ABSTRACT
Ungulates serve as the primary carrion source for facultative scavengers in European ecosystems. In the absence of large carnivores, such as wolves (Canis lupus), human hunting leftovers are the main source of carrion for these scavengers. Additionally, wild boars (Sus scrofa) are heavily culled in many ecosystems and are both a significant prey species for wolves as well as a key scavenger. Nowadays, wolves and wild boars are re‐establishing their historical home ranges. However, it remains unclear how their presence influences the population dynamics of facultative scavengers under different scenarios of human hunting strategies. We simulated the biomass densities of all states in the trophic web including European scavengers and wolves using an ordinary differential equations (ODE) model. The presence of wolves led to a positive trend in scavenger biomass in general. However, in general, we found that plant‐based resources were more important for scavenger dynamics than carrion, regardless of whether the carrion originated from human hunting or wolf predation. Only when wolves were absent but boars present, the human hunting strategy became important in determining scavenger dynamics via carrion supply. In conclusion, our model indicates that population dynamics of facultative scavengers are not mainly driven by the availability of carrion, but rather by the presence of and competition for vegetation. Furthermore, our simulations highlight the importance of adapting human hunting strategies in accordance with the re‐establishment of wolf and boar as these can cause fluctuating population patterns over the years.
Keywords: Lotka‐Volterra, population modelling, predator–prey dynamics, ungulates
Ungulates are the major source of carrion in European ecosystems, with human hunting leftovers being important in the absence of large carnivores like wolves. However, although our model shows that carrion is not the major food source for facultative scavengers, carrion can be important for these scavengers under some conditions.
1. Introduction
The decomposition of dead animal bodies – carrion – is an important ecological process that can have far‐reaching consequences for ecosystem functioning (Wenting et al. 2023, 2024). Most of the carrion in terrestrial ecosystems is consumed by scavengers (DeVault, Rhodes Jr, and Shivik 2003; Wilson and Wolkovich 2011). The major source of carrion in many ecosystems, including European temperate woodlands, consists of large ungulates (Beasley et al. 2019; Moleón et al. 2019; Greenspoon et al. 2023). This includes species like red deer (Cervus elaphus), fallow deer (Dama dama), and wild boar (Sus scrofa). Anthropogenic hunting is one of the major causes of death of free roaming ungulates, especially in areas where large carnivores no longer occur due to extermination (Gordon 2009; Found 2016; Williams et al. 2017).
Currently, however, populations of large carnivores are re‐establishing to their historical ranges across Europe (Chapron et al. 2014; Galaverni et al. 2016). An example is the grey wolf (Canis lupus), a social apex predator with large dispersal rates and large territories (Jędrzejewski et al. 2007), that expanded its distribution extensively over the past decades (Planillo et al. 2023). The re‐establishment of the wolf has been possible due to strict legal protection and the recovery of large herbivore populations (Chapron et al. 2014). The presence of the wolf can have cascading effects on ecosystem functioning (Allen et al. 2017), for example by indirectly changing the diet of grizzly bears (Ursus arctos horribilis) to more plant‐based (Ripple et al. 2015) and willow recovery through behavioural changes of herbivores (Marshall, Cooper, and Hobbs 2014). This is well‐studied in North American wolf habitats (Lesmerises, Dussault, and St‐Laurent 2012; Ripple and Beschta 2012; Ford and Goheen 2015; Gantchoff et al. 2022). The European situation is considerably less well‐studied (Nowak et al. 2017; Reinhardt et al. 2019), despite that there are essential differences between the European and North American continent. Since it is generally harder to predict trophic cascades in more human‐dominated landscapes such as European ecosystems (Hebblewhite et al. 2005; Muhly et al. 2013; Dorresteijn et al. 2015), insights obtained from North American wolf habitats might not be equally relevant in European wolf habitats (Focardi et al. 2017).
One of the most notable differences between European and American ecosystems is the importance of wild boar as both abundant ungulate, scavenger species, and prey species for wolves (Focardi et al. 2017). The wild boar is a wide‐spread non‐ruminant ungulate that is widely described as an ecosystem engineer due to its extensive rooting behaviour (Sandom, Hughes, and Macdonald 2013; Ballari and Barrios‐García 2014; Baruzzi and Krofel 2017; Barrios‐Garcia et al. 2023). It is a well‐known scavenger species (Selva et al. 2005; Selva and Fortuna 2007; Focardi et al. 2008) that can contribute considerably to carrion removal from ecosystems (Wenting, Rinzema, and van Langevelde 2022; Wenting et al. 2024; Newsome et al. 2023). Although wild boars are not tolerated by humans everywhere in Europe (Boonman‐Berson, Driessen, and Turnhout 2019), hence not everywhere present as prey species, they are reported as a noticeable part of the wolves' diet throughout European ecosystems in areas where they occur (Smietana and Klimek 1993; Ansorge, Kluth, and Hahne 2006; Nores, Llaneza, and Álvarez 2008; Lanszki et al. 2012; Špinkytė‐Bačkaitienė and Pėtelis 2012; Barja et al. 2023). That implies that the wild boar is an important prey species for wolves (Mattioli et al. 2011; Mori et al. 2017) and also an important scavenger in wolf habitats (Focardi et al. 2017).
The presence of large carnivores like wolves can influence the process of scavenging in ecosystems. Through only partially consuming their prey, wolves can indirectly facilitate scavengers (Vucetich, Vucetich, and Peterson 2012; Focardi et al. 2017; Boczulak et al. 2023). Wolves might facilitate consumption efficiency of vultures, corvids and smaller mammals by tearing open thick‐skinned carcasses (Moleón et al. 2014). Partial prey consumption is common behaviour for wolves, being the combined result of pack size, prey size, and completeness of consumption in first sitting (Sand et al. 2012; Vucetich, Vucetich, and Peterson 2012; Mech and Boitani 2019). In North America, it has been described that common ravens (Corvus corax) use activity patterns of wolves to benefit from wolf kills, as a feeding strategy in winter (Stahler, Heinrich, and Smith 2002; Walker et al. 2018). However, scavenger dynamics might not change in the same way in different systems because scavenger species adapt their behaviour based on the local circumstances. Klauder et al. (2021), for instance, found that red foxes (Vulpes vulpes) were least likely to visit wolf kills in Denali National Park and Preserve, Alaska. This contradicts to findings in Europe and elsewhere in North America, where red foxes are reported to visit up to 90% of wolf‐predated ungulates (Selva 2004; Wikenros, Ståhlberg, and Sand 2014; O'Malley et al. 2018). Thus, the potential impact of re‐establishing wolf populations on scavenger dynamics can be system specific (Laundré, Hernández, and Altendorf 2001; Levi and Wilmers 2012; Haswell, Kusak, and Hayward 2017; Kuijper et al. 2024), increasing the need to investigate potential influences of re‐establishing wolves under different circumstances.
It has been described that different causes of death of ungulates – e.g., originated from human hunting or predated by wolves – can differently influence scavengers. For instance, predator‐kills were mostly preferred by scavengers in the Białowieża Primaeval Forest, Poland (Selva 2004; Selva and Fortuna 2007). Carrion obtained from human hunting can also facilitate a wide range of scavenger species (Mateo‐Tomás et al. 2015), in some cases even more than wolf kills (Ho et al. 2023). It remains unclear to which extent such differences might be due to different human hunting strategies, e.g., hunting target (‘pressure’) or the fraction of carrion left for scavengers. Also, the actual importance of carrion versus other resources for facultative scavengers – that frequently consume but do not depend on carrion (Wilson and Wolkovich 2011) – remains unclear.
Thus, summarising, it remains unclear how human hunting strategies and the presence of wolves and/or wild boar (henceforth ‘boar’) influence the population dynamics of European facultative scavengers (henceforth ‘scavengers’). We focus on (vertebrate) species that consume plant‐based food and carrion primarily and are flexible in their diet and behaviour (Selva and Fortuna 2007; Wenting, Rinzema, and van Langevelde 2022; Wenting et al. 2024). These include corvids like common raven and carrion crow (Corvus corone), and mesocarnivores, for instance red fox, European badger (Melis melis), raccoon (Procyon lotor) and other mustelids including beach marten (Martes foina), pine marten (Martes martes) and European polecat (Mustela putorius) (Díaz‐Ruiz et al. 2013; Rooney and Montgomery 2013; Papakosta et al. 2014; Libois et al. 2019; Jain et al. 2022). In this study, we use a differential‐equations modelling approach to examine how different human hunting strategies combined with the presence or absence of wolf and boar influence the population dynamics of scavengers. We address two research questions: (1) What is the influence of human hunting strategies in interaction with the presence or absence of boar and wolf, on scavenger population dynamics? and (2) What is the relative importance of carrion for scavenger population dynamics under different human hunting strategies in interaction with the presence or absence of boar and wolf?
2. Methods
2.1. Model Description and Assumptions
We simulated the biomass densities of a trophic web of European scavengers and wolves (Figure 1) using an ordinary differential equations (ODE) model. We based our model on the model developed by Focardi et al. (2017) for scavenger/predator systems, but changed three main things: (1) we added a separate state for scavengers, (2) implemented human hunting on boar and deer, and (3) merged adult boar and piglets into one state to simplify the model. The other details of the model by Focardi et al. (2017) are similar to our model specifications that we explain here. In our model, vegetation is consumed by deer , boar and scavengers (Equation 1), which we further subdivided here into Equations (1a)–(1d). Here, vegetation includes all plant‐based materials. Deer are consumed by wolf , killed by hunters and die of other causes (Equation 2, subdivided over Equations (2a), (2b), (2c), (2d)). For boar the same applies as for deer, but they also consume deer carrion instead of only vegetation (Equation 3, subdivided over Equations (3a), (3b), (3c), (3d), (3e)). Scavengers consume both vegetation and the carrion from deer and boar, and die of natural causes (Equation 4, subdivided over Equations (4a), (4b), (4c), (4d)). Wolves thus consume deer and boar and die of natural causes (Equation 5, subdivided over Equations (5a), (5b), (5c)). For simplicity, we assumed no scavenging behaviour by wolves, nor did we assume that scavengers consume wolf carrion (as wolf carrion only makes up a small portion of the total amount of carrion).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
FIGURE 1.
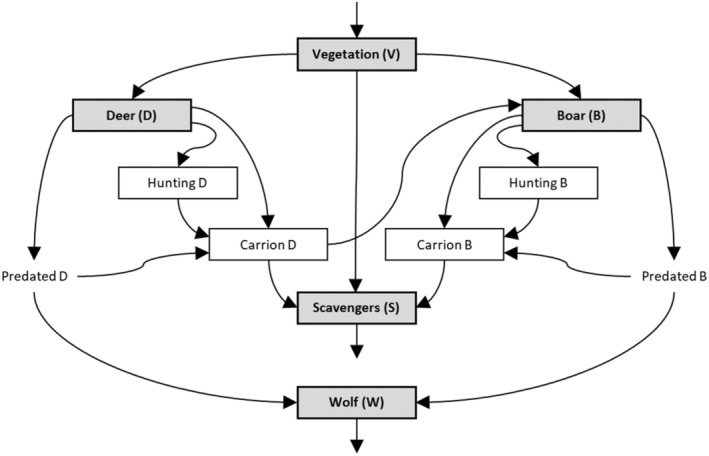
Trophic web of European scavengers and wolves. The consumers of vegetation (V) consist of two types of ungulates – deer (D) and boar (B) – and facultative scavengers (S). V represents vegetation and all other resources, including small prey of facultative scavengers, combined. The D species represent all Cervidae species, whereas the B represents wild boar (Sus scrofa). The S species represent all facultative scavengers, including vertebrates and invertebrates. Both D and B populations can be hunted, e.g., regular culling practices by humans. S species consume both D and B carrion, whereas B only scavenge on D carrion, i.e., we assume no cannibalism. Carrion from D is first consumed by B, then S. The large predator wolf (W) predates both on D and B, of which a fraction enters the carrion pool and is thus not consumed by W.
A description of all variables and the references underlying their parameter estimations are presented in Table 1, where in the text we only elaborate on the variables that are needed to understand the working of the equations. All vegetation biomass is modelled in one state and follows the regrowth equation of Turchin and Batzli (2001), where biomass is expressed in normalised values with respect to the carrying capacity and grows with rate (Equation 1a).
| (1a) |
TABLE 1.
Parameter values.
| Symbol | Meaning | Initial value | Value after sensitivity analysis | Unit | Reference/notes |
|---|---|---|---|---|---|
| R 0 | Regrowth rate of V | 4 | 4 | year−1 | Focardi et al. (2017) |
| k 0 | Carrying capacity of V | 10 | 10 | ton ha−1 | Normalised vegetation biomass density (as in Focardi et al. (2017)), unit is an approximation using Earth's total plant biomass (Bar‐On, Phillips, and Milo 2018) |
| A VD | Ingestion of resources by D per unit D for overwhelming V | 23.5 | 23.5 | year−1 | Based on individual food requirement of 986 kg year−1 (Mulley 2002) and average individual weight of 42 kg (Moore, Littlejohn, and Cowie 1988) |
| B XX | Half‐saturation density of resources in the foraging of D, B, S and W | 10 | 10 | ton ha−1 | Based on Focardi et al. (2017) |
| C VD | Conversion factor from consumed resource V to D | 0.017 | 0.025 | — | Based on Flajšman, Jerina, and Pokorny (2017), Mulley (2002), and Moore, Littlejohn, and Cowie (1988), see conversion coefficient calculations (Appendix S1) |
| M D | Death rate of D in the absence of hunting or predators | 0.125 | 0.125 | year−1 | Based on Müller et al. (2010); mean life expectancy in captivity is ~8 years |
| A XB | Ingestion of resources by B per unit B for overwhelming total resources and average carrion in diet | 20.15 | 20.15 | year−1 | Based on individual food requirement of 1209 kg year−1 (Nagy 2021; Treyer et al. 2012) and assumed average individual weight of 60 kg with 16% carrion in diet |
| C VB | Conversion factor from consumed resource V to B | 0.055 | 0.055 | — | Based on Chinn et al. (2022), Gethöffer, Sodeikat, and Pohlmeyer (2007), Treyer et al. (2012), Sá, Moreno, and Carciofi (2020), see conversion coefficient calculations (Appendix S1) |
| M B | Death rate of B in the absence of hunting or predators | 0.08 | 0.08 | year−1 | Based on Massei (1995): maximum age of 12 years |
| u a | Average portion of D carrion scavenged by B | 0.16 | 0.16 | — | Ballari and Barrios‐García (2014) |
| u | Maximum portion of D carrion scavenged by B | 0.2 | 0.2 | — | Unknown, tested during sensitivity analyses and estimated based on average 16% of Ballari and Barrios‐García (2014) |
| B u | Half‐saturation density of carrion portion in the scavenging of B on D carrion | 0.1 | 0.1 | — | Unknown, tested during sensitivity analyses and estimated based on average 16% of Ballari and Barrios‐García (2014) |
| C DB | Conversion factor from consumed resource D to B | 0.069 | 0.069 | — | Based on Chinn et al. (2022), Gethöffer, Sodeikat, and Pohlmeyer (2007), Treyer et al. (2012), see conversion coefficient calculations (Appendix S1) |
| q | Exponential decay rate of natural death rate of deer and boar with increasing predator density | 10 | 10 | — | Unknown, tested during sensitivity analyses |
| T D | Targeted population density for D by hunters | 0–1 | 0–1 | ton ha−1 | Varied to analyse effect of hunting regimes |
| H D | Hunting rate of D above aimpopD at overwhelming D | 0–1 | 0–1 | year−1 | Varied to analyse effect of hunting regimes |
| L D | Not harvested portion of hunted D | 0–1 | 0–1 | — | Varied to analyse effect of hunting regimes |
| T B | Targeted population density for B by hunters | 0–1 | 0–1 | ton ha−1 | Varied to analyse effect of hunting regimes |
| H B | Hunting rate of B above aimpopB at overwhelming B | 0–1 | 0–1 | year−1 | Varied to analyse effect of hunting regimes |
| L B | Not harvested portion of hunted B | 0–1 | 0–1 | — | Varied to analyse effect of hunting regimes |
| M S | Death rate of S | 0.2 | 0.2 | year−1 | Based on overall death rate as in Focardi et al. (2017) |
| C XS | Conversion factor from consumed resource D or B to S | 0.054 | 0.1 | — | Mean animal based conversion factor |
| M W | Death rate of W | 0.07 | 0.2 | year−1 | Based on Hannon and Ruth (2001); life expectancy in captivity is max. 14 years |
| r | Predation rate by W per unit W for overwhelming resources | 96.6 | 96.6 | year−1 | Based on individual food requirement of 1642.5 kg year−1 (Jędrzejewski et al. 2002) and average individual weight of 25 kg (Jędrzejewski et al. 2002) and corrected for unconsumed portion |
| v | Portion of predated resources by W not consumed by W | 0.32 | 0.32 | — | Based on Metz et al. (2011) and Wilmers, Crabtree, et al. (2003) |
| C XW | Conversion factor from consumed resource to W | 0.038 | 0.038 | — | Based on Jędrzejewski et al. (2002), Sidorovich et al. (2007), see conversion coefficient calculations (Appendix S1) |
| A XS | Ingestion of resources by S per unit S for overwhelming resources | 20 | 20 | year−1 | Unknown, tested during sensitivity analyses |
| C VS | Conversion factor from consumed resource V to S | 0.036 | 0.036 | — | Mean plant based conversion factor |
Note: See Appendix S1 for the conversion factor calculations.
The consumption rates of vegetation by deer (Equation 1b), boar (Equation 1c) and scavengers (Equation 1d) all follow a Holling type II functional response (Holling 1966), which is often used to describe the realistic ‘levelling‐off’ of a response with increasing resources (Skalski and Gilliam 2001).
| (1b) |
| (1c) |
| (1d) |
is the maximum amount of resources ingested per unit (e.g., is the maximum amount of vegetation ingested per unit of deer), with being the half‐saturation density of per unit (which we kept at the same value (Table 1) for all functional response equations in our model, as these values are very difficult to estimate (Skalski and Gilliam 2001)) to determine the actual ingestion rate via this functional response.
Equation (1b) has its functional response written in its most basic form, given that deer only consume vegetation in our model. However, boar (Equation 1c) also consume deer carrion , and scavengers (Equation 1d) also consume both deer and boar carrion, which influences their vegetation consumption rate per time step. Therefore, we extended upon the default Holling type II functional response equations of the vegetation consumption by boar and scavengers, which we list here as separate equations to be substituted in the main equations. For example for boar, we model the portion of deer carrion in their diet with a separate Holling type II functional response (Equation 1c2), based on the amount of available deer carrion.
| (1c2) |
Given that carrion is more nutritious than vegetation for boar, the conversion factor from a unit consumed vegetation biomass to a unit boar is smaller than the conversion factor from deer carrion to boar (Table 1; Appendix S1). As such, we model the maximum total consumption rate by boar so that it consumes less biomass, when more of its diet consists of carrion (Equation 1c1a).
| (1c1a) |
This way a unit of boar ‘aims to’ obtain approximately the same amount of boar biomass units in total via a Holling type II functional response (Equation 1c1), independent of the fraction of carrion in its diet.
| (1c1) |
For scavengers, their maximum total consumption rate is also computed via a Holling type II functional response (Equation 1d1), which considers the available vegetation, deer carrion and boar carrion biomass.
| (1d1) |
Given that we assume boars are the first and foremost scavengers to consume deer carrion (Wenting, Rinzema, and van Langevelde 2022; Wenting et al. 2024), only the deer carrion that is not consumed by boar are available for other scavengers. Deer (Equation 1d2) and boar carrion (Equation 1d3) are (i) produced by natural mortality, (ii) the fraction that is left by human hunters and (iii) the fraction that is left by wolves.
| (1d2) |
| (1d3) |
The functions that describe the growth of deer (Equation 2a), boar (Equation 3a) and scavengers (Equation 4a) from vegetation are all calculated by multiplying the consumed vegetation biomass by the conversion factor from a unit consumed vegetation biomass to a unit .
| (2a) |
| (3a) |
| (4a) |
The deer carrion growth function of boar (Equation 3b) is obtained by multiplying the portion of deer carrion in the boars' diet by the total consumed biomass per unit boar , the deer carrion to boar conversion factor and the total units of boar.
| (3b) |
The deer (Equation 4b) and boar carrion (Equation 4c) growth functions of scavengers are also obtained by multiplying the consumed carrion biomass by the carrion to scavenger conversion factor .
| (4b) |
| (4c) |
The consumed carrion biomass by scavengers is modelled with a Holling type I functional response (Holling 1966), meaning that scavengers will consume per unit until a maximum value that is equal to the total amount of available carrion. However, do note that itself is computed via a Holling type II functional response (Equation 1d1), so the overall carrion consumption by and subsequent growth of scavengers follows a Holling type II functional response in relation to resource availability.
The deer (Equation 5a) and boar growth functions of wolf (Equation 5b) are also similar in structure as the other growth functions, where the amount of predated deer and boar (both explained in the next paragraph) are multiplied by the conversion factor and multiplied by the fraction of the carrion that is not left behind by the wolves .
| (5a) |
| (5b) |
The predation of deer (Equation 2b) and boar by wolves (Equation 3c) are both also modelled with a Holling type II functional response, where is the maximum total predation rate per wolf unit. The wolves' total predation rate is divided over deer and boar based on their relative availability. We amplified this selection preference of wolf for the most abundant prey by squaring the deer and boar biomass densities, so that it was easier to simulate a system in which both deer and boar could co‐occur despite the higher vegetation conversion factors of boar versus deer (Table 1). This way we assumed that wolves became more specialistic hunters for a single prey species when that species was abundant compared to the other species (Becker et al. 2008; Sand et al. 2016; Zabihi‐Seissan, Prokopenko, and Vander Wal 2022).
| (2b) |
| (3c) |
Hunting of both deer (Equation 2c) and boar (Equation 3d) is zero when their biomass is equal or below the hunters' target biomass . When their biomass is higher, then only the amount above this target biomass is hunted with a hunting efficiency rate (to simulate the increasing difficulty to find animals to hunt when their density drops). This describes hunting regimes that are standard in European countries, where the hunting quota of animals are determined based on the yearly estimated population size and the target population size, but where quota are often not fully realised when these targets are strict (Dijkhuis et al. 2023).
| (2c) |
| (3d) |
Finally, the natural mortality of deer (Equation 2d), boar (Equation 3e), scavengers (Equation 4d) and wolves (Equation 5c) are modelled by multiplying a static death rate with the total biomass units of the respective populations. For both deer and boar, this natural mortality decreases with an exponential decay rate of multiplied by the wolves' predation pressure. We implemented this process to simulate that wolves more often target old and weak prey, thereby lowering the natural mortality rate of these prey animals (Becker et al. 2008; Kittle et al. 2017).
| (2d) |
| (3e) |
| (4d) |
| (5c) |
2.2. Parameter Estimation and Sensitivity Analysis
We aimed to develop an ODE model that resembles the actual processes of a temperate ecosystem, which is a non‐trivial task. Especially the estimation of parameter values is not straightforward, because (1) not all parameter values can be estimated directly from the literature and (2) even parameter values derived from the literature may cause non‐realistic simulations, given the simplifications of a model compared to reality. We approached this problem with a three‐step workflow. First, we searched the literature using keyword based on the explained meaning of the parameters (Table 1) to estimate the parameter values. Second, we built up the complexity our model step‐by‐step (first a model only with vegetation (by setting the initial values of all other states at zero), then vegetation + deer, then vegetation + boar, etc.; see R script via link in Data Accessibility Statement), to estimate the values of the other parameters and to finetune the parameters that we based on the literature. These values were estimated to avoid both chaotic time series and crashing populations, when these were unrealistic patterns for the simulated scenarios based on our expert knowledge. When we needed to update parameter values, we updated them such that it would strike a balance between changing as few parameters as possible with as small a deviation per parameter as possible (Table 1). Third, during each step of this workflow, we also performed sensitivity analyses on the parameters to check that the simulations were relatively robust to alterations of our estimated parameter values (see R script via link in Data Accessibility Statement). At each step of this workflow, we varied the parameters that were introduced at this step by a factor of 0.75, 0.875, 1, 1.125 and 1.25. Then we ran the simulations for all combinations of these parameter values at each step of our workflow (e.g., so simulations in a single step when 4 parameters were introduced). Then we examined the output of the simulations using: (1) timeseries line charts of the different states (e.g., ) with multiple lines and figure panels for the different parameter values of the sensitivity analysis and (2) 2D image plots of the end state of the different states (e.g., ) with two parameters that were varied during the sensitivity analysis along both the x‐ and y‐axis of the image plots and the other varied parameter values separated over multiple figure panels. When the qualitative patterns of the simulations were highly dependent on the parameter value range that we chose during our sensitivity analyses, then we updated our estimated parameter values in the same way as in step two to make the simulations more robust. Finally, at the end of each step, we visualised phase planes of each combination of two states to verify if the initial state values influenced the end states (which was never the case, i.e., all models converged to a single stable state).
After this three‐step workflow to estimate parameter values was complete, we let our simulation run with these same parameter values for four different scenarios: with and without both boar and wolf (i.e., wolf and boar, only wolf, only boar, neither), by iteratively setting the initial state value of boar and wolf at zero. For each of these four scenarios, we also varied two parameters of interest: (1) the hunters' target biomass for both the deer and population and (2) the fraction of carrion left by hunters. Finally, when our interpretations of the results were highly dependent on a single parameter value, we performed a sensitivity analysis for this parameter at this stage again to test the robustness of our conclusions.
2.3. Numerical Simulations
We performed the numerical simulations in R 4.3.1 (R Core Team 2023) with the deSolve package to solve the ODE model (Soetaert, Petzoldt, and Setzer 2010), the data. table package to process the data (Dowle and Srinivasan 2023), and the ggplot2 package to visualise (Wickham 2016). We used lsoda as the ODE solving algorithm (Petzold 1983), which switches automatically between stiff and non‐stiff methods. As such, this algorithm adaptively changes the time step size during integration to e.g., avoid overshooting. We let the simulations of all our different scenarios run for 250 time‐steps (years), because this was long enough to stabilise the different states from its initial values and still short enough to visually investigate the evolution of the states over time.
3. Results
3.1. Effect of Wild Boar on Scavenger Dynamics
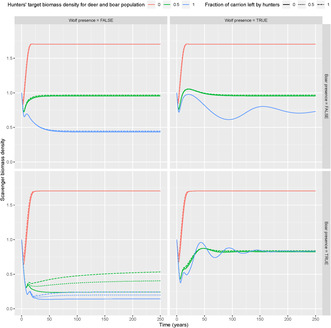
In the scenarios with a population target of 0, i.e., more hunting, all deer and boar became extinct (Appendix S2: Figures S2.1– S2.2), so, to assess the effect of boar on scavenger dynamics, we focused on the scenarios with a high or medium hunting target (Figure 2). When boar is present but wolf absent, we observed that the overall scavenger biomass was the lowest (Figure 2). In this scenario, there is more competition for vegetation resources between boar, deer and scavengers (Appendix S2: Figures S2.1–S2.3). Deer biomass is higher in the absence of boar (Appendix S2: Figure S2.1), but in the presence of boar, there is more biomass of deer and boar combined (Appendix S2: Figures S2.1 and S2.2). This means that competition for vegetation resources would drive scavenger biomass, rather than competition for carrion. This becomes also apparent from the lower vegetation biomass in the scenario with boar and without wolves (Appendix S2: Figure S2.3).
FIGURE 2.
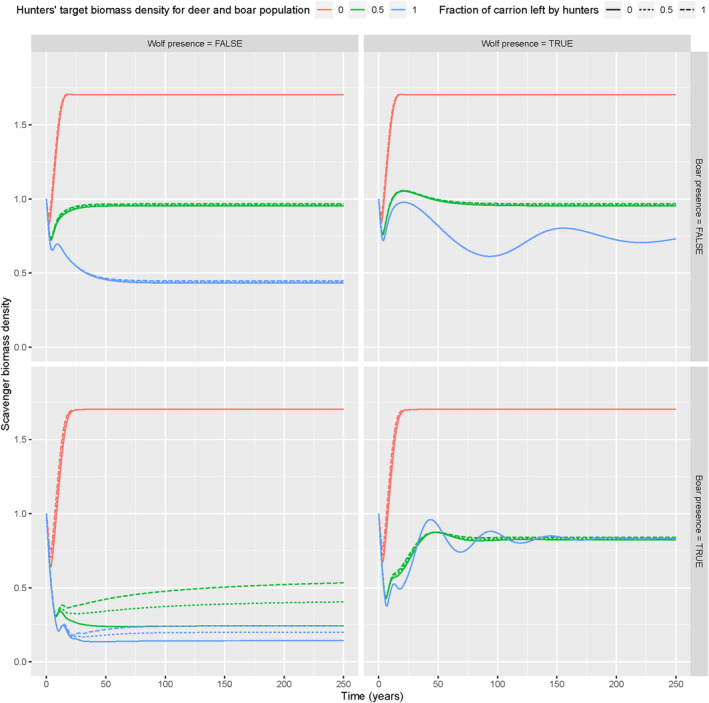
Scavenger biomass density ODE model simulations (y‐axis) over time (x‐axis), with boar (horizontal panels) and wolf present/absent (vertical panels), for different hunting target values (line colours) and fractions of carrion left by hunters (line types).
The importance of vegetation resources in determining scavenger biomass could be heavily influenced by the parameter value we used for the conversion factor of vegetation for scavengers . We assessed the importance of this parameter value with a sensitivity analysis. When was 30% higher, the same qualitative time series patterns of scavenger biomass occurred for all scenarios, with only the absolute scavenger biomass values becoming higher by a factor of 1–1.25 (Appendix S3: Figures S3.2 and S3.3). Similarly, we found the same patterns, but with lower absolute biomass values by a factor of 0.5–1, when was 30% lower (Appendix S3: Figures S3.1 and S3.2). That means that our results are robust to varying values of the conversion factor of vegetation for scavengers. Thus, the observation that vegetation resources, rather than carrion, are limiting scavenger biomass is robust. Our simulations showed that the effect of boar on scavenger biomass is negative in the absence of wolf but neutral in the presence of wolf (Figure 2).
3.2. Effect Re‐Establishing Wolf on Scavenger Dynamics
Our simulations showed a general positive trend in scavenger biomass in the presence of wolf (Figure 2). In the absence of boar, we found that wolf could only maintain their presence when the hunting target was high (so when there was little hunting) (Appendix S2: Figure S2.4). In the presence of boar, wolf could maintain their presence with both high and medium hunting targets (Appendix S2: Figure S2.4). In the scenarios where wolf could maintain their presence, we observed more fluctuations in the scavenger biomass around a stable equilibrium (Figure 2), which followed fluctuations in population dynamics of deer and boar (Appendix S2: Figures S2.1 and S2.2). This again is due to general predator prey dynamics, since the fluctuations in biomass of deer and boar followed the fluctuations of wolf biomass and vice versa (Appendix S2: Figures S2.1– S2.4).
3.3. Effect of Human Hunting Strategies on Scavenger Dynamics
The hunting target had, via the populations of deer and boar (Appendix S2: Figures S2.1 and S2.2), a huge effect on scavenger biomass in general (Figure 2). The lower the biomass of deer and boar, the higher the biomass of scavengers, resulting from decreasing competition for vegetation resources. We observed that more hunting resulted in less deer and boar (Appendix S2: Figures S2.1 and S2.2), which subsequently resulted in higher biomass of scavengers (Figure 2).
In the presence of both boar and wolf, medium and high hunting targets caused the same scavenger biomass (Figure 2). The higher the hunting target, the more the wolf took over from humans in killing deer and boar. This often resulted in deer and boar populations below the hunting target in this scenario, meaning that there was no human hunting needed in this scenario to maintain deer and boar population targets (Appendix S2: Figures S2.1 and S2.2). This, in turn, resulted in the same scavenger biomass (Figure 2), although population dynamics fluctuated more when the wolf dominated the hunting.
We found that the fraction of carrion left behind by hunters was only important for scavenger biomass when wolf was absent but boar present (Figure 2). The more carrion that was left behind by hunters, the higher the scavenger biomass (Figure 2). The reason that the extra growth scavengers gained from carrion was only important in this scenario is again due to competition for vegetation resources between scavengers, deer and boar. The vegetation resources were more limited in this scenario than in the three others (Appendix S2: Figure S2.3), and therefore higher fractions of carrion left behind by hunters, actually also resulted in lower populations of deer and boar in this scenario due to competition for vegetation resources with scavengers (Appendix S2: Figures S2.1 and S2.2).
3.4. Main Resource for Scavengers
To assess the main resource for scavengers under different scenarios, we first checked the importance of vegetation versus carrion for the growth of scavenger biomass. Overall, we found that vegetation resources caused way more growth of scavenger biomass compared to carrion (Figure 3). The only exception was when boar was present but wolf absent. Here, the scenarios with high and medium hunting targets resulted in more competition for vegetation resources and simultaneously for more deer and boar biomass that became available as carrion (Figure 3; Appendix S3: Figure S3.5). For that reason, carrion became more important in these scenarios (Figure 3). The sensitivity analysis of the conversion factor of vegetation resources for scavengers indicated that competition for vegetation resources was still a dominant process, rather than the availability of carrion in general, in determining the biomass of scavengers (Appendix S3: Figures S3.1–S3.3).
FIGURE 3.
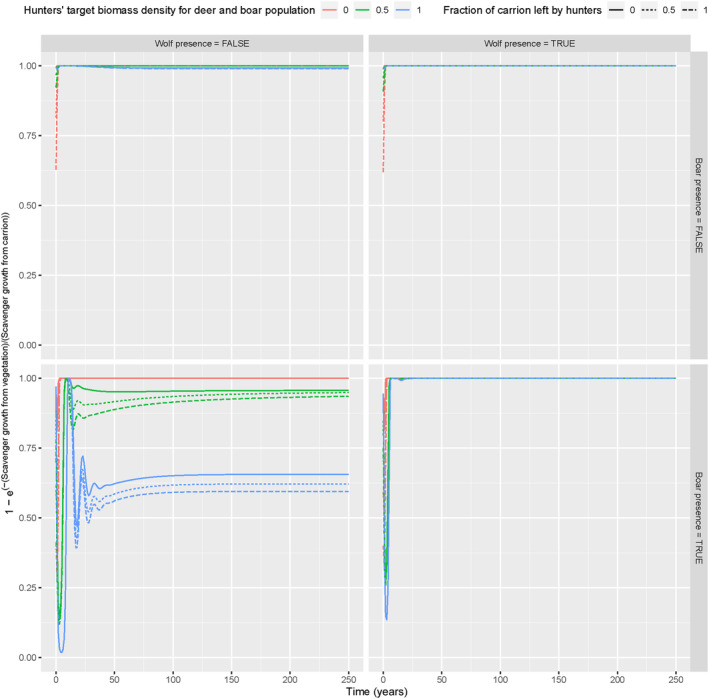
Scavenger growth from vegetation versus scavenger growth from carrion ODE model simulations (y‐axis, transformed from [0, ∞] to [0, 1] range) over time (x‐axis), with boar (horizontal panels) and wolf present/absent (vertical panels), for different hunting target values (line colours) and fractions of carrion left by hunters (line types).
When wolf was present but boar absent, the available carrion comes either from hunting or from predation (Appendix S2: Figure S2.5). The lower the hunting target, the more carrion was relatively obtained from hunting (Appendix S2: Figure S2.5). In the presence of boar, the fraction of carrion left behind by hunters matters in the case of medium hunting target (Appendix S2: Figure S2.5). In this scenario, we observed that higher fractions of carrion left behind by hunters, the larger the fraction of carrion that is originated from hunting.
In the presence of boar, we found that there was always more boar carrion than deer carrion available (Figure 4). This is because, in general, boar biomass was always higher than deer biomass in our simulations (Appendix S2: Figures S2.1 and S2.2). With a medium hunting target and in the absence of wolf, deer was not outcompeted by boar and scavengers (Appendix S2: Figures S2.1–S2.4). Also, deer was not outcompeted in the presence of wolf, but only when the hunting target was zero (Appendix S2: Figure S2.1). Only in the scenarios with medium hunting target, the fraction of carrion left behind by hunters influenced the fraction of deer versus boar carrion (Figure 4).
FIGURE 4.
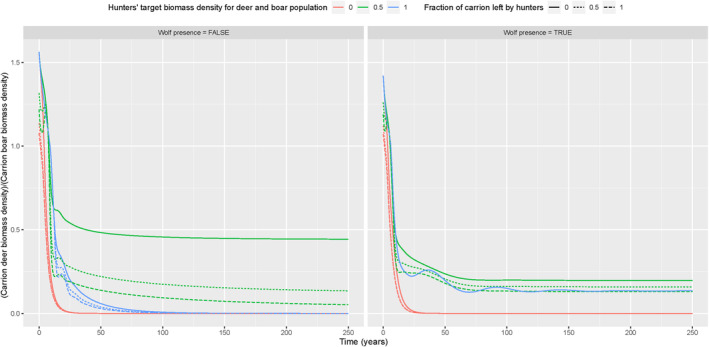
Deer carrion versus boar carrion biomass density ODE model simulations (y‐axis) over time (x‐axis), with wolf present/absent (panels), for different hunting target values (line colours) and fractions of carrion left by hunters (line types).
4. Discussion
In this study, we examined how different human hunting strategies, combined with the presence or absence of boar and wolf, influenced the dynamics of scavenger biomass in a system with only facultative scavengers. We did not aim to create fully realistic scenarios of specific existing natural systems, but intended to create a mathematical model to improve our theoretical understanding of all the interacting processes that are involved. Given the nature of a simulation study, we made many assumptions to simplify reality to obtain generalisable conclusions. These assumptions included that the wolves' diet was exclusively based on deer and boar predation, that there was only one shared vegetation resource for all populations, that wild boar did not scavenge on conspecifics, no scavenging by wolves, no human prosecution of wolves, and that the populations are limited by food (instead of space). Regardless of these assumptions, we found some patterns that provided new insights into the population dynamics of facultative scavengers when wolves and/or boar are re‐establishing under different human hunting strategies.
A key conclusion of our simulations is that carrion was not the most important resource in determining the biomass growth of facultative scavengers (Figure 3). These facultative scavengers are flexible in their diet and behaviour and can therefore adapt to local circumstances (Díaz‐Ruiz et al. 2013; Rooney and Montgomery 2013; Papakosta et al. 2014; Jain et al. 2022). As a result, carrion is not equally consumed among and within ecosystems and different local scavenger guilds, which results in high variability of the carrion decomposition process in general (Newsome et al. 2021; Wenting, Rinzema, and van Langevelde 2022; Wenting et al. 2024; Vandersteen et al. 2023). This implies that carrion is an ephemeral resource for facultative scavengers, which supplements their diet and behaviour but does not necessarily determine it (Wilson and Wolkovich 2011; Barton et al. 2013), which is in line with our results. Moreover, the presence of wolves also has indirect effects by changing intraguild dynamics between large and small prey species (Ripple and Beschta 2004; Jędrzejewski et al. 2012), ultimately changing dynamics among facultative scavenger guilds (Wikenros et al. 2013) and vegetation resources (Jędrzejewski et al. 2012; Kuijper et al. 2013).
Due to the direct competition for vegetation resources in our model by deer and boar with scavengers, we assumed that the competitive release hypothesis (Ketterson and Nolan Jr 1976; Le Bagousse‐Pinguet, Gross, and Straile 2012) applies to our study system. As such, a lower population of one group often positively impacts the populations of other groups (Berg et al. 2019; Van Moorter et al. 2021). This has been demonstrated for the European ecosystems where wolves are present (Chapman et al. 2011), which is reflected in our results (Appendix S2: Figures S2.1–S2.4).
The presence of wolf had an overall positive effect on the scavenger population and could take over the role of human hunting in controlling ungulate populations under some conditions (Figure 2). In our model, wolf was fully dependent on predation on deer and boar. It can supplement its diet with other resources, including livestock (Janeiro‐Otero et al. 2020) and carrion (Petroelje et al. 2019; Wirsing and Newsome 2021). Carrion consumption by wolves is extensively documented in some ecosystems (Mateo‐Tomás et al. 2015). In temperate ecosystems, on which our simulations were based, it has only been proven in areas where wolves were re‐established for multiple years, or where they were never extinct (Jędrzejewski et al. 2002; Selva 2004; Selva and Fortuna 2007). In other areas, where wolves recently re‐established, evidence is only anecdotical or absent. Thus, it is unknown whether recently re‐established wolves scavenge substantially or change their scavenging habits over time. Based on this, we decided to simplify the model by only focusing on scavenging by facultative scavengers and hence not to include scavenging behaviour of wolves.
Depending on the local circumstances, including the presence of large carnivores (that can induce fear), facultative scavengers establish a specific way of scavenging behaviour (Selva et al. 2005; Pereira, Owen‐Smith, and Moleón 2014; Kane et al. 2017). For example, the willingness of species to forage in open areas decreases with increasing predation pressure (Allen et al. 2015), in line with the ecology of fear (Haswell et al. 2018, 2020; Gaynor et al. 2021; Ramirez et al. 2024). This, in turn, might reduce the potential effects of habitat type on scavenging behaviour in general, meaning that scavengers might forage more in open landscapes instead of forests only, and vice versa (Wenting et al. 2024). We suppose that facultative scavengers, due to their adaptable nature, eventually adapt their scavenging habits when large carnivores re‐establish. However, the question is about the speed at which they will adapt their behaviour. This might cause some iterations in scavenger dynamics when wolves re‐establish, until scavengers have adapted their behaviour to the wolves' presence. However, the ultimate consequences are unclear and hard to predict, especially in human‐dominated landscapes (Hebblewhite et al. 2005; Dorresteijn et al. 2015).
Boar outcompeted deer in the scenarios with low hunting pressure and without wolves (Appendix S2: Figures S2.1 and S2.2). This is because we assumed boar to be more efficient in exploiting vegetation resources than deer, i.e., boar had a higher conversion factor of vegetation resources than deer (Table 1), mainly due to their higher reproductive rate (Appendix S1). For simplicity, we used only one vegetation resource for all species. Consequently, boar and deer competed directly for exactly the same resource. This is not realistic due to niche differentiation among those boar and deer species (Gebert and Verheyden‐Tixier 2001; Ballari and Barrios‐García 2014; Mikulka et al. 2018; Spitzer et al. 2020). The same applies to facultative scavengers; although they are predominantly omnivores, e.g., Red fox and European badger, that contain plant‐based resources in their diet, the vegetation they consume do not fully overlap with deer and boar (Castañeda et al. 2022; Jain et al. 2022). We assume this simplification to be the main limitation of our model for interpreting our results. However, although in reality the resources of all the species do not fully overlap, it is still reasonable that they do show some overlap. The absolute values of our results do not have any predictive power for reality, but the patterns that we modelled still do, which is exemplified by our sensitivity analyses on the vegetation conversion coefficients by scavengers (Appendix S3: Figures S3.1–S3.3). Therefore, our result that carrion might not be the main resource that determines the biomass growth of facultative scavengers is still valid.
We found that the presence of boar on scavenger biomass was negative when wolf was absent but neutral when wolf was present (Figure 2). However, scavenger biomass does not automatically reflect the functionality of the scavenger community and the potential effects that scavengers can have on ecological processes. Nonetheless, the simulations are in line with the alleged unique role of boars in carrion decomposition (Wenting, Rinzema, and van Langevelde 2022; Wenting et al. 2024). Also, based on our simulations, we expect that the co‐occurrence of both boar and wolf stimulates fundamental ecological processes – e.g., nutrient cycling and restoring biodiversity – the most.
Our simulations with and without boar's presence can be seen as an example of human influences that extend beyond hunting. Both boar and wolf are involved in human‐wildlife conflicts (Massei et al. 2015; Storie and Bell 2017; Kuijper et al. 2019; König et al. 2020). Wolf is, unlike boar, strictly protected by law in the EU, meaning that their presence needs to be tolerated (Trouwborst and Fleurke 2019). Boars are not tolerated everywhere, or their populations are extensively controlled (Thurfjell, Spong, and Ericsson 2013; Massei et al. 2015). Our simulations imply, however, that the coexistence of both boar and wolf would positively influence the scavenger dynamics in general by increasing the overall scavenger biomass densities. Consequently, the co‐existence of both species would, eventually, enhance the overall ecosystem functioning. We consider this as the most noticeable conclusion of our study.
When the hunting target was low, wolf could replace the effects of human hunting by keeping the populations of deer and boar below the hunting target (Appendix S2: Figures S2.1 and S2.2). That implies that human hunting in general should be reconsidered and adapted to re‐establishing wolf populations. This has not only ecological benefits, as our model implies (Figure 2), but would also reduce human‐wildlife conflicts since it has been widely documented that established wolves prefer wild prey over livestock (Meriggi and Lovari 1996; Sidorovich, Tikhomirova, and Jędrzejewska 2003; Ferretti et al. 2019).
In conclusion, our model indicates that population dynamics of facultative scavengers are not mainly driven by the availability of carrion but rather by the presence of and competition for vegetation and other resources. The co‐occurrence of boar and wolf can have positive effects on scavengers' population dynamics. Their population dynamics showed more fluctuations as human hunting, to control deer and boar densities, was taken over by wolves. Although this is in line with well‐documented natural predator–prey interactions (Wangersky and Cunningham 1957; Mougi and Iwasa 2010), it highlights the importance of changing the human hunting strategy in accordance with wolves' re‐establishment.
Author Contributions
Elke Wenting: conceptualization (lead), formal analysis (equal), methodology (equal), software (supporting), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Jasper A. J. Eikelboom: conceptualization (supporting), formal analysis (equal), methodology (equal), software (lead), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Henk Siepel: conceptualization (supporting), methodology (supporting), writing – original draft (supporting). Femke Broekhuis: conceptualization (supporting), writing – original draft (supporting). Frank van Langevelde: conceptualization (supporting), methodology (supporting), writing – original draft (supporting).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research Badges
This article has earned an Open Data badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.4121/a5a040e7‐de45‐4d60‐9ac4‐eec4e826aa8.
Supporting information
Appendix S1.
Appendix S2.
Appendix S3.
Acknowledgements
We thank Patrick Jansen, Henjo de Knegt, Dick Klees and Laurens Dijkhuis for their input during the modelling.
Funding: The authors received no specific funding for this work.
Elke Wenting and Jasper A.J. Eikelboom contributed equally to this work.
Contributor Information
Elke Wenting, Email: elke.wenting@ru.nl.
Jasper A. J. Eikelboom, Email: jasper.eikelboom@wur.nl.
Data Availability Statement
The complete R script of the ODE model, including all sensitivity analyses to produce the manuscript figures, is available via: https://doi.org/10.4121/a5a040e7‐de45‐4d60‐9ac4‐eec4e826aa85.
References
- Allen, B. L. , Allen L. R., Andrén H., et al. 2017. “Can We Save Large Carnivores Without Losing Large Carnivore Science?” Food Webs 12: 64–75. 10.1016/j.fooweb.2017.02.008. [DOI] [Google Scholar]
- Allen, M. L. , Elbroch L. M., Wilmers C. C., and Wittmer H. U.. 2015. “The Comparative Effects of Large Carnivores on the Acquisition of Carrion by Scavengers.” American Naturalist 185: 822–833. 10.1086/681004. [DOI] [PubMed] [Google Scholar]
- Ansorge, H. , Kluth G., and Hahne S.. 2006. “Feeding Ecology of Wolves Canis lupus Returning to Germany.” Acta Theriologica 51: 99–106. 10.1007/BF03192661. [DOI] [Google Scholar]
- Ballari, S. A. , and Barrios‐García M. N.. 2014. “A Review of Wild Boar Sus scrofa Diet and Factors Affecting Food Selection in Native and Introduced Ranges.” Mammal Review 44: 124–134. 10.1111/mam.12015. [DOI] [Google Scholar]
- Barja, I. , Navarro‐Castilla Á., Ortiz‐Jiménez L., et al. 2023. “Wild Ungulates Constitute the Basis of the Diet of the Iberian Wolf in a Recently Recolonized Area: Wild Boar and Roe Deer as Key Species for Its Conservation.” Animals 13: 3364. 10.3390/ani13213364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐On, Y. M. , Phillips R., and Milo R.. 2018. “The Biomass Distribution on Earth.” Proceedings of the National Academy of Sciences of the United States of America 115: 6506–6511. 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios‐Garcia, M. N. , Gonzalez‐Polo M., Simberloff D., and Classen A. T.. 2023. “Wild Boar Rooting Impacts Soil Function Differently in Different Plant Community Types.” Biological Invasions 25: 583–592. 10.1007/s10530-014-0818-7. [DOI] [Google Scholar]
- Barton, P. S. , Cunningham S. A., Lindenmayer D. B., and Manning A. D.. 2013. “The Role of Carrion in Maintaining Biodiversity and Ecological Processes in Terrestrial Ecosystems.” Oecologia 171: 761–772. 10.1007/s00442-012-2460-3. [DOI] [PubMed] [Google Scholar]
- Baruzzi, C. , and Krofel M.. 2017. “Friends or Foes? Importance of Wild Ungulates as Ecosystem Engineers for Amphibian Communities.” North‐Western Journal of Zoology 13: 320–325. [Google Scholar]
- Beasley, J. C. , Olson Z. H., Selva N., and DeVault T. L.. 2019. “Ecological Functions of Vertebrate Scavenging.” In Carrion Ecology and Management, edited by Olea P. P., Mateo‐Tomás P., and Sánchez‐Zapata J. A., 125–157. Cham: Springer. [Google Scholar]
- Becker, M. S. , Garrott R. A., White P. J., Gower C. N., Bergman E. J., and Jaffe R.. 2008. “Wolf Prey Selection in an Elk‐Bison System: Choice or Circumstance?” Terrestrial Ecology 3: 305–337. 10.1016/S1936-7961(08)00216-9. [DOI] [Google Scholar]
- Berg, J. E. , Hebblewhite M., St. Clair C. C., and Merrill E. H.. 2019. “Prevalence and Mechanisms of Partial Migration in Ungulates.” Frontiers in Ecology and Evolution 7: 325. 10.3389/fevo.2019.00325. [DOI] [Google Scholar]
- Boczulak, H. , Boucher N. P., Ladle A., Boyce M. S., and Fisher J. T.. 2023. “Industrial Development Alters Wolf Spatial Distribution Mediated by Prey Availability.” Ecology and Evolution 13: e10224. 10.1002/ece3.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonman‐Berson, S. , Driessen C., and Turnhout E.. 2019. “Managing Wild Minds: From Control by Numbers to a Multinatural Approach in Wild Boar Management in the Veluwe, The Netherlands.” Transactions of the Institute of British Geographers 44: 2–15. 10.1111/tran.12269. [DOI] [Google Scholar]
- Castañeda, I. , Doherty T. S., Fleming P. A., Stobo‐Wilson A. M., Woinarski J. C., and Newsome T. M.. 2022. “Variation in Red Fox Vulpes vulpes Diet in Five Continents.” Mammal Review 52: 328–342. 10.1111/mam.12292. [DOI] [Google Scholar]
- Chapman, B. B. , Brönmark C., Nilsson J. Å., and Hansson L. A.. 2011. “The Ecology and Evolution of Partial Migration.” Oikos 120: 1764–1775. 10.1111/j.1600-0706.2011.20131.x. [DOI] [Google Scholar]
- Chapron, G. , Kaczensky P., Linnell J. D. C., et al. 2014. “Recovery of Large Carnivores in Europe's Modern Human‐Dominated Landscapes.” Science 346: 1517–1519. 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]
- Chinn, S. M. , Schlichting P. E., Smyser T. J., Bowden C. F., and Beasley J. C.. 2022. “Factors Influencing Pregnancy, Litter Size, and Reproductive Parameters of Invasive Wild Pigs.” Journal of Wildlife Management 86: e22304. 10.1002/jwmg.22304. [DOI] [Google Scholar]
- DeVault, T. L. , Rhodes O. E. Jr., and Shivik J. A.. 2003. “Scavenging by Vertebrates: Behavioral, Ecological, and Evolutionary Perspectives on an Important Energy Transfer Pathway in Terrestrial Ecosystems.” Oikos 102: 225–234. 10.1034/j.1600-0706.2003.12378.x. [DOI] [Google Scholar]
- Díaz‐Ruiz, F. , Delibes‐Mateos M., García‐Moreno J. L., María López‐Martín J., Ferreira C., and Ferreras P.. 2013. “Biogeographical Patterns in the Diet of an Opportunistic Predator: The Red Fox Vulpes vulpes in the Iberian Peninsula.” Mammal Review 43: 59–70. 10.1111/j.1365-2907.2011.00206.x. [DOI] [Google Scholar]
- Dijkhuis, L. R. , Jansen P. A., den Ouden J., et al. 2023. “Estimating Red Deer Population Size Using Vantage Point Counts at Baited Sites.” Journal of Wildlife Management 87: e22489. 10.1002/jwmg.22489. [DOI] [Google Scholar]
- Dorresteijn, I. , Schultner J., Nimmo D. G., et al. 2015. “Incorporating Anthropogenic Effects Into Trophic Ecology: Predator–Prey Interactions in a Human‐Dominated Landscape.” Proceedings of the Royal Society B: Biological Sciences 282: 20151602. 10.1098/rspb.2015.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowle, M. , and Srinivasan A.. 2023. “_data.table: Extension of ‘data.frame’_. R package version 1.14.8.” https://CRAN.R‐project.org/package=data.table.
- Ferretti, F. , Lovari S., Mancino V., Burrini L., and Rossa M.. 2019. “Food Habits of Wolves and Selection of Wild Ungulates in a Prey‐Rich Mediterranean Coastal Area.” Mammalian Biology 99: 119–127. 10.1016/j.mambio.2019.10.008. [DOI] [Google Scholar]
- Flajšman, K. , Jerina K., and Pokorny B.. 2017. “Age‐Related Effects of Body Mass on Fertility and Litter Size in Roe Deer.” PLoS One 12: e0175579. 10.1007/s13364-017-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focardi, S. , Gaillard J. M., Ronchi F., and Rossi S.. 2008. “Survival of Wild Boars in a Variable Environment: Unexpected Life‐History Variation in an Unusual Ungulate.” Journal of Mammalogy 89: 1113–1123. 10.1644/07-MAMM-A-164.1. [DOI] [Google Scholar]
- Focardi, S. , Materassi M., Innocenti G., and Berzi D.. 2017. “Kleptoparasitism and Scavenging Can Stabilize Ecosystem Dynamics.” American Naturalist 190: 398–409. 10.1086/692798. [DOI] [PubMed] [Google Scholar]
- Ford, A. T. , and Goheen J. R.. 2015. “Trophic Cascades by Large Carnivores: A Case for Strong Inference and Mechanism.” Trends in Ecology & Evolution 30: 725–735. 10.1016/j.tree.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Found, R. 2016. “Managing Large Herbivores in Protected Areas.” Global Journal of Ecology 1: 001–011. 10.17352/gje.000001. [DOI] [Google Scholar]
- Galaverni, M. , Caniglia R., Fabbri E., Milanesi P., and Randi E.. 2016. “One, No One, Or One Hundred Thousand: How Many Wolves Are There Currently in Italy?” Mammal Research 61: 13–24. 10.1007/s13364-015-0247-8. [DOI] [Google Scholar]
- Gantchoff, M. G. , Beyer D. E., Erb J. D., et al. 2022. “Distribution Model Transferability for a Wide‐Ranging Species, the Gray Wolf.” Scientific Reports 12: 1–11. 10.1038/s41598-022-16121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, K. M. , Cherry M. J., Gilbert S. L., et al. 2021. “An Applied Ecology of Fear Framework: Linking Theory to Conservation Practice.” Animal Conservation 24: 308–321. 10.1111/acv.12629. [DOI] [Google Scholar]
- Gebert, C. , and Verheyden‐Tixier H.. 2001. “Variations of Diet Composition of Red Deer (Cervus elaphus L.) in Europe.” Mammal Review 31: 189–201. 10.1111/j.1365-2907.2001.00090.x. [DOI] [Google Scholar]
- Gethöffer, F. , Sodeikat G., and Pohlmeyer K.. 2007. “Reproductive Parameters of Wild Boar (Sus scrofa) in Three Different Parts of Germany.” European Journal of Wildlife Research 53: 287–297. 10.1007/s10344-007-0097-z. [DOI] [Google Scholar]
- Gordon, I. J. 2009. “What Is the Future for Wild, Large Herbivores in Human‐Modified Agricultural Landscapes?” Wildlife Biology 15: 1–9. 10.2981/06-087. [DOI] [Google Scholar]
- Greenspoon, L. , Krieger E., Sender R., et al. 2023. “The Global Biomass of Wild Mammals.” Proceedings of the National Academy of Sciences 120: e2204892120. 10.1073/pnas.2204892120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, B. , and Ruth M.. 2001. “Reestablishment of Wolves.” In Dynamic Modeling. Modeling Dynamic Systems. New York, NY: Springer. 10.1007/978-1-4613-0211-7_20. [DOI] [Google Scholar]
- Haswell, P. M. , Jones K. A., Kusak J., and Hayward M. W.. 2018. “Fear, Foraging and Olfaction: How Mesopredators Avoid Costly Interactions With Apex Predators.” Oecologia 187: 573–583. 10.1007/s00442-018-4133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell, P. M. , Kusak J., and Hayward M. W.. 2017. “Large Carnivore Impacts are Context‐Dependent.” Food Webs 12: 3–13. 10.1016/j.fooweb.2016.02.005. [DOI] [Google Scholar]
- Haswell, P. M. , Kusak J., Jones K. A., and Hayward M. W.. 2020. “Fear of the Dark? A Mesopredator Mitigates Large Carnivore Risk Through Nocturnality, but Humans Moderate the Interaction.” Behavioral Ecology and Sociobiology 74: 1–9. 10.1007/s00265-020-02831-2. [DOI] [Google Scholar]
- Hebblewhite, M. , White C. A., Nietvelt C. G., et al. 2005. “Human Activity Mediates a Trophic Cascade Caused by Wolves.” Ecology 86: 2135–2144. 10.1890/04-1269. [DOI] [Google Scholar]
- Ho, C. , Marzluff J. M., Stahler D. R., et al. 2023. “Scavengers Use Natural and Anthropogenic Resources Connecting Protected Areas With Surrounding Lands.” Frontiers in Bird Science 2: 1119507. 10.3389/fbirs.2023.1119507. [DOI] [Google Scholar]
- Holling, C. S. 1966. “The Functional Response of Invertebrate Predators to Prey Density.” Memoirs of the Entomological Society of Canada 98: 5–86. [Google Scholar]
- Jain, V. , Bugnyar T., Cunningham S. J., Gallego‐Abenza M., Loretto M. C., and Sumasgutner P.. 2022. “The Spatial and Temporal Exploitation of Anthropogenic Food Sources by Common Ravens (Corvus corax) in the Alps.” Movement Ecology 10: 1–15. 10.1186/s40462-022-00335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeiro‐Otero, A. , Newsome T. M., Van Eeden L. M., Ripple W. J., and Dormann C. F.. 2020. “Grey Wolf (Canis lupus) Predation on Livestock in Relation to Prey Availability.” Biological Conservation 243: 108433. 10.1016/j.biocon.2020.108433. [DOI] [Google Scholar]
- Jędrzejewski, W. , Niedziałkowska M., Hayward M. W., et al. 2012. “Prey Choice and Diet of Wolves Related to Ungulate Communities and Wolf Subpopulations in Poland.” Journal of Mammalogy 93: 1480–1492. 10.1644/10-MAMM-A-132.1. [DOI] [Google Scholar]
- Jędrzejewski, W. , Schmidt K., Theuerkauf J., Jędrzejewska B., and Kowalczyk R.. 2007. “Territory Size of Wolves Canis lupus: Linking Local (Białowieża Primeval Forest, Poland) and Holarctic‐Scale Patterns.” Ecography 30: 66–76. 10.1111/j.0906-7590.2007.04826.x. [DOI] [Google Scholar]
- Jędrzejewski, W. , Schmidt K., Theuerkauf J., et al. 2002. “Kill Rates and Predation by Wolves on Ungulate Populations in Białowieża Primeval Forest (Poland).” Ecology 83: 1341–1356. 10.1890/0012-9658(2002)083[1341:KRAPBW]2.0.CO;2. [DOI] [Google Scholar]
- Kane, A. , Healy K., Guillerme T., Ruxton G. D., and Jackson A. L.. 2017. “A Recipe for Scavenging in Vertebrates–the Natural History of a Behaviour.” Ecography 40: 324–334. 10.1111/ecog.02817. [DOI] [Google Scholar]
- Ketterson, E. D. , and Nolan V. Jr. 1976. “Geographic Variation and Its Climatic Correlates in the Sex Ratio of Eastern‐Wintering Dark‐Eyed Juncos (Junco hyemalis hyemalis).” Ecology 57: 679–693. 10.2307/1936182. [DOI] [Google Scholar]
- Kittle, A. M. , Anderson M., Avgar T., et al. 2017. “Landscape‐Level Wolf Space Use is Correlated With Prey Abundance, Ease of Mobility, and the Distribution of Prey Habitat.” Ecosphere 8: e01783. 10.1002/ecs2.1783. [DOI] [Google Scholar]
- Klauder, K. J. , Borg B. L., Sivy K. J., and Prugh L. R.. 2021. “Gifts of an Enemy: Scavenging Dynamics in the Presence of Wolves (Canis lupus).” Journal of Mammalogy 102: 558–573. 10.1093/jmammal/gyab020. [DOI] [Google Scholar]
- König, H. J. , Kiffner C., Kramer‐Schadt S., Fürst C., Keuling O., and Ford A. T.. 2020. “Human–Wildlife Coexistence in a Changing World.” Conservation Biology 34: 786–794. 10.1111/cobi.13513. [DOI] [PubMed] [Google Scholar]
- Kuijper, D. P. , De Kleine C., Churski M., Van Hooft P., Bubnicki J., and Jędrzejewska B.. 2013. “Landscape of Fear in Europe: Wolves Affect Spatial Patterns of Ungulate Browsing in Białowieża Primeval Forest, Poland.” Ecography 36: 1263–1275. 10.1111/j.1600-0587.2013.00266.x. [DOI] [Google Scholar]
- Kuijper, D. P. J. , Churski M., Trouwborst A., et al. 2019. “Keep the Wolf From the Door: How to Conserve Wolves in Europe's Human‐Dominated Landscapes?” Biological Conservation 235: 102–111. 10.1016/j.biocon.2019.04.004. [DOI] [Google Scholar]
- Kuijper, D. P. J. , Diserens T. A., Say‐Sallaz E., et al. 2024. “Wolves Recolonize Novel Ecosystems Leading to Novel Interactions.” Journal of Applied Ecology 61: 906–921. 10.1111/1365-2664.14602. [DOI] [Google Scholar]
- Lanszki, J. , Márkus M., Újváry D., Szabó Á., and Szemethy L.. 2012. “Diet of Wolves Canis lupus Returning to Hungary.” Acta Theriologica 57: 189–193. 10.1007/s13364-011-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laundré, J. W. , Hernández L., and Altendorf K. B.. 2001. “Wolves, Elk, and Bison: Reestablishing the “Landscape of Fear” in Yellowstone National Park, USA.” Canadian Journal of Zoology 79: 1401–1409. 10.1139/z01-094. [DOI] [Google Scholar]
- Le Bagousse‐Pinguet, Y. , Gross E. M., and Straile D.. 2012. “Release From Competition and Protection Determine the Outcome of Plant Interactions Along a Grazing Gradient.” Oikos 121: 95–101. 10.1111/j.1600-0706.2011.19778.x. [DOI] [Google Scholar]
- Lesmerises, F. , Dussault C., and St‐Laurent M. H.. 2012. “Wolf Habitat Selection Is Shaped by Human Activities in a Highly Managed Boreal Forest.” Forest Ecology and Management 276: 125–131. 10.1016/j.foreco.2012.03.025. [DOI] [Google Scholar]
- Levi, T. , and Wilmers C. C.. 2012. “Wolves–Coyotes–Foxes: A Cascade Among Carnivores.” Ecology 93: 921–929. 10.1890/11-0165.1. [DOI] [PubMed] [Google Scholar]
- Libois, R. , Schockert V., Lambinet C., et al. 2019. “Trophic Niche of Three Carnivores in Southern Belgium: Raccoon (Procyon lotor), European Badger (Meles meles) and Stone Marten (Martes foina).”
- Marshall, K. N. , Cooper D. J., and Hobbs N. T.. 2014. “Interactions Among Herbivory, Climate, Topography and Plant Age Shape Riparian Willow Dynamics in Northern Yellowstone National Park, USA.” Journal of Ecology 102: 667–677. 10.1111/1365-2745.12225. [DOI] [Google Scholar]
- Massei, G. 1995. “Feeding Ecology, Home Range and Habitat Use by the Wild Boar in a Mediterranean Coastal Area (Central Italy).” Doctoral dissertation, University of Aberdeen.
- Massei, G. , Kindberg J., Licoppe A., et al. 2015. “Wild Boar Populations Up, Numbers of Hunters Down? A Review of Trends and Implications for Europe.” Pest Management Science 71: 492–500. 10.1002/ps.3965. [DOI] [PubMed] [Google Scholar]
- Mateo‐Tomás, P. , Olea P. P., Moleón M., et al. 2015. “From Regional to Global Patterns in Vertebrate Scavenger Communities Subsidized by Big Game Hunting.” Diversity and Distributions 21: 913–924. 10.1111/ddi.12330. [DOI] [Google Scholar]
- Mattioli, L. , Capitani C., Gazzola A., Scandura M., and Apollonio M.. 2011. “Prey Selection and Dietary Response by Wolves in a High‐Density Multi‐Species Ungulate Community.” European Journal of Wildlife Research 57: 909–922. 10.1007/s10344-011-0503-4. [DOI] [Google Scholar]
- Mech, L. D. , and Boitani L., eds. 2019. Wolves: Behavior, Ecology, and Conservation. Chicago, IL: University of Chicago Press. [Google Scholar]
- Meriggi, A. , and Lovari S.. 1996. “A Review of Wolf Predation in Southern Europe: Does the Wolf Prefer Wild Prey to Livestock?” Journal of Applied Ecology 33: 1561–1571. 10.2307/2404794. [DOI] [Google Scholar]
- Metz, M. C. , Vucetich J. A., Smith D. W., Stahler D. R., and Peterson R. O.. 2011. “Effect of Sociality and Season on Gray Wolf (Canis lupus) Foraging Behavior: Implications for Estimating Summer Kill Rate.” PLoS One 6: e17332. 10.1371/journal.pone.0017332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulka, O. , Zeman J., Drimaj J., et al. 2018. “The Importance of Natural Food in Wild Boar (Sus scrofa) Diet During Autumn and Winter.” Folia Zoologica 67: 165–172. 10.25225/fozo.v67.i3-4.a3.2018. [DOI] [Google Scholar]
- Moleón, M. , Sánchez‐Zapata J. A., Selva N., Donázar J. A., and Owen‐Smith N.. 2014. “Inter‐Specific Interactions Linking Predation and Scavenging in Terrestrial Vertebrate Assemblages.” Biological Reviews 89: 1042–1054. 10.1111/brv.12097. [DOI] [PubMed] [Google Scholar]
- Moleón, M. , Selva N., Quaggiotto M. M., Bailey D. M., Cortés‐Avizanda A., and DeVault T. L.. 2019. “Carrion Availability in Space and Time.” In Carrion Ecology and Management, 23–44. Cham: Springer International. 10.1007/978-3-030-16501-7_2. [DOI] [Google Scholar]
- Moore, G. H. , Littlejohn R. P., and Cowie G. M.. 1988. “Liveweights, Growth Rates, and Mortality of Farmed Red Deer at Invermay.” New Zealand Journal of Agricultural Research 31: 293–300. 10.1080/00288233.1988.10423418. [DOI] [Google Scholar]
- Mori, E. , Benatti L., Lovari S., and Ferretti F.. 2017. “What Does the Wild Boar Mean to the Wolf?” European Journal of Wildlife Research 63: 1–5. 10.1007/s10344-016-1060-7. [DOI] [Google Scholar]
- Mougi, A. , and Iwasa Y.. 2010. “Evolution Towards Oscillation or Stability in a Predator–Prey System.” Proceedings of the Royal Society B: Biological Sciences 277: 3163–3171. 10.1098/rspb.2010.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhly, T. B. , Hebblewhite M., Paton D., Pitt J. A., Boyce M. S., and Musiani M.. 2013. “Humans Strengthen Bottom‐Up Effects and Weaken Trophic Cascades in a Terrestrial Food Web.” PLoS One 8: e64311. 10.1371/journal.pone.0064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, D. W. , Gaillard J. M., Bingaman Lackey L., Hatt J. M., and Clauss M.. 2010. “Comparing Life Expectancy of Three Deer Species Between Captive and Wild Populations.” European Journal of Wildlife Research 56: 205–208. 10.1007/s10344-009-0342-8. [DOI] [Google Scholar]
- Mulley, R. 2002. “The Feed Requirements of Adult Red Deer.” NZGA: Research and Practice Series 9: 51–55. [Google Scholar]
- Nagy, K. 2021. “Food Requirements of Wild Animals: Predictive Equations for Free‐Living Mammals, Reptiles, and Birds.” Nutrition Abstracts and Reviews 71: 21R–31R. [Google Scholar]
- Newsome, T. , Cairncross R., Cunningham C. X., et al. 2023. “Scavenging With Invasive Species.” Biological Reviews 99: 562–581. 10.1111/brv.13035. [DOI] [PubMed] [Google Scholar]
- Newsome, T. M. , Barton B., Buck J. C., et al. 2021. “Monitoring the Dead as an Ecosystem Indicator.” Ecology and Evolution 11: 5844–5856. 10.1002/ece3.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nores, C. , Llaneza L., and Álvarez Á.. 2008. “Wild Boar Sus scrofa Mortality by Hunting and Wolf Canis lupus Predation: An Example in Northern Spain.” Wildlife Biology 14: 44–51. 10.2981/0909-6396(2008)14[44:WBSSMB]2.0.CO;2. [DOI] [Google Scholar]
- Nowak, S. , Mysłajek R. W., Szewczyk M., Tomczak P., Borowik T., and Jędrzejewska B.. 2017. “Sedentary But Not Dispersing Wolves Canis lupus Recolonizing Western Poland (2001–2016) Conform to the Predictions of a Habitat Suitability Model.” Diversity and Distributions 23: 1353–1364. 10.1111/ddi.12621. [DOI] [Google Scholar]
- O'Malley, C. , Elbroch L. M., Lendrum P. E., and Quigley H.. 2018. “Motion‐Triggered Video Cameras Reveal Spatial and Temporal Patterns of Red Fox Foraging on Carrion Provided by Mountain Lions.” PeerJ 6: e5324. 10.7717/peerj.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakosta, M. , Kitikidou K., Bakaloudis D., and Vlachos C.. 2014. “Dietary Variation of the Stone Marten (Martes foina): A Meta‐Analysis Approach.” Wildlife Biology in Practice 10: 85–101. 10.2461/wbp.2014.10.11. [DOI] [Google Scholar]
- Pereira, L. M. , Owen‐Smith N., and Moleón M.. 2014. “Facultative Predation and Scavenging by Mammalian Carnivores: Seasonal, Regional and Intra‐Guild Comparisons.” Mammal Review 44, no. 1: 44–55. 10.1111/mam.12005. [DOI] [Google Scholar]
- Petroelje, T. R. , Belant J. L., Beyer D. E. Jr., and Svoboda N. J.. 2019. “Subsidies From Anthropogenic Resources Alter Diet, Activity, and Ranging Behavior of an Apex Predator (Canis lupus).” Scientific Reports 9: 13438. 10.1038/s41598-019-49879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, L. 1983. “Automatic Selection of Methods for Solving Stiff and Nonstiff Systems of Ordinary Differential Equations.” SIAM Journal on Scientific and Statistical Computing 4: 136–148. 10.1137/0904010. [DOI] [Google Scholar]
- Planillo, A. , Wenzler‐Meya M., Reinhardt I., et al. 2023. “Understanding Habitat Selection of Range‐Expanding Populations of Large Carnivores: 20 Years of Grey Wolves (Canis lupus) Recolonizing Germany.” Diversity and Distributions 30: 71–86. 10.1111/ddi.13789. [DOI] [Google Scholar]
- R Core Team . 2023. “R: A Language and Environment for Statistical Computing.” R Foundation for Statistical Computing. https://www.R‐project.org/.
- Ramirez, J. I. , Kuijper D. P., Olofsson J., et al. 2024. “Applied Ecology of Fear: A Meta‐Analysis on the Potential of Facilitating Human‐Wildlife Coexistence Through Nonlethal Tools.” Ecological Solutions and Evidence 5: e12322. 10.1002/2688-8319.12322. [DOI] [Google Scholar]
- Reinhardt, I. , Kluth G., Nowak C., et al. 2019. “Military Training Areas Facilitate the Recolonization of Wolves in Germany.” Conservation Letters 12: e12635. 10.1111/conl.12635. [DOI] [Google Scholar]
- Ripple, W. J. , and Beschta R. L.. 2004. “Wolves and the Ecology of Fear: Can Predation Risk Structure Ecosystems?” Bioscience 54: 755–766. 10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2. [DOI] [Google Scholar]
- Ripple, W. J. , and Beschta R. L.. 2012. “Trophic Cascades in Yellowstone: The First 15 Years After Wolf Reintroduction.” Biological Conservation 145: 205–213. 10.1016/j.biocon2011.11.005. [DOI] [Google Scholar]
- Ripple, W. J. , Beschta R. L., Fortin J. K., and Robbins C. T.. 2015. “Wolves Trigger a Trophic Cascade to Berries as Alternative Food for Grizzly Bears.” Journal of Animal Ecology 84: 652–654. 10.1111/1365-2656.12339. [DOI] [PubMed] [Google Scholar]
- Rooney, E. , and Montgomery W. I.. 2013. “Diet Diversity of the Common Buzzard (Buteo buteo) in a Vole‐Less Environment.” Bird Study 60: 147–155. 10.1080/00063657.2013.772085. [DOI] [Google Scholar]
- Sá, A. G. A. , Moreno Y. M. F., and Carciofi B. A. M.. 2020. “Food Processing for the Improvement of Plant Proteins Digestibility.” Critical Reviews in Food Science and Nutrition 60: 3367–3386. 10.1080/10408398.2019.1688249. [DOI] [PubMed] [Google Scholar]
- Sand, H. , Eklund A., Zimmermann B., Wikenros C., and Wabakken P.. 2016. “Prey Selection of Scandinavian Wolves: Single Large or Several Small?” PLoS One 11: e0168062. 10.1371/journal.pone.0168062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand, H. , Vucetich J. A., Zimmermann B., et al. 2012. “Assessing the Influence of Prey–Predator Ratio, Prey Age Structure and Packs Size on Wolf Kill Rates.” Oikos 121: 1454–1463. 10.1111/j.1600-0706.2012.20082.x. [DOI] [Google Scholar]
- Sandom, C. J. , Hughes J., and Macdonald D. W.. 2013. “Rewilding the Scottish Highlands: Do Wild Boar, Sus scrofa, Use a Suitable Foraging Strategy to Be Effective Ecosystem Engineers?” Restoration Ecology 21: 336–343. 10.1111/j.1526-100X.2012.00903.x. [DOI] [Google Scholar]
- Selva, N. 2004. “The Role of Scavenging in the Predator Community of Białowieża Primeval Forest.” Ph.D. dissertation, Polish Academy of Sciences. Warsaw, Poland.
- Selva, N. , and Fortuna M. A.. 2007. “The Nested Structure of a Scavenger Community.” Proceedings of the Royal Society B: Biological Sciences 274: 1101–1108. 10.1098/rspb.2006.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva, N. , Jędrzejewska B., Jędrzejewski W., and Wajrak A.. 2005. “Factors Affecting Carcass Use by a Guild of Scavengers in European Temperate Woodland.” Canadian Journal of Zoology 83: 1590–1601. 10.1139/z05-158. [DOI] [Google Scholar]
- Sidorovich, V. E. , Stolyarov V. P., Vorobei N. N., Ivanova N. V., and Jędrzejewska B.. 2007. “Litter Size, Sex Ratio, and Age Structure of Gray Wolves, Canis lupus, in Relation to Population Fluctuations in Northern Belarus.” Canadian Journal of Zoology 85: 295–300. 10.1139/Z07-001. [DOI] [Google Scholar]
- Sidorovich, V. E. , Tikhomirova L. L., and Jędrzejewska B.. 2003. “Wolf Canis lupus Numbers, Diet and Damage to Livestock in Relation to Hunting and Ungulate Abundance in Northeastern Belarus During 1990–2000.” Wildlife Biology 9: 103–111. 10.2981/wlb.2003.032. [DOI] [Google Scholar]
- Skalski, G. T. , and Gilliam J. F.. 2001. “Functional Responses With Predator Interference: Viable Alternatives to the Holling Type II Model.” Ecology 82: 3083–3092. 10.1890/0012-9658(2001)082[3083:FRWPIV]2.0.CO;2. [DOI] [Google Scholar]
- Smietana, W. , and Klimek A.. 1993. “Diet of Wolves in the Bieszczady Mountains, Poland.” Acta Theriologica 38: 245–251. [Google Scholar]
- Soetaert, K. , Petzoldt T., and Setzer R. W.. 2010. “Solving Differential Equations in R: Package deSolve.” Journal of Statistical Software 33: 1–25. 10.18637/jss.v033.i09.20808728 [DOI] [Google Scholar]
- Špinkytė‐Bačkaitienė, R. , and Pėtelis K.. 2012. “Diet Composition of Wolves (Canis lupus L.) in Lithuania.” Acta Biologica Universitatis Daugavpiliensis 12: 100–105. [Google Scholar]
- Spitzer, R. , Felton A., Landman M., Singh N. J., Widemo F., and Cromsigt J. P.. 2020. “Fifty Years of European Ungulate Dietary Studies: A Synthesis.” Oikos 129: 1668–1680. 10.1111/oik.07435. [DOI] [Google Scholar]
- Stahler, D. , Heinrich B., and Smith D.. 2002. “Common Ravens, Corvus corax, Preferentially Associate With Grey Wolves, Canis lupus, as a Foraging Strategy in Winter.” Animal Behaviour 64: 283–290. 10.1006/anbe.2002.3047. [DOI] [Google Scholar]
- Storie, J. T. , and Bell S.. 2017. “Wildlife Management Conflicts in Rural Communities: A Case‐Study of Wild Boar (Sus scrofa) Management in Ērgļu Novads, Latvia.” Sociologia Ruralis 57: 64–86. 10.1111/soru.12122. [DOI] [Google Scholar]
- Thurfjell, H. , Spong G., and Ericsson G.. 2013. “Effects of Hunting on Wild Boar Sus scrofa Behaviour.” Wildlife Biology 19: 87–93. 10.2981/12-027. [DOI] [Google Scholar]
- Treyer, D. , Linderoth P., Liebl T., Pegel M., Weiler U., and Claus R.. 2012. “Influence of Sex, Age and Season on Body Weight, Energy Intake and Endocrine Parameter in Wild Living Wild Boars in Southern Germany.” European Journal of Wildlife Research 58: 373–378. 10.1007/s10344-011-0557-3. [DOI] [Google Scholar]
- Trouwborst, A. , and Fleurke F. M.. 2019. “Killing Wolves Legally: Exploring the Scope for Lethal Wolf Management Under European Nature Conservation Law.” Journal of International Wildlife Law & Policy 22: 231–273. 10.1080/13880292.2019.1686223. [DOI] [Google Scholar]
- Turchin, P. , and Batzli G. O.. 2001. “Availability of Food and the Population Dynamics of Arvicoline Rodents.” Ecology 82: 1521–1534. 10.1890/0012-9658(2001)082[1521:AOFATP]2.0.CO;2. [DOI] [Google Scholar]
- van Moorter, B. , Singh N. J., Rolandsen C. M., et al. 2021. “Seasonal Release From Competition Explains Partial Migration in European Moose.” Oikos 130: 1548–1561. [Google Scholar]
- Vandersteen, J. , Fust C., Crowther M. S., et al. 2023. “Carcass Use by Mesoscavengers Drives Seasonal Shifts in Australian Alpine Scavenging Dynamics.” Wildlife Research 50: 1031–1045. 10.1071/WR22100. [DOI] [Google Scholar]
- Vucetich, J. A. , Vucetich L. M., and Peterson R. O.. 2012. “The Causes and Consequences of Partial Prey Consumption by Wolves Preying on Moose.” Behavioral Ecology and Sociobiology 66: 295–303. 10.1007/s00265-011-1277-0. [DOI] [Google Scholar]
- Walker, L. E. , Marzluff J. M., Metz M. C., et al. 2018. “Population Responses of Common Ravens to Reintroduced Grey Wolves.” Ecology and Evolution 8: 11158–11168. 10.1002/ece3.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangersky, P. J. , and Cunningham W. J.. 1957. “Time Lag in Prey‐Predator Population Models.” Ecology 38: 136–139. 10.2307/1932137. [DOI] [Google Scholar]
- Wenting, E. , Jansen P. A., Laugeman M. J., and van Langevelde F.. 2023. “Leakage of Nutrients Into the Soil Due to Carrion Decomposition Can Enhance Plant Growth.” Journal of Soil Science and Plant Nutrition 23: 6874–6879. 10.1007/s42729-023-01430-0. [DOI] [Google Scholar]
- Wenting, E. , Jansen P. A., Pattipeilohy L., van Lunteren P., Siepel H., and van Langevelde F.. 2024. “Influence of Tree Cover on Carcass Detection and Consumption by Facultative Vertebrate Scavengers.” Ecology and Evolution 14: e10935. 10.1002/ece3.10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenting, E. , Rinzema S. C., and van Langevelde F.. 2022. “Functional Differences in Scavenger Communities and the Speed of Carcass Decomposition.” Ecology and Evolution 12: e8576. 10.1002/ece3.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag. [Google Scholar]
- Wikenros, C. , Sand H., Ahlqvist P., and Liberg O.. 2013. “Biomass Flow and Scavengers Use of Carcasses After Re‐Colonization of an Apex Predator.” PLoS One 8: e77373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenros, C. , Ståhlberg S., and Sand H.. 2014. “Feeding Under High Risk of Intraguild Predation: Vigilance Patterns of Two Medium‐Sized Generalist Predators.” Journal of Mammalogy 95: 862–870. 10.1644/13-MAMM-A-125. [DOI] [Google Scholar]
- Williams, S. T. , Williams K. S., Lewis B. P., and Hill R. A.. 2017. “Population Dynamics and Threats to an Apex Predator Outside Protected Areas: Implications for Carnivore Management.” Royal Society Open Science 4: 161090. 10.1098/rsos.161090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmers, C. C. , Crabtree R. L., Smith D. W., Murphy K. M., and Getz W. M.. 2003. “Trophic Facilitation by Introduced Top Predators: Grey Wolf Subsidies to Scavengers in Yellowstone National Park.” Journal of Animal Ecology 72: 909–916. 10.1046/j.1365-2656.2003.00766.x. [DOI] [Google Scholar]
- Wilson, E. E. , and Wolkovich E. M.. 2011. “Scavenging: How Carnivores and Carrion Structure Communities.” Trends in Ecology & Evolution 26: 129–135. 10.1016/j.tree.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Wirsing, A. J. , and Newsome T. M.. 2021. “Scavenging Effects of Large Canids.” Integrative and Comparative Biology 61: 117–131. 10.1093/icb/icab012. [DOI] [PubMed] [Google Scholar]
- Zabihi‐Seissan, S. , Prokopenko C. M., and Vander Wal E.. 2022. “Wolf Spatial Behavior Promotes Encounters and Kills of Abundant Prey.” Oecologia 200: 11–22. 10.1007/s00442-022-05218-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Appendix S3.
Data Availability Statement
The complete R script of the ODE model, including all sensitivity analyses to produce the manuscript figures, is available via: https://doi.org/10.4121/a5a040e7‐de45‐4d60‐9ac4‐eec4e826aa85.


