Abstract
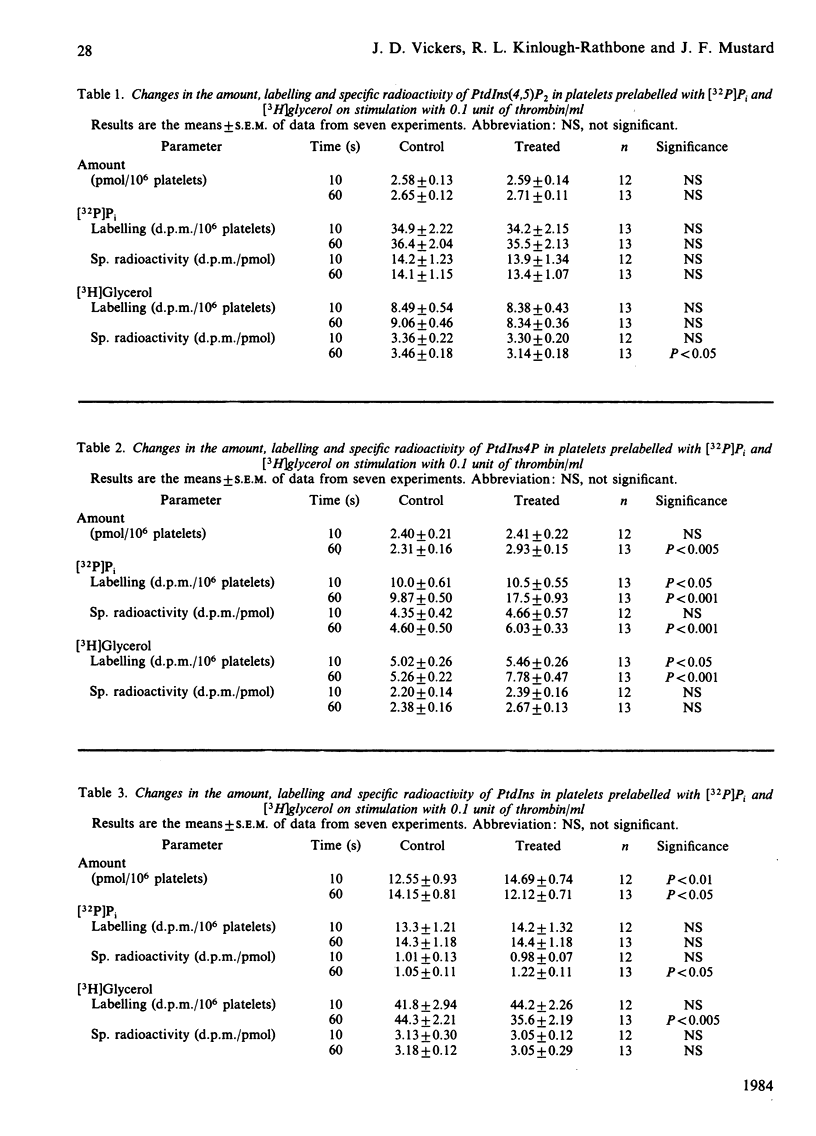
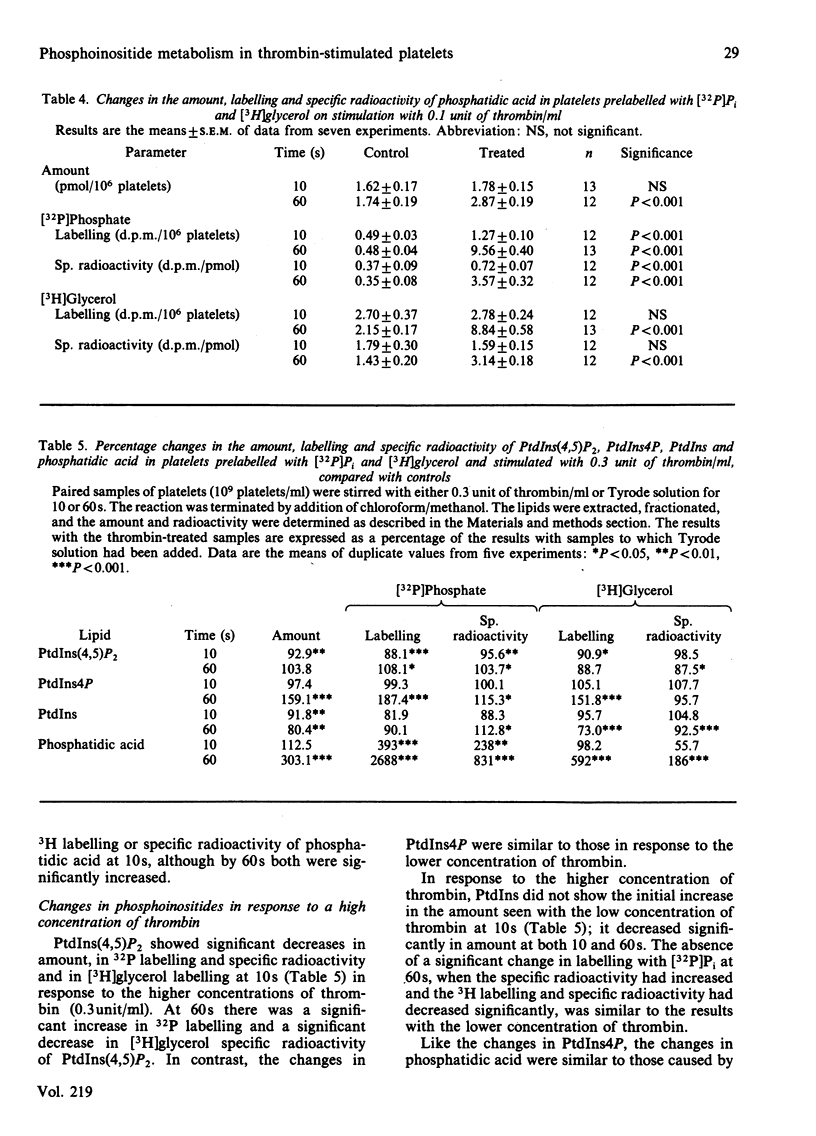
Experiments with washed platelets from rabbits demonstrate that stimulation with a low concentration of thrombin (0.1 unit/ml) that causes maximal aggregation and partial release of granule contents does not significantly decrease the amount of phosphatidylinositol 4,5-bisphosphate [ PtdIns (4,5)P2] at 10s; this contrasts with ADP stimulation. The amount of PtdIns (4,5)P2 was significantly decreased by a higher concentration of thrombin (0.3 unit/ml). Increased turnover of the PtdIns (4,5)P2 at 60s was indicated by changes in labelling with [3H]glycerol in platelets stimulated with both concentrations of thrombin. An unexpected observation with the lower thrombin concentration was a significant increase in the amount of phosphatidylinositol ( PtdIns ) at 10s. This contrasts with data from other laboratories, which indicate that thrombin causes a significant decrease in PtdIns . At 60s, with the lower concentration of thrombin, PtdIns was significantly decreased. With the higher concentration of thrombin there was a significant decrease in the amount of PtdIns at 10s, in keeping with the data from other laboratories. The initial increase in PtdIns may not have been observed by other investigators because higher concentrations of thrombin were used. The reaction involved in this initial increase in the amount of PtdIns does not appear to be increased degradation of PtdIns4P or PtdIns (4,5)P2, since their total amount was unchanged at 10s. The magnitude of the increase in PtdIns is such that more than the existing pool of phosphatidic acid would have to be converted into PtdIns to account for the increase. It is suggested that synthesis of phosphatidic acid de novo from dihydroxyacetone phosphate and glycerol 3-phosphate might be the source of phosphatidic acid, which leads to increased PtdIns at 10s with the lower concentration of thrombin. Thus it appears that the initial response of platelets to thrombin does not require an early change in PtdIns (4,5)P2 and may involve stimulation of synthesis de novo of PtdIns via phosphatidic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Ardlie N. G., Perry D. W., Packham M. A., Mustard J. F. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971 Apr;136(4):1021–1023. doi: 10.3181/00379727-136-35419. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Majerus P. W. Thrombin-induced hydrolysis of phosphatidylinositol in human platelets. J Biol Chem. 1980 Mar 10;255(5):1790–1792. [PubMed] [Google Scholar]
- Berridge M. J. 5-Hydroxytryptamine stimulation of phosphatidylinositol hydrolysis and calcium signalling in the blowfly salivary gland. Cell Calcium. 1982 Oct;3(4-5):385–397. doi: 10.1016/0143-4160(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phosphatidylinositol-specific phospholipase-C of platelets: association with 1,2-diacyglycerol-kinase and inhibition by cyclic-AMP. Biochem Biophys Res Commun. 1979 Sep 12;90(1):92–98. doi: 10.1016/0006-291x(79)91594-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981 Jun;100(4):1688–1695. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N. Is phosphatidylinositol now out of the calcium gate? Nature. 1982 Jan 28;295(5847):281–282. doi: 10.1038/295281a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Dawson R. M. Transfer of arachidonic acid between phospholipids in rat liver microsomes. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1399–1405. doi: 10.1016/0006-291x(79)91222-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Haldar J., Mustard J. F. Changes in 32 p-labelling of platelet phospholipids in response to ADP. Br J Haematol. 1972 Nov;23(5):571–585. doi: 10.1111/j.1365-2141.1972.tb07092.x. [DOI] [PubMed] [Google Scholar]
- McKean M. L., Smith J. B., Silver M. J. Formation of lysophosphatidylcholine by human platelets in response to thrombin. Support for the phospholipase A2 pathway for the liberation of arachidonic acid. J Biol Chem. 1981 Feb 25;256(4):1522–1524. [PubMed] [Google Scholar]
- Michell R. H. Is phosphatidylinositol really out of the calcium gate? Nature. 1982 Apr 8;296(5857):492–493. doi: 10.1038/296492a0. [DOI] [PubMed] [Google Scholar]
- Okuma M., Yamashita S., Numa S. Enzymic studies on phosphatidic acid synthesis in human platelets. Blood. 1973 Mar;41(3):379–389. [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol lipids and cell stimulation in mammalian salivary gland. Cell Calcium. 1982 Oct;3(4-5):369–383. doi: 10.1016/0143-4160(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell F. A., Deykin D. Mobilization of arachidonic acid in human platelets. Kinetics and Ca2+ dependency. Biochim Biophys Acta. 1977 Sep 28;488(3):370–380. doi: 10.1016/0005-2760(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E. Inositol lipid metabolism in the responses of stimulated platelets. Cell Calcium. 1982 Oct;3(4-5):311–322. doi: 10.1016/0143-4160(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Changes in phosphatidylinositol-4,5-bisphosphate 10 seconds after stimulation of washed rabbit platelets with ADP. Blood. 1982 Dec;60(6):1247–1250. [PubMed] [Google Scholar]


