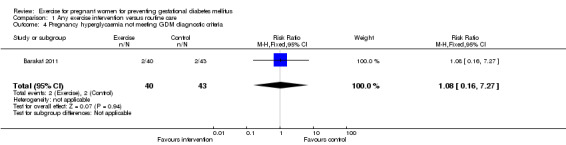
Comparison 1 Any exercise intervention versus routine care, Outcome 4 Pregnancy hyperglycaemia not meeting GDM diagnostic criteria.
1.5. Analysis.
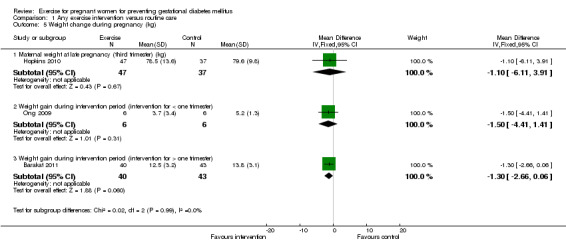
Comparison 1 Any exercise intervention versus routine care, Outcome 5 Weight change during pregnancy (kg).
1.6. Analysis.
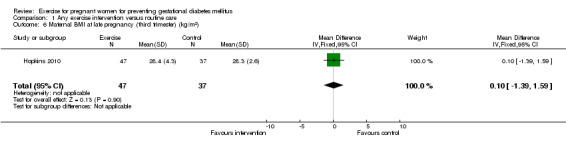
Comparison 1 Any exercise intervention versus routine care, Outcome 6 Maternal BMI at late pregnancy (third trimester) (kg/m2).
1.7. Analysis.
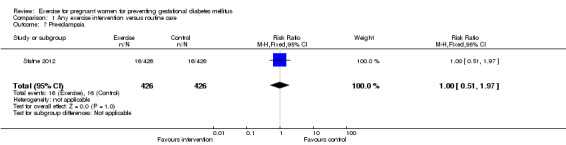
Comparison 1 Any exercise intervention versus routine care, Outcome 7 Pre‐eclampsia.
Adherence to the exercise intervention was reported as excellent in four of the five included trials (Barakat 2011, Callaway 2010; Hopkins 2010; Ong 2009). Barakat 2011 reported 85% of women in the intervention group adhered to the exercise intervention without providing information on the definition of adherence. In Callaway 2010, 15 women (71%) achieved the prescribed exercise goal at 20 weeks' gestation, 16 women (73%) at 28 weeks' gestation and 10 women (53%) at 36 weeks' gestation. In Hopkins 2010, women in the intervention group completed 75% [SD] 17% of total exercise prescribed. In Ong 2009, 94% of all scheduled exercise sessions were completed by women in the intervention group. In Stafne 2012, 55% (n = 217) women in the intervention group were adherent, which was defined as exercising three days per week or more at moderate to high intensity, while10% (n = 33) women in the control group were found to be exercising three days per week or more at moderate to high intensity during the study period.
Insulin sensitivity was reported in four trials (Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). Different methods and different time points were chosen to measure insulin sensitivity in the four trials, so it was not possible to statistically combine these results. In Stafne 2012 and Callaway 2010, insulin resistance was estimated using "Homeostasis model" (HOMA‐IR). Stafne 2012 found a significant lower insulin resistance in women receiving the exercise intervention at 32 to 36 weeks' gestation when compared with women in the control group, however, this difference became non‐significant after adjusting for baseline imbalance in insulin resistance between the two study groups. Callaway 2010 found no significant difference in insulin sensitivity at 12 weeks, 20 weeks, 28 weeks and 36 weeks' gestation between women receiving the additional exercise intervention and those having routine antenatal care. Hopkins 2010 used minimal model analysis of parameters of insulin sensitivity. MINMOD Millennium computer program was used to calculate insulin sensitivity index, acute insulin response, glucose effectiveness based fasting insulin and glucose values. No difference was seen in any parameters of glucose regulation during pregnancy between women in the exercise intervention group and control group at trial entry (19+/‐ 1.1 weeks' gestation) or late pregnancy (35+/‐0.8 weeks' gestation) (Hopkins 2010). In Ong 2009, insulin sensitivity was determined from the OGTT using a validated oral glucose insulin sensitivity (OGIS) index and reported no significant difference between women in the two study groups at 28 weeks' gestation.
There were no data available on other maternal perinatal outcomes and no trial reported longer‐term outcomes and health services cost.
Fetal/neonatal outcomes
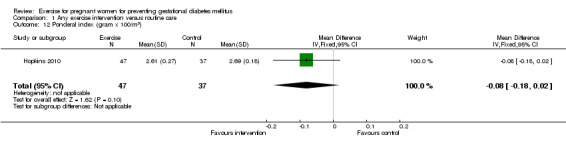
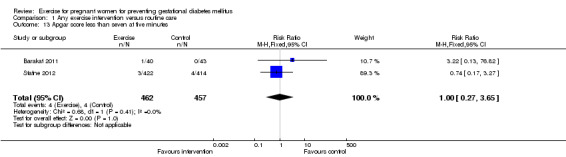
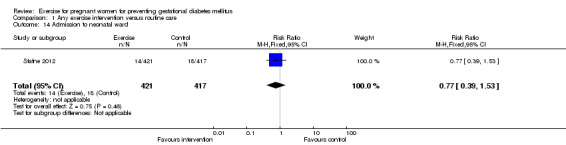
For fetal and neonatal secondary outcomes, no significant difference was found between babies born to women in the exercise intervention group and control group for birthweight (two trials, 167 infants, MD ‐102.87 gram, 95% CI ‐235.34 to 29.60) (Analysis 1.8); macrosomia (two trials, 934 infants, RR 0.91, 95% CI 0.68 to 1.22) (Analysis 1.9); small‐for‐gestational age (one trial, 84 infants, RR 1.05, 95% CI 0.25 to 4.40) (Analysis 1.10); or gestational age at birth (two trials, 167 infants, MD ‐0.04 week; 95% CI ‐0.37 to 0.29) (Analysis 1.11). Babies born to women receiving exercise interventions had a slightly lower ponderal index, however, the difference was only of borderline significance (one trial, 84 infants, MD ‐0.08 gram x 100/m³, 95% CI ‐0.18 to 0.02) (Analysis 1.12). No significant differences were seen in the incidences of Apgar score less than seven in five minutes (two trials, 919 infants, RR 1.00, 95% CI 0.27 to 3.65) (Analysis 1.13) and admission to neonatal ward (one trial, 838 infants, RR 0.77, 95% CI 0.39 to 1.53) (Analysis 1.14) between the two study groups.
1.8. Analysis.
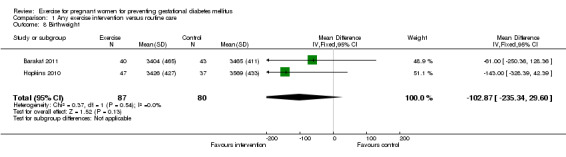
Comparison 1 Any exercise intervention versus routine care, Outcome 8 Birthweight.
1.9. Analysis.
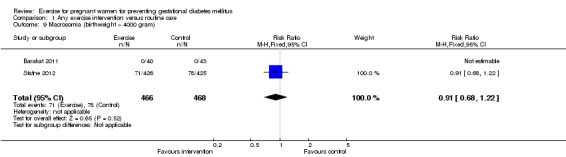
Comparison 1 Any exercise intervention versus routine care, Outcome 9 Macrosomia (birthweight > 4000 gram).
1.10. Analysis.
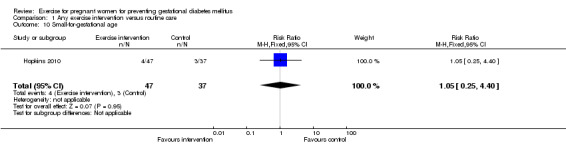
Comparison 1 Any exercise intervention versus routine care, Outcome 10 Small‐for‐gestational age.
1.11. Analysis.
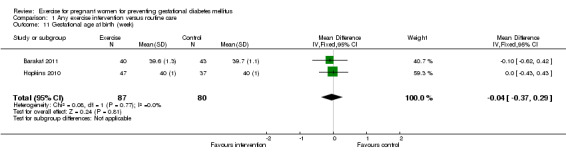
Comparison 1 Any exercise intervention versus routine care, Outcome 11 Gestational age at birth (week).
1.12. Analysis.
Comparison 1 Any exercise intervention versus routine care, Outcome 12 Ponderal index (gram x 100/m3).
1.13. Analysis.
Comparison 1 Any exercise intervention versus routine care, Outcome 13 Apgar score less than seven at five minutes.
1.14. Analysis.
Comparison 1 Any exercise intervention versus routine care, Outcome 14 Admission to neonatal ward.
There were no data available on the other neonatal and infant review outcomes. No trial has reported on childhood or adulthood outcomes and health services cost.
Discussion
Summary of main results
Based on the current available evidence from five randomised trials with data available from 992 women and their babies, we found exercise interventions, including individualised exercise advice with regular follow‐up, home‐based stationary cycling either supervised or unsupervised, or providing regular supervised group exercise sessions had no significant effect on preventing gestational diabetes mellitus (GDM) or improving insulin sensitivity during pregnancy compared with standard antenatal care with normal daily activities.
We found women who received the exercise intervention had a trend of increased caesarean section rate, although the difference was only of borderline significance. This may result from the inclusion of outcome data from Barakat 2011, which found a doubled risk of caesarean section in women receiving exercise intervention. This increased risk for women in the exercise group in Barakat 2011 may result from the baseline imbalance in parity, where more women in the exercise group were primiparous when compared with women in the control group. Another possible explanation for this increased risk of caesarean section is the closer monitoring of women in the exercise group during the study period.
We did not find any significant differences in operative vaginal birth between women receiving additional exercise intervention and routine care. No significant differences were seen in any of the other reported maternal and infant secondary outcomes between the two study groups.
Overall completeness and applicability of evidence
The evidence for exercise during pregnancy for GDM prevention is incomplete. No trial reported on the primary outcomes for the review of large‐for‐gestational age and perinatal mortality.
Many reported secondary outcomes, including pregnancy hyperglycaemia not meeting GDM diagnostic criteria, maternal weight change during pregnancy, maternal BMI at late pregnancy, small‐for‐gestational age, ponderal index, were limited to single trials with small sample sizes (Barakat 2011; Hopkins 2010). No trial reported any longer‐term outcomes for the women and their children. It is important to note that all of the five included trials were conducted in high‐income countries (two were in Australia, one each from in New Zealand, Norwary and Spain), hence it is limited for other settings.
Quality of the evidence
In this review, we included five trials with 922 women and their babies providing outcome data (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009; Stafne 2012). Four of the five included trials had small sample sizes (Barakat 2011; Callaway 2010; Hopkins 2010; Ong 2009) and the overall risk of bias for the five included trials was judged to be moderate.
The methods of generating random sequence and allocation concealment were unclear in three trials (Barakat 2011; Hopkins 2010; Ong 2009). Risk of performance bias is not easy to avoid since behavioural interventions such as these cannot easily be blinded from participants or investigators. This was seen in Callaway 2010 and Stafne 2012 where it was noted that women in the control group voluntarily increased the amount of physical activity they undertook. Similarly, some attrition after randomisation is to be expected as some women will develop contraindications to exercise during their pregnancy. However, there was also attrition due to women being lost to follow‐up, with a substantial differential between intervention and control groups in Hopkins 2010 and Stafne 2012.
In Barakat 2011 and Stafne 2012, baseline imbalances were noted in maternal education level, parity, exercise habits before gestation and insulin resistance between the two study groups.
Potential biases in the review process
The baseline physical activity levels of the women were unclear in three included trials (Callaway 2010; Hopkins 2010; Ong 2009). Barakat 2011 reported women's pre‐pregnancy exercise pattern while Stafne 2012 reported women's exercise pattern at around 20 weeks' gestation. A potential source of bias may be introduced by the different activity levels of women at trial entry in the different studies.
We were not able to examine if publication bias was present due to the small number of trials included in this review. We will test if there is any publication bias by using funnel plots when additional eligible trials become available.
Agreements and disagreements with other studies or reviews
We found no significant difference in the risk of developing GDM when comparing women receiving additional exercise interventions during pregnancy with those having standard antenatal care. The four included trials which reported outcome data on insulin sensitivity, used different methods to assess insulin sensitivity and showed no difference in insulin sensitivity at different gestation weeks between women in the two treatment groups. We did not see any differences between women and their babies in the two study groups for any of the other reported secondary outcomes.
Another Cochrane review (Kramer 2006) and one non‐Cochrane systematic review (Streuling 2011) on the effect of exercise during pregnancy were identified through searching the literature. Kramer 2006 assessed the effect of exercise during pregnancy on physical fitness, the course of labour and delivery and other pregnancy outcomes. A total of 14 small trials involving 1014 healthy pregnant women, of moderate to high risk of bias were reviewed (Kramer 2006). Streuling 2011 aimed to assess the effect of physical activity during pregnancy on gestational weight gain; 12 trials of varying risk of bias, involving 906 healthy pregnant women were reviewed.
Neither review (Kramer 2006; Streuling 2011) reported on the effects of exercise on preventing pregnancy glucose intolerance. Both reviews (Kramer 2006; Streuling 2011) reported on maternal gestational weight gain, and this was the only outcome reported in Streuling 2011. Kramer 2006 found increasing exercise in sedentary women had no significant effect on total maternal gestational weight gain, which was consistent with our results; while Streuling 2011 reported significant lower gestational weight gain in women in the intervention group compared with control group (12 trials, 906 women, MD ‐0.61 kg, 95% CI ‐1.17 to ‐0.06). In a sensitivity analysis, by excluding three trials with high risk of bias, this difference remained significant (MD ‐0.93, 95% CI ‐1.35 to ‐0.50) (Streuling 2011). This difference may result from the inclusion of one trial which found a significant difference in gestational weight gain between women in the exercise group and the control group (Sedaghati 2007) in Streuling 2011, but not included in our review or in Kramer 2006.
For other pregnancy outcomes, Kramer 2006 found the exercise intervention had no significant effect on caesarean section rate, infant birthweight, and gestational age at birth, which was consistent with our results.
Authors' conclusions
Implications for practice.
There is a limited and incomplete body of evidence from randomised trials assessing the effects of exercise for preventing gestational diabetes or glucose intolerance in pregnancy, which is insufficient to inform or guide practice.
Implications for research.
Further well‐designed trials with sufficient power to assess the effects of exercise for pregnant women on GDM prevention and other pregnancy outcomes are needed. Different types and intensities of exercise interventions should be compared in future trials. Outcomes such as longer‐term health outcomes for women and their children and health service costs should be included.
Acknowledgements
Thanks to Associate Professor Lene AH Haakstad and Professor Kari Bo (Norwegian School of Sport Sciences, Department of Sports Medicine, Ulleval Stadion, Oslo, Norway) for providing additional information for their trials.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
Appendices
Appendix 1. WOMBAT Perinatal Trials Registry and ClinicalTrials.gov search strategy
We searched trials in the Women and Babies Health and Wellbeing: Action through Trials (WOMBAT) Perinatal Trials Registry using the terms of "gestational diabetes mellitus", "pregnancy", "pregnant", "glucose intolerance", "exercise", "lifestyle", "behavioural intervention". We reviewed all relevant trials listed under the search results.
We searched trials in the ClinicalTrials.gov trial registry using the terms of "exercise", "lifestyle", "behavioural intervention", "pregnancy", "pregnant", "glucose intolerance", "gestational diabetes mellitus". Then we categorised retrieved trials by topic. We selected the condition category of "Nutritional and Metabolic Diseases" and we reviewed trials listed under the conditions of "Diabetes Mellitus", "Diabetes, Gestational", "Glucose Intolerance", "Glucose Metabolism Disorders", "Hyperglycemia", "Metabolic Diseases", "Obesity", "Overnutrition".
Data and analyses
Comparison 1. Any exercise intervention versus routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational diabetes mellitus | 3 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.66, 1.84] |
| 2 Caesarean section | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.97, 1.84] |
| 3 Operative vaginal birth | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.17] |
| 4 Pregnancy hyperglycaemia not meeting GDM diagnostic criteria | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.16, 7.27] |
| 5 Weight change during pregnancy (kg) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Maternal weight at late pregnancy (third trimester) (kg) | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐6.11, 3.91] |
| 5.2 Weight gain during intervention period (intervention for < one trimester) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.41, 1.41] |
| 5.3 Weight gain during intervention period (intervention for > one trimester) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.66, 0.06] |
| 6 Maternal BMI at late pregnancy (third trimester) (kg/m2) | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.39, 1.59] |
| 7 Pre‐eclampsia | 1 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.51, 1.97] |
| 8 Birthweight | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐102.87 [‐235.34, 29.60] |
| 9 Macrosomia (birthweight > 4000 gram) | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.22] |
| 10 Small‐for‐gestational age | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.25, 4.40] |
| 11 Gestational age at birth (week) | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.37, 0.29] |
| 12 Ponderal index (gram x 100/m3) | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.18, 0.02] |
| 13 Apgar score less than seven at five minutes | 2 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.27, 3.65] |
| 14 Admission to neonatal ward | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barakat 2011.
| Methods | Randomised controlled trial. | |
| Participants | 100 healthy women having uncomplicated and singleton pregnancies. Exclusion criteria:
Setting: Madrid, Spain. |
|
| Interventions | Women in the intervention group (50 women randomised, 40 women completed study):
Women in the control group (50 women randomised, 43 women completed study):
|
|
| Outcomes | Primary: 50 g maternal glucose screen, maternal weight gain and GDM. Secondary: maternal age, BMI, smoking habits, alcohol intake, occupational activity, time standing per day, time of domestic task, educational level, parity, gestational age, type of delivery, blood pressure, birthweight, Apgar score and adherence (women in the intervention group). |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as “women were randomly assigned either to an exercise group or a control group”. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported on whether outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/50 (20%) women from the exercise group did not complete study, reported reasons were: risk of premature labour (n = 3), incompetent cervix was diagnosed (n = 2) and personal reasons such as change of residence (n = 5). 7/50 (14%) participants of the control group did not complete study, reported reasons were: pregnancy‐induced hypertension (n = 1), risk for premature labour (n = 2) and personal reasons (n = 4). |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | High risk | Baseline characteristics were only available for women who completed study (83/100). Among the women who completed study, baseline imbalance exited in maternal education level, parity and exercise habits before gestation between the two study groups. |
Callaway 2010.
| Methods | Randomised controlled trial. | |
| Participants | 50 obese women (BMI ≥ 30 kg/m²) aged 18‐45 years, who were willing and able to be randomised to an exercise intervention. Exclusion criteria: non‐English speaking, contraindication or inability to exercise, medical or obstetric contraindication to exercise including haemodynamically significant heart disease, restrictive lung disease, incompetent cervix (cerclage), multiple gestation, severe anaemia, chronic bronchitis, type 1 diabetes, orthopaedic limitations, poorly controlled seizure disorder, poorly controlled hyperthyroidism, or a heavy smoker. Setting: the Royal Brisbane and Women’s Hospital (a tertiary referral teaching hospital), Queensland, Australia. |
|
| Interventions |
|
|
| Outcomes | Primary outcomes: energy expenditure at 12, 20, 28 and 36 weeks' gestation; insulin resistance at 12, 20, 28 and 36 weeks' gestation. Other outcomes: gestational diabetes mellitus (according to ADIPS criteria (see notes)). |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as: "randomisation was done by a random number allocation technique", no further details about the random number allocation technique, probably done adequately. |
| Allocation concealment (selection bias) | Low risk | Reported as: "randomisation was done by a random number allocation technique conducted by a third party at another location outside the hospital”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on whether outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
|
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
Hopkins 2010.
| Methods | Randomised controlled trial. | |
| Participants | 98 healthy nulliparous women between 20‐40 years of age, with a singleton pregnancy of less than 20 weeks' gestation. Exclusion criteria: alcohol consumption or tobacco use at recruitment; a personal or family history of T2DM; development of any medical condition for which participation in an exercise program was contraindicated by the American College of Obstetricians and Gynecologists (e.g. pre‐eclampsia, fetal growth restriction, preterm birth). Setting: Auckland, New Zealand. |
|
| Interventions | Women in the intervention group (n = 47)
Women in the control group (n = 37)
|
|
| Outcomes | Maternal insulin sensitivity and body composition; infant birthweight, SGA, crown‐heel length, head circumference and chest circumference; neonatal BMI, ponderal index, growth related peptides and offspring body composition. | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “all participants were randomly assigned to exercise or control groups”, no further details were available. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on whether outcome assessors were blinded to group allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 14 (14.3%) participants (two from the intervention group and 12 from the control group) lost to follow‐up during the study period.
Another 2 participants in the control group withdrew at the allocation stage as a result of increased work commitments (did not complete baseline testing) and wanting to take part in another exercise program during pregnancy. No information reported on whether or not these 2 participants were included in the final data analysis. |
| Selective reporting (reporting bias) | High risk | None of the prespecified primary outcomes of this review were reported in this trial. |
| Other bias | Low risk | No obvious risk of other bias, although not clear why groups are unbalanced (37 women in the control group and 47 women in the intervention group). |
Ong 2009.
| Methods | Randomised control trial. | |
| Participants | 12 obese women (mean BMI [SD]: 35.1 [3.5] kg/m²) with a singleton pregnancy, a normal 18‐week anatomy scan and no evidence of cardiovascular disease or pre‐existent diabetes. No information on exclusion criteria. Setting: Crawley, Western Australia, Australia. |
|
| Interventions | Women in the intervention group (n = 6)
Women in the control group (n = 6)
All women
|
|
| Outcomes | Maternal weight change, glucose tolerance, insulin sensitivity. | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “women were randomly allocated into either an exercise intervention group or a control group”, no other information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available on whether outcome assessors were blinded to group allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up or post randomisation exclusion. |
| Selective reporting (reporting bias) | High risk | None of the prespecified primary outcomes of this review were reported in this trial. |
| Other bias | Low risk | No obvious risk of other bias. |
Stafne 2012.
| Methods | Randomised control trial. | |
| Participants | 855 white women aged 18 years or older with a singleton live fetus. Exclusion criteria: High‐risk pregnancies or diseases that could interfere with participation (or both). Women who lived too far from the hospitals to attend weekly training groups (more than 30‐minute drive). Setting: Stavanger, Norway. |
|
| Interventions | Women in the intervention group (429 women randomised, 375 women completed study):
Women in the control group (426 women randomised, 327 women completed study):
All women:
|
|
| Outcomes | Primary outcomes: prevalence of gestational diabetes and insulin resistance. Secondary outcomes: maternal weight, BMI and pregnancy complications and outcomes (e.g. newborn weight, gestational age, Apgar scores). |
|
| Notes | GDM diagnosis based on WHO criteria:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by a web‐based computerised procedure in blocks of 30. |
| Allocation concealment (selection bias) | Low risk | Described as “concealed randomisation was performed at the Unit for Applied Clinical Research, Norwegian University of Technology and Science (which was outside the recruiting hospitals)”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors for glucose and insulin levels were blinded for group allocation; no information on whether outcome assessors for other outcomes were blinded for group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 375/429 women in intervention group and 327/426 women in control group completed study. The Intervention group: 33/429 (7.7%) women lost to follow‐up during pregnancy: 5 gave birth before follow‐up; 1 medical reasons, 4 illness; 1 moved; 2 work and/or family reasons; 20 gave no reason. 396 women assessed at 32‐36 weeks' gestational age (then 10 answered questionnaire by mail; 11 did not complete OGTT). The control group: 61/426 (14.3%) women lost to follow‐up during pregnancy: 6 gave birth before follow‐up;1 medical reasons, 2 illness; 4 moved; 2 work and/or family reasons; 46 gave no reason. 365 women assessed at 32‐36 weeks' gestational age (then 24 answered questionnaire by mail; 14 did not complete OGTT). |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | High risk |
|
ACOG: American College of Obstetricians and Gynecologists ADA: Amerian Diabetes Association ADIPS: Australasian Diabetes in Pregnancy Society BGL: blood glucose level BMI: body mass index BP: blood pressure DM: diabetes mellitus GDM: gestational diabetes mellitus HR: heart rate HRmax: max heart rate IOM: Institute of Medicine IUGR: intrauterine growth restriction LGA: large‐for‐gestational age OGTT: oral glucose tolerance test SD: standard deviation SGA: small‐for‐gestational age T2DM: type 2 diabetes mellitus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 1997 | Participants were women with abnormal 1‐hour 50‐gram oral glucose challenge test result (> 140 mg/dL). |
| Clapp 1997 | A literature review, but not a report for randomised controlled trial. |
| Clapp 2002 | All participants received the same exercise intervention but different dietary interventions (high glycaemic carbohydrate diet was compared with low glycaemic carbohydrate diet). |
| Clapp 2002a | No outcome relating to pregnancy glucose tolerance reported. |
| Gaston 2009 | This trial aimed to assess whether maternal‐fetal disease information was a good source of exercise motivation during pregnancy. No clinically relevant outcome was reported. |
| Haakstad 2011 | No outcome relating to pregnancy glucose tolerance reported. |
| Hui 2006 | Participants in the intervention group received both exercise and dietary interventions, data for the effects of exercise intervention on pregnancy outcomes were not able to be extracted separately. |
| Kim 2010a | Participants were women with recent diagnosis of GDM. |
| Luoto 2010 | This trial aimed to assess the effect of diet and exercise counselling on preventing GDM. Data for the effects of exercise counselling for GDM prevention were not able to be extracted separately. |
| Quinlivan 2007 | Exercise was not a part of the study intervention. |
GDM: gestational diabetes mellitus
Characteristics of ongoing studies [ordered by study ID]
Chasan‐Taber 2009.
| Trial name or title | A randomised controlled trial of prenatal physical activity to prevent gestational diabetes. |
| Methods | RCT. |
| Participants | 364 sedentary women, with diagnosis of GDM in a prior pregnancy according to American Diabetes Association criteria. |
| Interventions | 12‐week individually tailored exercise intervention. |
| Outcomes | GDM, serum biomarkers associated with insulin resistance, adoption and maintenance of exercise during pregnancy. |
| Starting date | |
| Contact information | Lisa Chasan‐Taber: LCT@schoolph.umass.edu |
| Notes |
Ko 2008.
| Trial name or title | Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome. |
| Methods | RCT. |
| Participants | 100 pregnant women 18‐45 years old receiving prenatal care at Madigan Army Medical Center. |
| Interventions | Intervention group will exercise 3 times per week at moderate‐vigorous intensity for 45 minutes per session. Control group women will continue their usual physical activity throughout pregnancy. |
| Outcomes | Primary outcome: central adiposity. Secondary outcome: leptin levels, glucose, insulin, cholesterol, fetal adiposity and neonatal adiposity. |
| Starting date | October 2007. |
| Contact information | Cynthia W Ko: United States, Washington Madigan Army Medical Center Fort Lewis, Washington, United States, 98431. |
| Notes |
Melo 2008.
| Trial name or title | Exercise and pregnancy: randomised clinical trial. |
| Methods | |
| Participants | 150 women aged between 10‐50 years, with singleton pregnancy (gestational age < 13 weeks), fetus alive and no previous practice of physical activity. |
| Interventions | Walking 3 times a week during 1 hours (moderate activity). |
| Outcomes | Maternal primary outcome: preterm labour, weight gain, pre‐eclampsia, gestational diabetes. Perinatal primary outcome: birthweight, Apgar scores, body composition, admission at neonatal intensive care unit. |
| Starting date | April 2008. |
| Contact information | Adriana Melo: asomelo@gmail.com Melania Amorim: melamorim@uol.com.br |
| Notes |
Newnham 2011.
| Trial name or title | Preventing gestational diabetes mellitus using a home‐based supervised exercise program during pregnancy. |
| Methods | RCT. |
| Participants | 200 women at 12‐13 weeks' gestation, with a history of gestational diabetes in a previous pregnancy. |
| Interventions | In addition to routine, regular antenatal care, women in the intervention group will be required to participate in 3 60‐minute exercise sessions each week, starting at 14 weeks' gestation, for a total of 14 weeks (i.e. to be completed by 28 weeks of gestation). All exercise sessions will be home‐based and fully supervised by an experienced exercise physiologist. |
| Outcomes | Primary outcome measures: diagnosis of gestational diabetes mellitus (after the 14 week intervention period (28 weeks' gestation)). |
| Starting date | April 2011. |
| Contact information | John Newnham: JNewnham@obsgyn.uwa.edu.au Kym Guelfi: kym.guelfi@uwa.edu.au |
| Notes | ClinicalTrials.gov identifier: NCT01283854 |
Oostdam 2009.
| Trial name or title | The effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. |
| Methods | RCT. |
| Participants | 160 pregnant women who are at increased risk for GDM. |
| Interventions | Supervised exercise program on 2 days per week during the remaining duration of the pregnancy. Each session will last for 60 minutes, consist of aerobic and strength exercises. |
| Outcomes | Primary maternal outcome: fasting plasma glucose and relative increase in insulin resistance; primary neonatal outcome: birthweight. Secondary outcome measures are: maternal serum triglycerides, maternal weight gain during pregnancy, maternal physical activity level, fetal growth. |
| Starting date | 1/10/2007. |
| Contact information | Corresponding author: Mireille Nm van Poppel: mnm.vanpoppel@vumc.nl |
| Notes |
Ramirez‐Velez 2009.
| Trial name or title | Clinical trial to assess the effect of physical exercise on endothelial function and insulin resistance in pregnant women. |
| Methods | RCT. |
| Participants | 64 women, gestational age between 16‐20 weeks at trial entry, who have not participated in a structured exercise program, including significant amounts of walking for the past 4 months are eligible for the trial and live fetus at the routine ultrasound scan and a normal pregnancy. |
| Interventions | Walking (10 minutes), aerobic exercise (30 minutes), stretching (10 minutes) and relaxation exercise (10 minutes). Exercise will be performed at 3 sessions per week. All sessions will be supervised by a physical therapist and a physical educator. Aerobic activities will be performed at moderate intensity (60%‐70% of maximal heart rate) measured by the 6‐20 Borg's rating scale. Each session starts with 5 minutes of warm up, followed by 30 minutes of aerobic activity, including 5 minutes cool down. This is followed by 15 minutes of circuit strength training of the upper limbs, lower limbs, and deep abdominal stabilisation muscles. The last 5 minutes consists of stretching and relaxation exercises. |
| Outcomes | Primary outcome: brachial artery flow‐mediated dilation. Secondary outcome: high sensitivity C‐Reactive Protein, nitrates, nitrites and cyclic GMP, blood lipid profile, anthropometric indicators, functional capacity, maternal and neonatal outcomes. |
| Starting date | October 2008. |
| Contact information | |
| Notes |
Shen 2008.
| Trial name or title | Impact of diet and exercise activity on pregnancy outcomes (IDEA). |
| Methods | RCT. |
| Participants | 1600 healthy pregnant women with < 20 weeks' gestation. |
| Interventions | Aerobic exercise or walk for 3‐5 times/day for 30‐45 minutes/time from 20 weeks to 36 weeks of pregnancy. Dietary education on nutrition for healthy pregnancy through weekly class during pregnancy. |
| Outcomes | Primary outcome: excessive weight gain during pregnancy. Secondary outcome: macrosomia, requirement of delivery procedures. |
| Starting date | July 2006. |
| Contact information | Garry Shen: gshen@ms.umanitoba.ca |
| Notes |
cyclic GMP: cyclic guanosine monophosphate GDM: gestational diabetes mellitus RCT: randomised controlled trial
Differences between protocol and review
In order to avoid duplicate information from another Cochrane review by Kramer 2006, we decided to only include studies that reported relevant outcomes on gestational diabetes mellitus, pregnancy glucose tolerance, insulin sensitivity or insulin resistance during pregnancy.
Outcome measure of 'maternal BMI at late pregnancy' was added in the review.
Weight gain during pregnancy was divided into subgroups of 'weight gain during intervention period' and 'maternal weight at late pregnancy (third trimester).
Contributions of authors
Shanshan Han wrote drafts of the protocol and review, with Caroline Crowther and Philippa Middleton contributing to data extraction and all drafts.
Sources of support
Internal sources
Australian Research Centre for Health of Women and Babies (ARCH), Robinson Institute, The University of Adelaide, Australia.
External sources
Australian Department of Health and Ageing and NHMRC, Australia.
Declarations of interest
None known.
New
References
References to studies included in this review
Barakat 2011 {published data only}
Callaway 2010 {published data only}
- Byrne NM, Groves AM, McIntyre HD, Callaway LK. Changes in resting and walking energy expenditure and walking speed during pregnancy in obese women. American Journal of Clinical Nutrition 2011;94(3):819‐30. [DOI] [PubMed] [Google Scholar]
- Callaway L. A randomized controlled trial using exercise to reduce gestational diabetes and other adverse maternal and neonatal outcomes in obese pregnant women ‐ the pilot study. Australian Clinical Trials Registry (www.actr.org.au) (accessed 21 June 2007).
- Callaway L, McIntyre D, Colditz P, Byrne N, Foxcroft K, O'Connor B. Exercise in obese pregnant women: a randomized study to assess feasibility. Hypertension in Pregnancy 2008;27(4):549. [Google Scholar]
- Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes. Feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010;33(7):1457‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2010 {published data only}
- Hofman P, Hopkins S. Randomised controlled study of the effects of exercise during pregnancy on maternal insulin sensitivity and neonatal outcomes. Australian Clinical Trials Register (http://www.actr.org/actr) (accessed 6 December 2005).
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Effects of exercise training on maternal hormonal changes in pregnancy. Clinical Endocrinology 2011;74(4):495‐500. [DOI] [PubMed] [Google Scholar]
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. Journal of Clinical Endocrinology and Metabolism 2010;95(5):2080‐8. [DOI] [PubMed] [Google Scholar]
Ong 2009 {published data only}
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home‐based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes and Metabolism 2009;35(5):418‐21. [DOI] [PubMed] [Google Scholar]
Stafne 2012 {published data only}
- Morkved S. Effects of regular exercise during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
- Stafne SN, Salvesen KA, Romundstad PR, Eggebo TM, Carlsen SM, Morkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstetrics and Gynecology 2012;119(1):29‐36. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 1997 {published data only}
- Chen B, Steiner JL, Holcomb WL. Effects of a short‐term moderate exercise program on glucose tolerance in pregnancy. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S173. [Google Scholar]
Clapp 1997 {published data only}
- Clapp JF 3rd. Diet, exercise, and feto‐placental growth. Archives of Gynecology and Obstetrics 1997;260:101‐8. [Google Scholar]
Clapp 2002 {published data only}
- Clapp JF 3rd. Maternal carbohydrate intake and pregnancy outcome. Proceedings of the Nutrition Society 2002;61(1):45‐50. [DOI] [PubMed] [Google Scholar]
Clapp 2002a {published data only}
- Clapp JF 3rd, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. American Journal of Obstetrics and Gynecology 2002;186(1):142‐7. [DOI] [PubMed] [Google Scholar]
Gaston 2009 {published data only}
- Gaston A, Prapavessis H. Maternal‐fetal disease information as a source of exercise motivation during pregnancy. Health Psychology 2009;28(6):726‐33. [DOI] [PubMed] [Google Scholar]
Haakstad 2011 {published data only}
- Haakstad L, Bo K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. European Journal of Contraception and Reproductive Health Care 2011;16:116‐25. [DOI] [PubMed] [Google Scholar]
- Haakstad L, Bo K. Effect of supervised aerobic dance exercise in prevention of excessive weight gain in pregnancy: a single blind randomized controlled trial. International Journal of Gynaecology and Obstetrics 2009;107(Suppl 2):S198. [Google Scholar]
Hui 2006 {published data only}
- Hui AL, Ludwig SM, Gardiner P, Sevenhuysen G, Murray R, Morris M, et al. Community‐based exercise and dietary intervention during pregnancy: a pilot study. Canadian Journal of Diabetes 2006;30(2):169‐75. [Google Scholar]
Kim 2010a {published data only}
- Kim C. Walking exercise and nutrition to reduce diabetes risk for you (WENDY). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 06 July 2011).
Luoto 2010 {published data only}
- Luoto R. Primary prevention of gestational diabetes among women at risk: a cluster‐randomized controlled trial. Current Controlled Trials (http://www.controlled‐trials.com/ISRCTN33885819) (accessed 2010).
- Luoto RM, Kinnunen TI, Aittasalo M, Ojala K, Mansikkamaki K, Poropainen E, et al. Prevention of gestational diabetes: design of a cluster‐randomized controlled trial and one‐year follow‐up. BMC Pregnancy and Childbirth 2010;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinlivan 2007 {published data only}
- Quinlivan J. A randomised trial of a multidisciplinary team care approach involving obstetric, dietary and clinical psychological input in obese pregnant women to reduce the incidence of gestational diabetes. Australian Clinical Trials Register (www.actr.org.au) (accessed 6 December 2005).
References to ongoing studies
Chasan‐Taber 2009 {published data only}
- Chasan‐Taber L. B.A.B.Y. Study: an exercise intervention to prevent gestational diabetes. Annals of Behavioral Medicine 2011 2011;41(Suppl 1):S135. [Google Scholar]
- Chasan‐Taber L, Marcus BH, Stanek E 3rd, Ciccolo JT, Marquez DX, Solomon CG, et al. A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design and methods. Journal of Women's Health 2009;18(6):851‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan‐Taber L, Silveira M, Marcus BH, Braun B, Stanek E, Markenson G. Feasibility and efficacy of a physical activity intervention among pregnant women: the behaviours affecting baby and you (B.A.B.Y.) study. Journal of Physical Activity and Health 2011;8(Suppl 2):S228‐S238. [DOI] [PubMed] [Google Scholar]
Ko 2008 {published data only}
- Ko CW. Effect of physical activity on metabolic syndrome in pregnancy and fetal outcome. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 9 April 2008).
Melo 2008 {published data only}
- Melo A. Exercise and pregnancy: randomised clinical trial. Current Controlled Trials (www.controlled‐trials.com/) (accessed 17 March 2010).
Newnham 2011 {published data only}
- Newnham J. Fournier P. Guelfi K. Grove R. Wallman K. Doherty D. Preventing gestational diabetes mellitus using a home‐based supervised exercise program during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/), (accessed 25 July 2011). [NCT01283854]
Oostdam 2009 {published data only}
- Oostdam N, Poppel MN, Eekhoff EM, Wouters MG, Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy and Childbirth 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez‐Velez 2009 {published data only}
- Ramirez‐Velez R, Aguilar AC, Mosquera M, Garcia RG, Reyes LM, Lopez‐Jaramillo P. Clinical trial to assess the effect of physical exercise on endothelial function and insulin resistance in pregnant women. Trials 2009;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2008 {published data only}
- Shen G. Impact of diet and exercise activity on pregnancy outcomes (IDEA). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Additional references
ACOG 2001
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins‐‐Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician‐gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstetrics & Gynecology 2001;98(3):525‐38. [PubMed] [Google Scholar]
ADA 2003
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S103‐S105. [DOI] [PubMed] [Google Scholar]
Alwan 2009
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amorim 2007
- Amorim Adegboye AR, Linne YM, Lourenco PMC. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005627.pub2] [DOI] [PubMed] [Google Scholar]
Barker 1994
- Barker D. Mothers, babies and diseases in later life. London: BMJ Publishing Group, 1994. [Google Scholar]
Bellamy 2009
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet 2009;373(9677):1773‐9. [DOI] [PubMed] [Google Scholar]
Bottalico 2007
- Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Seminars in Perinatology 2007;31(3):176‐84. [DOI] [PubMed] [Google Scholar]
Ceysens 2006
- Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chasan‐Taber 2008
- Chasan‐Taber L, Schmidt MD, Pekow P, Sternfeld B, Manson JE, Solomon CG, et al. Physical activity and gestational diabetes mellitus among Hispanic women. Journal of Women's Health 2008;17(6):999‐1008. [DOI] [PubMed] [Google Scholar]
Chodick 2010
- Chodick G, Elchalal U, Sella T, Heymann AD, Porath A, Kokia E, et al. The risk of overt diabetes mellitus among women with gestational diabetes: a population‐based study. Diabetic Medicine 2010;27(7):779‐85. [DOI] [PubMed] [Google Scholar]
Clapp 1991
- Clapp JF 3rd, Capeless EL. The changing glycaemic response to exercise during pregnancy. American Journal of Obstetrics and Gynecology 1991;165(6 Pt 1):1678‐83. [DOI] [PubMed] [Google Scholar]
Clapp 1998
- Clapp JF 3rd. Effect of dietary carbohydrate on the glucose and insulin response to mixed caloric intake and exercise in both nonpregnant and pregnant women. Diabetes Care 1998;21(Suppl 2):B107‐B112. [PubMed] [Google Scholar]
Clapp 2000
- Clapp JF 3rd, Kiess W. Effects of pregnancy and exercise on concentrations of the metabolic markers tumor necrosis factor alpha and leptin. American Journal of Obstetrics & Gynecology 2000;182(2):300‐6. [DOI] [PubMed] [Google Scholar]
Clapp 2006
- Clapp JF. Effects of diet and exercise on insulin resistance during pregnancy. Metabolic Syndrome and Related Disorders 2006;4(2):84‐90. [DOI] [PubMed] [Google Scholar]
ClinicalTrials 2011
- ClinicalTrials.gov. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 6 July 2011).
Coustan 2010
- Coustan DR, Lowe LP, Metzger BE, Dyer AR, International Association of Diabetes and Pregnancy Study Groups. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. American Journal of Obstetrics & Gynecology 2010;202(6):654 e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477‐86. [DOI] [PubMed] [Google Scholar]
Cypryk 2008
- Cypryk K, Szymczak W, Czupryniak L, Sobczak M, Lewinski A. Gestational diabetes mellitus ‐ an analysis of risk factors. Endokrynologia Polska 2008;59(5):393‐7. [PubMed] [Google Scholar]
Dabelea 2005
- Dabelea D, Snell‐Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005;28(3):579‐84. [DOI] [PubMed] [Google Scholar]
Dempsey 2004a
- Dempsey JC, Sorensen TK, Williams MA, Lee IM, Miller RS, Dashow EE, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. American Journal of Epidemiology 2004;159(7):663‐70. [DOI] [PubMed] [Google Scholar]
Dempsey 2004b
- Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson ML, Miller RS, et al. A case‐control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Research and Clinical Practice 2004;66(2):203‐15. [DOI] [PubMed] [Google Scholar]
Dempsey 2005
- Dempsey JC, Butler CL, Williams MA. No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exercise and Sport Sciences Reviews 2005;33(3):141‐9. [DOI] [PubMed] [Google Scholar]
Devlieger 2008
- Devlieger R, Casteels K, Assche FA. Reduced adaptation of the pancreatic B cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstetricia et Gynecologica Scandinavica 2008;87(12):1266‐70. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerrero‐Romero 2010
- Guerrero‐Romero F, Aradillas‐García C, Simental‐Mendia LE, Monreal‐Escalante E, Cruz Mendoza E, Rodríguez‐Moran M. Birth weight, family history of diabetes, and metabolic syndrome in children and adolescents. Journal of Pediatrics 2010;156(5):719‐23. [DOI] [PubMed] [Google Scholar]
HAPO Study Cooperative Research Group 2008
- HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991‐2002. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Harder 2009
- Harder T, Roepke K, Diller N, Stechling Y, Dudenhausen JW, Plagemann A. Birth weight, early weight gain, and subsequent risk of type 1 diabetes: systematic review and meta‐analysis. American Journal of Epidemiology 2009;169(12):1428‐36. [DOI] [PubMed] [Google Scholar]
Hedderson 2010
- Hedderson M, Gunderson E, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstetrics and Gynecology 2010;115(3):597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henriksen 2008
- Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstetricia et Gynecologica Scandinavica 2008;87(2):134‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 1998
- Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus‐‐management guidelines. The Australasian Diabetes in Pregnancy Society. Medical Journal of Australia 1998;169(2):93‐7. [DOI] [PubMed] [Google Scholar]
Jastrow 2010
- Jastrow N, Roberge S, Gauthier RJ, Laroche L, Duperron L, Brassard N, et al. Effect of birth weight on adverse obstetric outcomes in vaginal birth after cesarean delivery. Obstetrics & Gynecology 2010;115(2 Pt 1):338‐43. [DOI] [PubMed] [Google Scholar]
Ju 2008
- Ju H, Rumbold AR, Willson KJ, Crowther CA. Borderline gestational diabetes mellitus and pregnancy outcomes. BMC: Pregnancy and Childbirth 2008;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862‐8. [DOI] [PubMed] [Google Scholar]
Kim 2010b
- Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health 2010;100(6):1047‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knowler 2002
- Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2006
- Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD000180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2005
- Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. American Journal of Obstetrics and Gynecology 2005;192(4):989‐97. [DOI] [PubMed] [Google Scholar]
Lo 2006
- Lo JC, Feigenbaum SL, Escobar GJ, Yang J, Crites YM, Ferrara A. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population‐based study. Diabetes Care 2006;29(8):1915‐7. [DOI] [PubMed] [Google Scholar]
Melzer 2010
- Melzer K, Schutz Y, Boulvain M, Kayser B. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Medicine 2010;40(6):493‐507. [DOI] [PubMed] [Google Scholar]
Metzger 1998
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop‐Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998;21(Suppl 2):B161‐B167. [PubMed] [Google Scholar]
Metzger 2007
- Metzger BE, Buchanan TA, Coustan DR, Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):S251‐S260. [DOI] [PubMed] [Google Scholar]
Morisset 2010
- Morisset AS, St‐Yves A, Veillette J, Weisnagel SJ, Tchernof A, Robitaille J. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes Metabolism Research and Reviews 2010;26(1):17‐25. [DOI] [PubMed] [Google Scholar]
Morrison 2008
- Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. Journal of Pediatrics 2008;152(2):201‐6. [DOI] [PubMed] [Google Scholar]
Mottola 2008
- Mottola MF. The role of exercise in the prevention and treatment of gestational diabetes mellitus. Current Diabetes Reports 2008;8(4):299‐304. [DOI] [PubMed] [Google Scholar]
Mulla 2010
- Mulla WR, Henry TQ, Homko CJ. Gestational diabetes screening after HAPO: has anything changed?. Current Diabetes Reports 2010;10(3):224‐8. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Diabetes in pregnancy: management of diabetes and its complications from pre‐conception to the postnatal period. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
Oken 2006
- Oken E, Ning Y, Rifas‐Shiman SL, Radesky JS, Rich‐Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstetrics and Gynecology 2006;108(5):1200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petitt 1985
- Petitt DJ, Bennett PH, Knowler WC, Baird HR, Aleck KA. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy. Long‐term effects on obesity and glucose tolerance in the offspring. Diabetes 1985;34(Suppl 2):119‐22. [DOI] [PubMed] [Google Scholar]
Petry 2010
- Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition 2010;104(6):775‐87. [DOI] [PubMed] [Google Scholar]
Pivarnik 2006
- Pivarnik JM, Chambliss HO, Clapp JF, Dugan SA, Hatch MC, Lovelady CA, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Medicine and Science in Sports and Exercise 2006;38(5):989‐1006. [DOI] [PubMed] [Google Scholar]
Redden 2010
Reece 2009
- Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373(9677):1789‐97. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rizzo 1997
- Rizzo TA, Metzger BE, Dooley SL, Cho NH. Early malnutrition and child neurobehavioral development: insights from the study of children of diabetic mothers. Child Development 1997;68(1):26‐38. [PubMed] [Google Scholar]
Schlüssel 2008
- Schlüssel MM, Souza EB, Reichenheim ME, Kac G. Physical activity during pregnancy and maternal‐child health outcomes: a systematic literature review. Cadernos de Saude Publica 2008;24(Suppl 4):S531‐S544. [DOI] [PubMed] [Google Scholar]
Sedaghati 2007
- Sedaghati P, Ziaee V, Ardjmand A. The effect of an ergometric training program on pregnants weight gain and low back pain. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2007;166:209‐13. [Google Scholar]
Silva 2010
- Silva JC, Pacheco C, Bizato J, Souza BV, Ribeiro TE, Bertini AM. Metformin compared with glyburide for the management of gestational diabetes. International Journal of Gynaecology and Obstetrics 2010;111(1):37‐40. [DOI] [PubMed] [Google Scholar]
Simmons 2004
- Simmons D, Walters BN, Rowan JA, McIntyre HD. Metformin therapy and diabetes in pregnancy. Medical Journal of Australia 2004;180(9):462‐4. [PubMed] [Google Scholar]
Snapp 2008
- Snapp CA, Donaldson SK. Gestational diabetes mellitus: physical exercise and health outcomes. Biological Research for Nursing 2008;10(2):145‐55. [DOI] [PubMed] [Google Scholar]
Soler 1978
- Soler NG, Soler SM, Malins JM. Neonatal morbidity among infants of diabetic mothers. Diabetes Care 1978;1(6):340‐50. [DOI] [PubMed] [Google Scholar]
Solomon 1997
- Solomon CG, Willett WC, Carey VJ, Rich‐Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278(13):1078‐83. [PubMed] [Google Scholar]
Streuling 2011
- Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, Kries R. Physical activity and gestational weight gain: a meta‐analysis of intervention trials. British Journal of Obstetrics and Gynaecology 2011;118(3):278‐84. [DOI] [PubMed] [Google Scholar]
Whincup 2008
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300(24):2886‐97. [DOI] [PubMed] [Google Scholar]
WOMBAT 2011
- Women and babies health and wellbeing: action through trials. http://www.wombatcollaboration.net/ (accessed 6 July 2011) 2011.
Yogev 2009
- Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Seminars in Fetal and Neonatal Medicine 2009;14(2):77‐84. [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Archives of Internal Medicine 2006;166(5):543‐8. [DOI] [PubMed] [Google Scholar]


