Abstract
Plexiform schwannomas representing a rare subset, comprise 5% of all schwannomas. However, their occurrence in the thyroid gland is exceptionally rare. A 32-year-old male presented with an incidentally discovered, asymptomatic thyroid mass. Imaging revealed an approximately 5 cm heterogeneous solid mass on the right thyroid lobe extending to the upper mediastinum and directly invading the upper trachea. Under the suspicion of thyroid malignancy, the patient underwent right thyroidectomy. Histological examination confirmed a plexiform schwannoma with S100-positive spindle cells. Currently, the patient is undergoing outpatient follow-up, with no reported complications. To our knowledge, this is the first documented case of plexiform schwannoma of the thyroid gland within the English literature. This case highlights the diverse and unpredictable clinical manifestations of thyroid masses, emphasizing the importance of a multidisciplinary approach for diagnosing and managing rare entities, such as thyroid gland schwannomas.
Keywords: Head and neck neoplasms, Neurilemmoma, Thyroid neoplasms
Introduction
Schwannomas are benign encapsulated neoplasms that originate from Schwann cells and are typically found in peripheral nerves [1]. While schwannomas can develop along nerve sheaths throughout the body, 25% to 45% of extracranial schwannomas occur in the head and neck region [1,2]. Several subtypes of schwannomas have been recognized and depicted in the latest Blue Book published by the World Health Organization [3]. Although the occurrence of schwannomas within the thyroid gland is unusual and extremely rare, regardless of known subtypes [4], they can still affect individuals of all ages, with a peak incidence observed in those between 40 and 60 years of age. Furthermore, there is no notable sex or ethnic predilection [5].
The clinical symptoms of thyroid schwannomas are highly dependent on the size, location, and growth direction of the tumor [5,6]. Most patients remain asymptomatic until the tumor reaches a substantial size and affects adjacent structures, such as the recurrent laryngeal nerve or trachea [7,8]. Here, we report a rare pathological type of thyroid plexiform schwannoma in a 32-year-old male to share our unusual clinical experience.
Case
Ethics statement: This study was exempted from review by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: YUMC2023-11-046-HE003). Written informed consent was obtained from the patient to participate in the study.
A 32-year-old male with no significant medical history presented to the Department of Endocrinology at Yeungnam University Hospital after incidental discovery of a thyroid mass during an ultrasound examination at a local hospital. The patient did not report any symptoms related to hypothyroidism or hyperthyroidism and denied experiencing any symptoms associated with a thyroid mass, such as hoarseness or dysphagia. Blood pressure and heart rate were 120/78 mmHg and 96 beats per minute, respectively. During physical examination, a soft and non-tender mass was palpable on the right side of the patient’s neck. No palpable cervical or supraclavicular lymph nodes were observed. Initial laboratory findings indicated thyroid-stimulating hormone, free thyroxine, and triiodothyronine levels of 0.712 µIU/mL, 1.48 ng/dL, and 1.32 ng/mL, respectively.
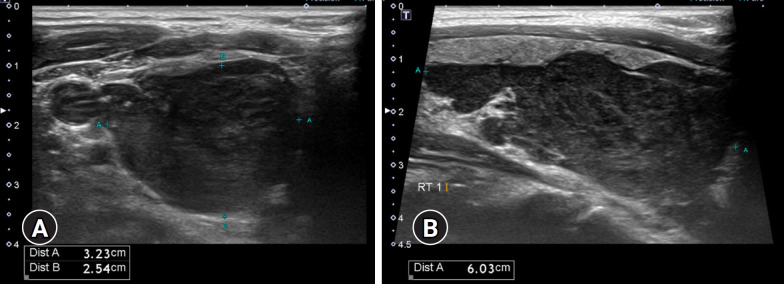
The initial thyroid ultrasound revealed a 3.23×2.54×6.04 cm heterogeneous and hypoechoic right thyroid mass with irregular margins (Fig. 1). Fine-needle aspiration (FNA) was performed, indicating Bethesda II classification with a few benign-appearing follicular cells.
Fig. 1.
Thyroid ultrasound. (A) Transverse and (B) longitudinal views reveal a right thyroid mass measuring 3.23×2.54×6.04 cm, characterized by a solid, heterogeneous, and hypoechoic appearance with irregular margins. No cervical lymphadenopathy is visible.
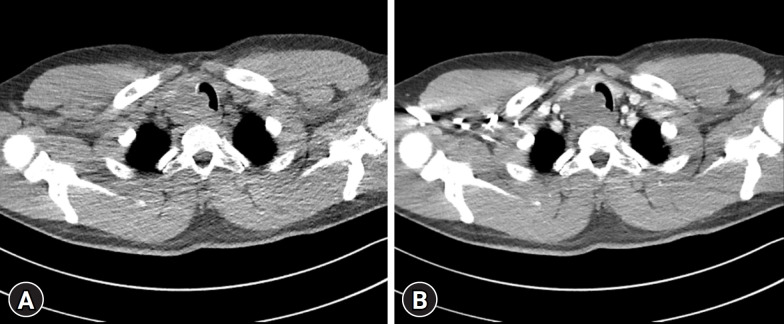
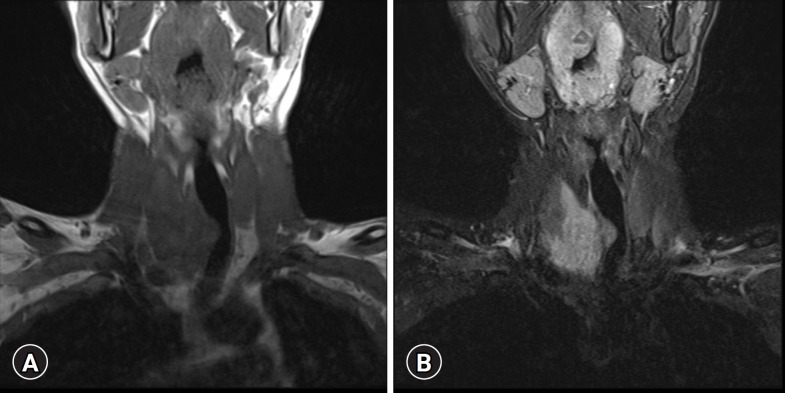
Computed tomography (CT) revealed a lobulated low-density mass in the inferior portion of the right thyroid gland, extending to the upper mediastinum and directly invading the upper trachea with luminal narrowing (Fig. 2). Magnetic resonance imaging (MRI) of the neck revealed an isointense to hypointense signal on T1-weighted images and hyperintense signal on T2-weighted images (Fig. 3).
Fig. 2.
Neck computed tomography image reveals a lobulated low-density mass approximately 5 cm in size in the inferior portion of the right thyroid gland, extending to the upper mediastinum, and direct invasion of the upper trachea, causing luminal narrowing. No enhancement is visible in the mass (A) before and (B) after contrast administration.
Fig. 3.
Magnetic resonance imaging shows a right thyroid mass. (A) Hypointense signal on coronal T1-weighted images. (B) Hyperintense signals on coronal T2-weighted images without a target sign.
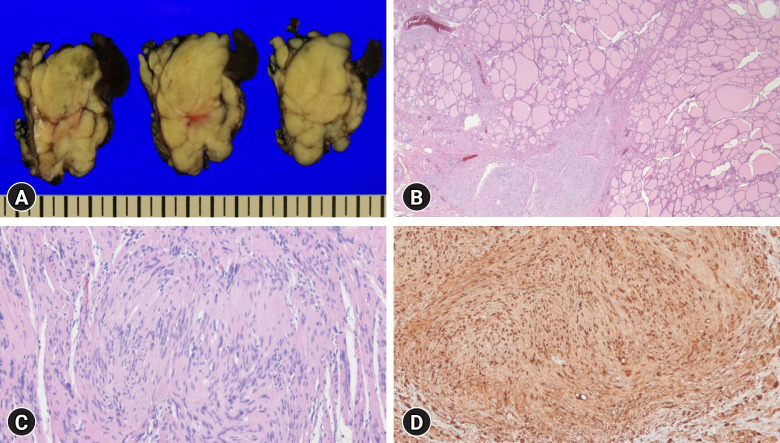
The patient was referred to the Department of Otorhinolaryngology to undergo right thyroidectomy for diagnostic purposes and to relieve the tracheal compression caused by the mass. Surgical findings revealed a firm encapsulated mass measuring approximately 5×5 cm on the right lobe of the thyroid gland. The mass directly invaded the trachea, necessitating primary closure of the tracheal defect. The right recurrent laryngeal nerve encapsulated within the thyroid mass was excised along with the mass, followed by ansa neurorrhaphy. The pathology of the specimen revealed a multinodular yellowish mass adjacent to the thyroid gland (Fig. 4A). Histological evaluation revealed a poorly encapsulated spindle cell tumor, with some of the tumor exhibiting features suggestive of infiltration into the thyroid gland (Fig. 4B). Cytologically, the tumor consisted of short, wavy spindle cells without substantial pleomorphism or atypia (Fig. 4C). Immunohistochemical staining revealed that these cells were positive for S100 protein (Fig. 4D), the Ki-67 score was low (low proliferative index), and trimethylation of lysine 27 on histone H3 (H3K27me3) remained intact. The overall findings were consistent with a diagnosis of plexiform schwannoma.
Fig. 4.
Gross and histologic findings of the resected specimen. (A) Gross appearance of the resected specimen shows a multinodular yellowish mass adjacent to the thyroid gland. (B) Low-power microscopic examination reveals a poorly encapsulated mass with a tongue-like projection into the thyroid gland (hematoxylin and eosin [H&E] stain, ×10). (C) The tumor cells have spindled, wavy, and tapering nuclei. Typical Verocay bodies, formed by two rows of nuclear palisading separated by eosinophilic fibrillary cell processes, are present (H&E stain, ×100). (D) Tumor cells exhibit diffuse positivity for S100 protein (immunohistochemical stain, ×100).
The patient is currently undergoing follow-up as an outpatient, and to date, no notable complications have been reported.
Discussion
A plexiform schwannoma is a rare subtype of schwannoma, accounting for approximately 5% of all schwannoma occurrences [9]. It is characterized by an intraneural plexiform growth pattern. Pathologically, it resembles a conventional schwannoma; both are benign tumors with no significant clinical differences between the two types. However, plexiform schwannomas must be distinguished from plexiform neurofibromas and malignant peripheral nerve sheath tumors, which present similar patterns [9]. Plexiform schwannomas are usually present as cutaneous masses and are common in head and neck lesions but are rare in the thyroid gland. Most cases of plexiform schwannomas are not associated with known genetic conditions, although there have been occasional reports of such tumors in patients diagnosed with neurofibromatosis type 2 or schwannomatosis. The exact cause of schwannomas is not clear but is often associated with mutations in the NF2 gene, which is responsible for regulating cell growth and division [10]. Recently, a novel SOX10 mutation was suggested to be associated with plexiform schwannomas [11]. In the current case, the patient presented with an incidentally discovered thyroid mass that was initially characterized by irregular margins and a solid hypoechoic texture on ultrasonography. In addition, the patient did not exhibit specific clinical features associated with any other genetically derived syndromes related to schwannomas. To the best of our knowledge, this is the first documented case of plexiform schwannoma of the thyroid gland in the English literature. However, we were unable to conduct both somatic and germline genetic studies to investigate the potential genetic factors contributing to the pathogenesis of this condition.
Previous studies indicated that most thyroid schwannomas are localized on the right lobe of the thyroid, as observed in this case, with fewer cases in the left lobe [6]. This right lobe predilection may be associated with the neurological asymmetry within the thyroid glands [7]. The clinical symptoms of thyroid schwannomas vary based on the mass size and location. Despite the involvement of the trachea and recurrent laryngeal nerve in our case, there were no symptoms such as dyspnea or hoarseness, presumably due to the slow-growing nature of the tumor, which allowed the patient sufficient time to adapt. In contrast, other cases presented with symptoms such as progressive hoarseness, dysphagia, or dyspnea caused by tracheal compression due to retrosternal tumor growth [6].
The diagnosis of thyroid schwannomas can be challenging. Their extreme rarity makes recognition difficult, and they can mimic a thyroid nodule or malignancy [2]. Ultrasound typically shows schwannomas as cystic nodules with a well-defined, round, or ovoid shape, a solid or mixed solid, and hypoechogenicity. If ultrasound reveals a target sign within the tumor, indicating the presence of two different tissues, it has diagnostic value [12]. FNA findings in schwannoma cases are often inconclusive owing to poor sensitivity and nondiagnostic specimen rates of up to 50% [6,13]. In another case report, the FNA result was classified as Bethesda II; however, the patient was later diagnosed with thyroid schwannoma [4]. The main cause of the discrepancy in our case may have been the infiltration of the tumor into the thyroid gland, which did not allow optimal targeting during the FNA procedure; normal thyroid follicular cells might have been collected, making diagnosis difficult. Additionally, a dense interstitial component is often found in schwannomas, which can impede the collection of sufficient aspirated material. Other potential factors contributing to the lower sensitivity of FNA include prominent hypocellular Antoni B areas and the presence of frequent cystic portions [14].
A unique multinodular appearance with the typical histological features of schwannomas can readily lead to a diagnosis of the plexiform subtype. Therefore, it is important to note that plexiform schwannomas may lack an intact fibrous capsule and can exhibit infiltrative foci into adjacent visceral organs, as observed in our case, potentially leading to misinterpretation as a worrisome malignant feature. Despite benign cytology, surgery was performed in this case because these findings made it difficult to exclude the possibility of malignancy. Previous studies noted that plexiform neurofibromas may also lack a discrete fibrous capsule and can invade neighboring structures [9,15]. Differential diagnosis should consider other benign peripheral nerve sheath tumors, such as plexiform neurofibromas or perineuriomas. Neurofibromas consist of Schwann cells mixed with shredded carrot-like stromal collagen, whereas a perineurioma is defined as a tumor entirely composed of S100-negative perineurial cells. Nuclear atypia, increased mitotic activity, and cellularity may warrant the diagnosis of malignant peripheral nerve sheath tumors. Therefore, identifying the loss of H3K27me3 is helpful [16].
A CT scan of a schwannoma typically reveals a mass with a well-defined margin that appears hypodense to isodense compared to muscle and may rarely be accompanied by unusual, calcified components [17]. On MRI, schwannomas usually appear hypointense to isointense, resembling muscle on T1-weighted images; on T2-weighted images, they exhibit significantly increased signal intensity [18,19]. The target sign or reverse target sign is a characteristic feature of schwannomas on MRI because of the histological distinction between the central core and the peripheral tissue [19]. However, these signs are not always clearly detected, and less than half of the cases exhibit this feature [20].
Surgical resection is the primary treatment for thyroid schwannomas and is considered curative in most cases. Typically, lobectomy or tumor excision is performed, resulting in an excellent prognosis and minimal postoperative complications [1,6,14].
This case serves as a reminder of the diverse and unexpected clinical presentations of thyroid masses and the importance of a multidisciplinary approach involving imaging, pathology, endocrinology, and surgical expertise when diagnosing and managing rare entities, such as schwannomas within the thyroid gland. Further research and case studies are necessary to enhance our understanding of these uncommon thyroid neoplasms.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: all authors; Investigation: IRP, MCK, SYS; Writing-original draft: IRP, MCK; Writing-review & editing: SMC.
References
- 1.Colreavy MP, Lacy PD, Hughes J, Bouchier-Hayes D, Brennan P, O'Dwyer AJ, et al. Head and neck schwannomas: a 10 year review. J Laryngol Otol. 2000;114:119–24. doi: 10.1258/0022215001905058. [DOI] [PubMed] [Google Scholar]
- 2.De Simone B, Del Rio P, Sianesi M. Schwannoma mimicking a neoplastic thyroid nodule. Updates Surg. 2014;66:85–7. doi: 10.1007/s13304-012-0189-5. [DOI] [PubMed] [Google Scholar]
- 3.WHO Classification of Tumours Editorial Board . Soft tissue and bone tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2020. [Google Scholar]
- 4.Dhar H, Dabholkar JP, Kandalkar BM, Ghodke R. Primary thyroid schwannoma masquerading as a thyroid nodule. J Surg Case Rep. 2014;2014:rju094. doi: 10.1093/jscr/rju094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagavalli S, Yehuda M, McPhaul LW, Gianoukakis AG. A cervical schwannoma masquerading as a thyroid nodule. Eur Thyroid J. 2017;6:216–20. doi: 10.1159/000454877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbarah S, Abbarh S, AlHarthi B. Unusual thyroid nodule: a case of symptomatic thyroid schwannoma. Cureus. 2020;12:e11425. doi: 10.7759/cureus.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki T, Kumeda S, Iwasa T, Inokawa K, Hori T, Makiuchi M. Primary neurilemoma of the thyroid gland: report of a case. Surg Today. 1993;23:265–8. doi: 10.1007/BF00309239. [DOI] [PubMed] [Google Scholar]
- 8.Vázquez-Benítez G, Pérez-Campos A, Masgrau NA, Pérez-Barrios A. Unexpected tumor: primary asymptomatic schwannoma in thyroid gland. Endocr Pathol. 2016;27:46–9. doi: 10.1007/s12022-015-9409-0. [DOI] [PubMed] [Google Scholar]
- 9.Berg JC, Scheithauer BW, Spinner RJ, Allen CM, Koutlas IG. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol. 2008;39:633–40. doi: 10.1016/j.humpath.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Williams EA, Ravindranathan A, Gupta R, Stevers NO, Suwala AK, Hong C, et al. Novel SOX10 indel mutations drive schwannomas through impaired transactivation of myelination gene programs. Neuro Oncol. 2023;25:2221–36. doi: 10.1093/neuonc/noad121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wali AA, Yang R, Merbs SL, Rodriguez FJ, Eberhart CG, Lucas CG. Orbital SOX10-mutant schwannoma with plexiform growth: expanding the histopathological spectrum of a new molecular group. J Neuropathol Exp Neurol. 2023;82:963–5. doi: 10.1093/jnen/nlad080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao HN, Ma BY, Yan F, Peng YL. Multimodal ultrasound imaging of primary thyroid schwannoma: a case report. Medicine (Baltimore) 2021;100:e25517. doi: 10.1097/MD.0000000000025517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HL, Yu SY, Li GK, Wei WI. Extracranial head and neck Schwannomas: a study of the nerve of origin. Eur Arch Otorhinolaryngol. 2011;268:1343–7. doi: 10.1007/s00405-011-1491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simo D, Selvaggi F, Cieri M, Angelucci D, Claudi R, Giuliani C, et al. Surgical approach for nodular neck lesions mimicking primitive thyroid neoplasms Report of three cases. Ann Ital Chir. 2014;85:474–8. [PubMed] [Google Scholar]
- 15.Woodruff JM, Scheithauer BW, Kurtkaya-Yapicier O, Raffel C, Amr SS, LaQuaglia MP, et al. Congenital and childhood plexiform (multinodular) cellular schwannoma: a troublesome mimic of malignant peripheral nerve sheath tumor. Am J Surg Pathol. 2003;27:1321–9. doi: 10.1097/00000478-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 expression is a highly sensitive marker for sporadic and radiation-induced MPNST. Am J Surg Pathol. 2016;40:479–89. doi: 10.1097/PAS.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moroni AL, Righini C, Faure C, Serra-Tosio G, Lefournier V. CT Features of an unusual calcified schwannoma of the superior laryngeal nerve. AJNR Am J Neuroradiol. 2007;28:981–2. [PMC free article] [PubMed] [Google Scholar]
- 18.Crist J, Hodge JR, Frick M, Leung FP, Hsu E, Gi MT, et al. Magnetic resonance imaging appearance of schwannomas from head to toe: a pictorial review. J Clin Imaging Sci. 2017;7:38. doi: 10.4103/jcis.JCIS_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Bhalla AS, Sharma R, Kumar A, Thakar A, Goyal A. Diffusion-weighted imaging in extracranial head and neck schwannomas: a distinctive appearance. Indian J Radiol Imaging. 2016;26:231–6. doi: 10.4103/0971-3026.184418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, Chen XX, Wu HL, Zhu JF, Chen Y, Yu LF, et al. Sonographic features and diagnosis of peripheral schwannomas. J Clin Ultrasound. 2017;45:127–33. doi: 10.1002/jcu.22438. [DOI] [PubMed] [Google Scholar]