Abstract
Thoracoabdominal aortic aneurysms (TAAAs) are complex and if untreated have high mortality and morbidity rates. Open surgical repair is the historical treatment approach; however, postoperative complications remain high with spinal cord ischemia notably one of the more serious and common complications. The avoidance of thoracotomy or laparotomy with the advent of endovascular aortic repair (EVAR) and thoracic endovascular aortic repair (TEVAR) have decreased the morbidity and mortality with TAAA repair, especially in patients with significant comorbidities such as a history of aortic surgery, underlying cardiac disease, and chronic obstructive pulmonary disease. Endovascular treatment options have grown to include fenestrated EVAR, multibranched EVAR, and physician-modified fenestration stent grafts. These techniques have achieved lower mortality rates than traditional open repair, but complications such as limb ischemia, spinal cord ischemia, and long-term durability must be considered. This review provides an overview of the most common endovascular techniques for TAAAs as well as short- and midterm outcomes.
Keywords: thoracoabdominal aortic aneurysms, endovascular repair, fenestrated EVAR, branched EVAR, physician-modified fenestrated stent graft
Aortic disease with the combined involvement of the thoracic and abdominal aorta is complex and ever-evolving. Consequently, management of both acute and chronic thoracoabdominal disease requires a fluid treatment plan with extensive planning and acknowledgment of the likelihood of multiple interventions to achieve an optimal outcome. 1 2 Although there is preservation of the integrity of all layers of the aorta, aneurysms remain of particular concern due to the increased wall tension and subsequent rupture risk at these increased vessel diameters. Aortic aneurysm rupture continues to carry a significant risk of morbidity and mortality in the United States with mortality of a ruptured abdominal aortic aneurysm (AAA) approaching 90%. 3 4
The gold standard of treatment of thoracoabdominal aortic aneurysms (TAAAs) has been open surgical repair. 5 Despite improvements in surgical techniques, spinal cord protection, and perioperative critical care support, mortality and perioperative complication rates remain high. 5 6 7 Spinal cord ischemia (SCI) is notably a morbid complication that requires attention perioperatively, and often remains unavoidable and permanent. 5 8 Other complications include mesenteric ischemia, limb ischemia, renal failure, pulmonary hemorrhage, and pneumonia. 5 8 9 Further increasing the surgical risk, patients requiring aortic intervention often have a history of prior aortic disease and/or surgery, cardiac disease, and lung disease. 5 9 Open TAAA surgical repair is highly invasive and extremely challenging, as it involves both the thoracic and abdominal cavities, reconstruction of visceral branches, and repair or reconstruction of the diseased aorta. 8 Even at high-volume aortic centers, 30-day mortality rates are estimated at 5 to 19%, which increases to 20 to 40% for emergency cases. 8 Early mortality rates depend on the location and extent of the TAAA and range from 2 to 21% at experienced centers depending on the Crawford classification of the underlying disease ( Table 1 ). 5 10 11 Resultantly, thoracic endovascular aortic repair (TEVAR) and endovascular aortic repair (EVAR) are being employed with increasing frequency as a less invasive treatment option for patients with TAAA.
Table 1. Early mortality rates of TAAA repair by Crawford classification 5 10 11 .
| Crawford types | Early mortality rate (%) |
|---|---|
| Type I | 5–8 |
| Type II | 8–13 |
| Type III | 8–21 |
| Type IV | 2–6 |
Abbreviation: TAAA, thoracoabdominal aortic aneurysm.
The purpose of this literature review is to provide brief overview of current options of endovascular repair of TAAAs as well as brief short- and midterm outcomes of such techniques.
Patient Selection and Imaging Evaluation
Indications for intervention on TAAAs include 12 13 :
(1) Rupture
(2) Acute dissection
(3) Persistent symptomatic state
(4) Rapid growth (> 1 cm/year)
(5) Absolute size criterion (descending aortic diameter with history of connective tissue disorder > 6.0 cm, and without history of connective tissue disorder > 6.5 cm)
In elective cases, a standardized, thorough workup for preoperative evaluation and risk factor management is crucial for optimizing early and late outcomes. This includes the use of computed tomographic angiography of the aorta and branch vessels with three-dimensional reconstruction. When possible, employment of advanced imaging processing software can allow the surgical team to develop a more precise operative plan. 14 15 True centerline measurements and multiplanar reconstructions that include evaluation of proximal and distal landing zones, degree of aortic tortuosity, relationship of aneurysm to arch and visceral branch vessels, and size and quality of access vessels, are helpful adjuncts in accurate evaluation and planning. 12 14 15
Aortic Aneurysm Assessment
The standardization of measurement of aortic aneurysm diameter is a crucial preoperative step before considering any endovascular approach. Various studies have looked at three-dimensional image postprocessing, which can provide detailed analyses of aortic morphology including methods such as volume rendering, double oblique multiplanar reformatting, and curved planar reformatting along central lumen lines. 14 In addition to the Crawford classification of TAAAs ( Table 2 ), several schemata have been developed to propose advanced aortic characterization, with the TEVAR protocol being one of the most complete schemata proposed to date. 14 16 The TEVAR protocol obtains measurements that divides the aorta in 12 zones and provides a consistent and reproducible interpretation of thoracic aortic aneurysms ( Fig. 1 ). In addition to measuring the diameter of the aorta at the centerline, the TEVAR protocol allows for estimation of irregularities and tortuosity based on the landmarks close to the aneurysm. 16 This protocol can be utilized to differentiate progressive disease such as penetrating aortic ulcer, intramural aortic hematoma, and dissection. 14 16 Recently, the International College of Angiology has proposed a newer, more comprehensive schema known as the International College of Angiology Aortic Research Schema (ICAARS). ICAARS combines the TEVAR protocol and four other schemata (Contrast Enhanced - Cardiovascular Magnetic Resonance Angiography (CE-CMRA) Protocol, Unified Protocol, Expanded Unified Protocol, Protocol by Agarwal et al) to yield a more thorough, compressive aortic assessment ( Table 3 ). 14 The ICAARS divides the aorta into thoracic and abdominal sections and subsequently into 14 segments, as well as describing the morphology of the aneurysm. 14 This is an effort to describe aortic aneurysmal morphology beyond the typical saccular or fusiform subtypes, and addresses the aortic sinus region, isthmus, and descending regions of the aorta, not included in the TEVAR protocol. 14
Table 2. Crawford classification of TAAA.
| C | |
|---|---|
| Crawford types | Description |
| Type I | Distal of the left subclavian artery to the visceral aorta (suprarenal) |
| Type II | Distal of the left subclavian artery to the infrarenal aorta |
| Type III | Midthoracic aorta (below T6) to the infrarenal aorta |
| Type IV | Diaphragm (T12) to the infrarenal aorta |
| Type V | Midthoracic aorta (below T6) to the visceral aorta (suprarenal) |
Abbreviation: TAAA, thoracoabdominal aortic aneurysm.
Fig. 1.
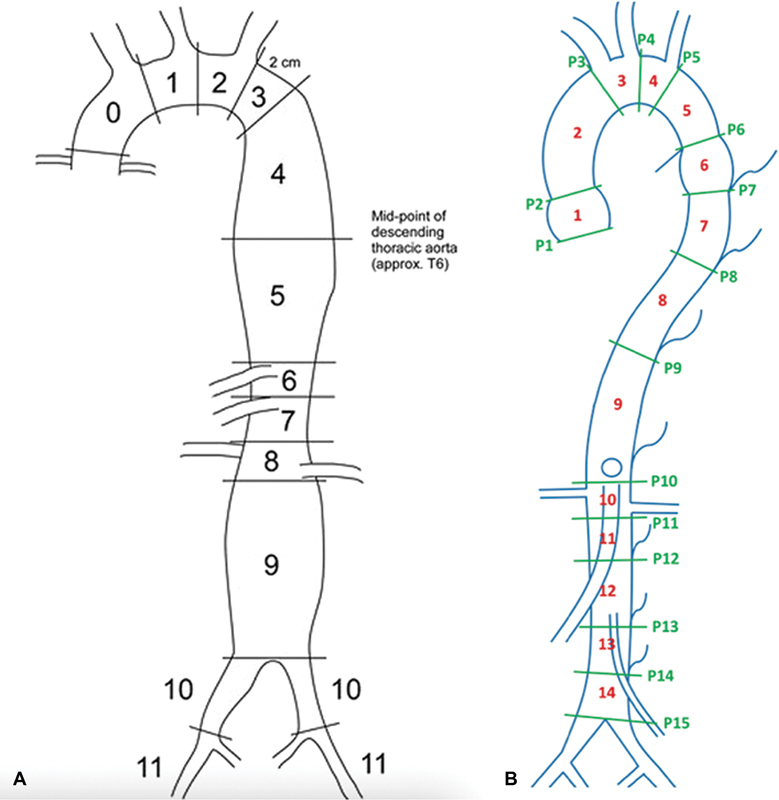
( A ) Twelve zones of aorta based on the thoracic endovascular aortic repair (TEVAR) protocol (1, 2); ( B ) 14 zones of aorta based on the International College of Angiology Aortic Research Schema (ICAARS) schema. 17
Table 3. Comparison of TEVAR and IARS protocols 17 .
| TEVAR vs. ICAARs protocol | |
|---|---|
| TEVAR | ICAARS |
|
Aorta divided into 12 zones:
Zone 0 : proximal edge of covered endograft Zone 1 : proximal transverse aorta Zone 2- distal transverse aorta Zone 3 : < 2 cm within the origin of the left subclavian artery without covering it Zone 4 : proximal end of endograft > 2 cm distal to left subclavian artery within proximal descending thoracic aorta Zone 5 : distal descending thoracic aorta but proximal to celiac artery Zone 6 : celiac segment and proximal juxtavisceral segment Zone 7 : SMA segment and juxtavisceral segment Zone 8 : covering at least 1 renal artery Zone 9 : infrarenal arteries Zone 10 : common iliac arteries Zone 11: external iliac arteries |
Aorta divided into 14 zones: Zone 1: aortic root to ascending aorta Zone 2: proximal transverse aorta Zone 3: distal transverse aorta Zone 4: distal to left common carotid to include left subclavian Zone 5: distal to left subclavian to spindle Zone 6: distal to spindle to proximal descending aorta Zone 7: proximal descending to distal descending Zone 8: distal descending aorta to juxtavisceral Zone 9: juxtavisceral including celiac artery but supra renal Zone 10: juxtarenal and SMA Zone 11: proximal infrarenal to mid infrarenal Zone 12: midinfrarenal to distal infrarenal including IMA Zone 13: distal infrarenal to suprailiac aorta Zone 14: suprailiac proximal to bifurcation |
| Measures diameter of aorta at centerline and estimation of irregularities and tortuosity | Allows for measurements of aneurysms that overlap to include thoracoabdominal aorta or includes continuation of abdominal aorta into its bifurcation into common iliac arteries |
| Utilized to determine candidates of revascularization through measurement of aneurysm pre- and postoperatively | Describes morphology of aneurysm (saccular, fusiform, juxtavisceral, apple-on-a-stick, aortoiliac) |
| Focus on aortic arch and lower sections of abdominal aorta | Focus includes sinus region, isthmus descending sections of aorta |
Abbreviations: ICAARS, International College of Angiology Aortic Research Schema; IMA, inferior mesenteric artery; SMA, superior mesenteric artery; TEVAR, thoracic endovascular aortic repair.
Endograft Sizing
After an aortic assessment approach is chosen, it is imperative that proximal and distal landing zones are thoroughly assessed and accurately measured. Most grafts for TAAAs require 10 to 20% oversizing of the seal zone diameter. 12 14 Given normal aortic anatomy, the distal landing zone is usually smaller in diameter than the proximal zone, which often necessitates either multiple grafts or employment of newer tapered grafts to adjust for the size discrepancy.
An additional consideration when sizing the endograft is the tendency of the stent to follow the greater curvature of the aorta and the natural tortuosity of the vessel. Although there is some degree of aortic straightening with stiffer endografts, this tendency to follow the greater curvature can lead to underestimation of the length of an endovascular graft and is important to consider to ensure adequate coverage. 12 Additionally, when planning the length of an endograft, it is recommended that a proximal and distal seal zone of 2 cm is employed whenever possible for aneurysmal disease. 14 17 If there is severe angulation, calcification, or thrombus, the seal zone length may need to be extended to ensure durable repair of the pathologic segment. Importantly, given patient limitations and clinical conditions, this seal zone should be individualized to each patient at the time of planning and deployment.
Current Endovascular Technology
Conventional endovascular devices are usually unsuitable for TAAAs because of inadequate landing zones and the risk of mesenteric and SCI with long stent graft deployment. In emergency situations, the sandwich technique with snorkel and/or chimney grafts can be used with increased risks of endoleak and of reduced blood flow from the multiple periscopes squeezed into the aortic lumen. 12 The endovascular options for preserving abdominal visceral branches include, fenestrated EVAR (FEVAR), multi-branched EVAR (BEVAR), and physician-modified fenestrated endograft (PMFG). PMFG and hybrid procedures such as surgical debranching and EVAR can be performed at the time of emergent operation, while custom-made FEVAR and BEVAR devices require time to procure and are not an option for emergency surgery. 5 12 Custom-made FEVAR and BEVAR grafts seem to be the ideal option for elective TAAA repair. 5
Fenestrated EVAR
Since the 1990s, FEVAR has been performed to secure the proximal landing zone for type Ia endoleaks (proximal seal failure). In 2001, successful FEVAR for pararenal AAA was reported and in 2005, FEVAR for TAAA was reported. 12 18 19 The most common type of fenestrated stent graft is composed of a Zenith platform, and the number of fenestrations is determined by the number of reconstructed branches (Cook Medical Inc., Bloomington, IN) ( Fig. 2 ).
Fig. 2.
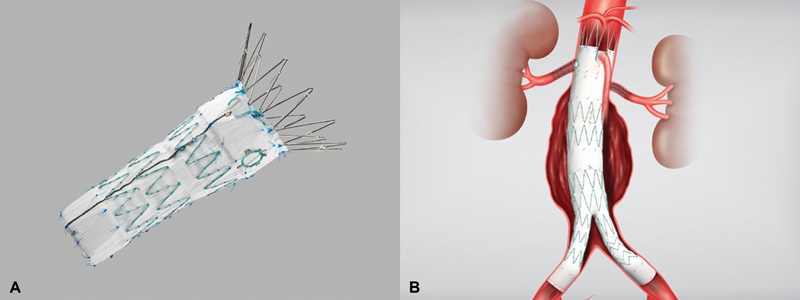
( A ) Fenestrated endovascular stent. ( B ) Fenestrated endovascular stent with bifurcation into common iliac vessels (Cook Medical Inc., Bloomington, IN).
Device design and sizing are crucial for a successful FEVAR and accurate intraoperative positioning of the fenestration to the accompanying visceral branches. In addition, the extent of stent graft coverage must be weighed against the risk of SCI; therefore, preoperative designing is necessary to obtain a seal zone that is not excessive or insufficient. There are several methods of preoperative planning, though methods that employ the aortic centerline approach are most common, especially in complex TAAAs. 20 The aortic centerline is calculated semiautomatically with an operator assessing whether the center line runs along the proper path, modifying as necessary.
Current outcomes of FEVAR in TAAAs, as seen in the WINDOWS trial, have demonstrated 30-day mortality at 1.4 to 7.8% with technical success achieved in 87 to 98% of cases. The rate of SCI has been reported to be 2 to 10% and the visceral vessel patency rate at 1 year was 90 to 98% with estimated overall survival at 2 years of 78 to 92%. Crawford type II TAAA was associated with higher mortality and longer hospitalization than Crawford type III and risk factors for poor long-term survival following TAAA treatment include age, chronic pulmonary obstructive disease, and Crawford type II TAAA. 21 22 23 24 25
FEVAR has been associated with type III endoleaks (defect or misalignment of endograft material), requiring secondary intervention more frequently compared with other EVAR types. This occurs likely due to the short junction between the main body of the stent graft and the bridging-covered stent for the visceral arteries. The rate of freedom from secondary intervention has been reported as 79 to 96.7% at 1 year and 63 to 88.0% at 3 years. 25 The main device is commonly inserted via the common femoral artery and the required number of sheaths for branched reconstruction is inserted in the contralateral common femoral artery; therefore, more common complications include limb ischemia and compartment syndrome secondary to prolonged lower extremity ischemia during device deployment. 1 5 26 Technical difficulties, especially in complex TAAAs, lead to longer operative times that can then lead to longer ischemic time of the lower extremities. Therefore, attention needs to be directed to this situation prompting development of techniques that can decrease and/or prevent this serious complication.
Endovascular Procedure of FEVAR
Femoral arteries are identified and are either bilaterally surgically exposed or percutaneously accessed. The devices are inserted via the femoral artery after systemic heparinization. Custom-made fenestrated stent grafts have radiopaque markers to denote the direction of the stent graft and the location of the fenestrations. The graft is deployed in the optimal position under fluoroscopic guidance. A guiding sheath is inserted from the contralateral femoral artery and cannulated into the main stent graft from its distal opening and then into each visceral branch through the fenestrations, using a guidewire and catheter. The stent graft is completely deployed, and covered stents are delivered into the visceral arteries via the guiding sheaths and deployed between the fenestrated stent graft and each visceral branch. 5
Multi-Branched EVAR
A multibranched TAAA stent graft became commercially available in 2008 and has rapidly gained popularity. 5 BEVAR strategies have allowed for creation of branched endografts, allowing management of aortic pathologies with increasing complexity. Accurate deployment positioning can be improved by performing fluoroscopic-guided aortography at an oblique projection of 40 to 60 degrees. 14 The t-Branch (Cook Medical Inc.), is the most commonly used multibranched stent graft and is designed with four directional sleeves for the celiac axis, superior mesenteric artery, and both renal arteries ( Fig. 3 ). It is estimated that just over 50% of TAAA population are potential candidates for the device in a single-stage procedure, and patient pool increases when considering staged procedures. 27 The t-Branch is designed with a smaller waist at the site of the main body visceral sleeve. The number of side branches, diameter of the side branches (6 or 8 mm), and the proximal and distal diameters can be modified by surgeons. One method is to use a combination of reinforced fenestrations and balloon-expandable covered stents. 12 28 The proximal end of the covered stent is then flared at the site of the reinforced fenestrations to create a gasket seal.
Fig. 3.
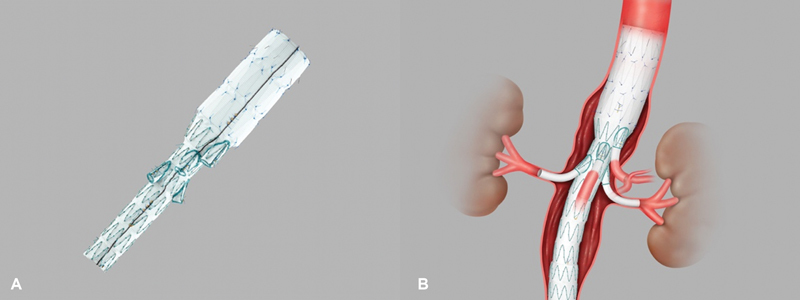
( A ) t-Branch multibranched endovascular stent (Cook Medical). ( B ) t-Branch multibranch stent with bilateral renal stent coverage (Cook Medical Inc., Bloomington, IN).
The BEVAR approach has certain advantages as compared with other endovascular approaches. Cuffed branches can achieve better seal and easier access to target vessels compared with fenestrations when the gap between the endograft wall and the ostium of the target vessel is > 10 mm. What is more, cuffed branches incorporating bridging stents are more flexible, accommodate more complex anatomical conditions, and reduce type III endoleaks arising from separation of the aortic and visceral components. 3 5 27
Current t-Branch clinical outcome reports include a 30-day mortality of 4.0 to 9.1%, with technical success achieved in 82 to 98.9%. The occurrence of SCI ranges from 3 to 35.7% and the visceral vessel patency rate at 1 year is 95 to 99%. 3 29 Overall estimated survival at 1 year is approximately 82 to 88%. 5 For TAAAs with larger diameters and challenging angles, complications including increased endoleak frequency and visceral stent mobility have been noted for fenestrated stent grafts. 5 12 Compared with FEVAR, type III endoleaks are unlikely to occur with the t-Branch device as the main device has structural sleeves extending to the visceral arteries providing a longer overlap zone between the bridging stent. 30 Branched stent grafts can provide better sealing and fixation using an overlapping segment in the branch rather than with a thin joint between a reinforced fenestration and mating visceral stent graft. 5 Studies have reported the rate of freedom from secondary intervention 1 year after t-Branch intervention at 79 to 100%. 3 5 30 What is more, the t-Branch device is associated with a lower risk of lower extremity ischemia and subsequent compartment syndrome because of the stent graft delivery sheath is removed following deployment of the main body and prior to sheath cannulation, a time-consuming step. Therefore, lower extremity ischemic time is shorter than that for the fenestrated device. 30
Addressing Spinal Cord Ischemia and Cerebral Infarction in BEVAR
It has been reported that the incidence of SCI is increased in TAAA repair using the t-Branch device in patients with risk factors including maximum short axis of > 65 mm, a coverage length of > 360 mm, and > 5 sacrificed intercostal arteries. 30 Therefore, SCI prevention techniques such as spinal cord drainage and staged surgery in which the main device is deployed first, and visceral stenting is deployed days to weeks after having been utilized. Staged repairs are more frequently utilized in patients who require endograft coverage from the aortic arch or proximal descending through the visceral segment of the aorta. Due to concerns for aneurysmal rupture during the interval between staged interventions, it is recommended that the side branch reconstruction be performed within 2-4 weeks, depending on the size of the TAAA. 31 In addition, cerebral infarction sometimes occurs during the t-Branch procedure most likely because of embolization secondary to the insertion of a long sheath and pull-through wire via the upper extremities. 30 Therefore, attention to the quality of the aortic arch during assessment of patient candidacy for a t-Branch device is crucial.
Endovascular Procedure of BEVAR (t-Branch)
The femoral artery is surgically exposed on only one side and a 4-Fr sheath is inserted percutaneously into the contralateral femoral artery. The left axillary artery is exposed surgically, and a 6-Fr sheath is placed. The stent graft is deployed but does not require the same precise deployment of the main body as that for the FEVAR since the branched sleeve is positioned 10 to 20 mm above the orifice of the visceral vessels and there is enough room for adjustment and cannulation. After main body deployment, the stent graft and delivery sheath are removed and the femoral arteriotomy site is closed, allowing for perfusing of the lower limb. A pull-through wire is inserted between the axillary sheath and the 4-Fr sheath in the contralateral femoral artery. The 6-Fr left axillary artery sheath is exchanged for a 10-Fr Ansel sheath (Cook Medical Inc.) and guided into the stent graft over the pull-through wire. Tension is applied to both ends of the pull-through wire allowing the Ansel sheath to tract around the acute aortic arch curvature. A covered stent such as Fluency (Bard Peripheral Vascular; Bard Inc., Tempe, AZ) or Viabahn (W.L Gore & Associates, Flagstaff, AZ) is deployed to each visceral branch. The bridging covered stent is lined with a self-expandable stent to prevent kinking. 5 30
Physician-Modified Fenestration Stent Graft
The physician-modified graft utilizes the Cook TX2 or Zenith (Cook Medical Inc.) devices. The device is unsheathed on a back table and reinforced fenestrations are created with Atrium SST PTFE (Atrium Medical Corp., Hudson, NH) and platinum coils. Permanent and temporary diameter-reducing ties are created, and once modifications are complete the device is reinserted into the delivery sheath. PMFGs have been reported in the management of complex aortic aneurysms—predominantly juxtarenal aneurysms—and as such, only a few limited series or individual cases have been reported. 32 The 30-day mortality is reported to range from 2 to 9% with technical success achieved in 88 to 98% of cases. 33 34 35 PMFGs were associated with less blood loss, less fluid requirements, and shorter total operative time than hybrid repairs. To highlight one study by Oderich et al, postoperative mortality rates were 3.3% in the PMFG group versus 19% in the hybrid group. 32
Conclusion
There remains minimal long-term data and regulatory approval for most endovascular procedures of TAAAs, and so conventional open surgical repair remains standard therapy for TAAAs in low-risk patients, despite unsatisfactory short- and long-term outcomes. The complications of FEVAR are noted to be serious and the incidence of BEVAR-related SCI is high. There is no completely reliable procedure or even schema in measuring and customizing grafts for each patient. It is imperative for the operating surgeon to understand and consider every treatment modality to select the best and safest treatment option for each patient. The continued development and increasing interest in endovascular repair of TAAA shows promising future insight on outcomes and the desire to standardize treatment approaches.
Footnotes
Conflict of Interest None declared.
References
- 1.Law Y, Tsilimparis N, Rohlffs F et al. Fenestrated or branched endovascular aortic repair for postdissection thoracoabdominal aortic aneurysm. J Vasc Surg. 2019;70(02):404–412. doi: 10.1016/j.jvs.2018.10.117. [DOI] [PubMed] [Google Scholar]
- 2.Huu A L, Green S Y, Coselli J S. Thoracoabdominal aortic aneurysm repair: from an era of revolution to an era of evolution. Semin Thorac Cardiovasc Surg. 2019;31(04):703–707. doi: 10.1053/j.semtcvs.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, Li Y, Peng R et al. Multibranched stent-grafts for the treatment of thoracoabdominal aortic aneurysms: a systematic review and meta-analysis. J Endovasc Ther. 2016;23(04):626–633. doi: 10.1177/1526602816647723. [DOI] [PubMed] [Google Scholar]
- 4.Crawford E S, DeNatale R W. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3(04):578–582. doi: 10.1067/mva.1986.avs0030578. [DOI] [PubMed] [Google Scholar]
- 5.Baba T, Ohki T, Maeda K. Current status of endovascular treatment for thoracoabdominal aortic aneurysms. Surg Today. 2020;50(11):1343–1352. doi: 10.1007/s00595-019-01917-3. [DOI] [PubMed] [Google Scholar]
- 6.Rigberg D A, McGory M L, Zingmond D Set al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience J Vasc Surg 20064302217–222., discussion 223 [DOI] [PubMed] [Google Scholar]
- 7.Coselli J S, Bozinovski J, LeMaire S A.Open surgical repair of 2286 thoracoabdominal aortic aneurysms Ann Thorac Surg 20078302S862–S864., discussion S890–S892 [DOI] [PubMed] [Google Scholar]
- 8.Coselli J S, LeMaire S A, Conklin L D, Köksoy C, Schmittling Z C.Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair Ann Thorac Surg 200273041107–1115., discussion 1115–1116 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Davis C A, III, Miller C C, IIIet al. Cardiac function predicts mortality following thoracoabdominal and descending thoracic aortic aneurysm repair Eur J Cardiothorac Surg 20032401119–124., discussion 124 [DOI] [PubMed] [Google Scholar]
- 10.Conrad M F, Crawford R S, Davison J K, Cambria R P.Thoracoabdominal aneurysm repair: a 20-year perspective Ann Thorac Surg 20078302S856–S861., discussion S890–S892 [DOI] [PubMed] [Google Scholar]
- 11.Patel V I, Ergul E, Conrad M F et al. Continued favorable results with open surgical repair of type IV thoracoabdominal aortic aneurysms. J Vasc Surg. 2011;53(06):1492–1498. doi: 10.1016/j.jvs.2011.01.070. [DOI] [PubMed] [Google Scholar]
- 12.Orr N, Minion D, Bobadilla J L. Thoracoabdominal aortic aneurysm repair: current endovascular perspectives. Vasc Health Risk Manag. 2014;10:493–505. doi: 10.2147/VHRM.S46452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elefteriades J A, Botta D M., JrIndications for the treatment of thoracic aortic aneurysms Surg Clin North Am 20098904845–867., ix [DOI] [PubMed] [Google Scholar]
- 14.Alslaim H, Sanampudi S, Raissi D et al. A comprehensive research schema for the characterization of aortic aneurysms. Int J Angiol. 2022;32(01):34–42. doi: 10.1055/s-0042-1744275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal P P, Chughtai A, Matzinger F R, Kazerooni E A. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29(02):537–552. doi: 10.1148/rg.292075080. [DOI] [PubMed] [Google Scholar]
- 16.Society for Vascular Surgery Ad Hoc Committee on TEVAR Reporting Standards Fillinger M F, Greenberg R K, McKinsey J F, Chaikof E L.Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg 201052041022–1033., 1033.e15 [DOI] [PubMed] [Google Scholar]
- 17.Adams J D, Garcia L M, Kern J A.Endovascular repair of the thoracic aorta Surg Clin North Am 20098904895–912., ix [DOI] [PubMed] [Google Scholar]
- 18.Stanley B M, Semmens J B, Lawrence-Brown M M, Goodman M A, Hartley D E. Fenestration in endovascular grafts for aortic aneurysm repair: new horizons for preserving blood flow in branch vessels. J Endovasc Ther. 2001;8(01):16–24. doi: 10.1177/152660280100800103. [DOI] [PubMed] [Google Scholar]
- 19.Anderson J L, Adam D J, Berce M, Hartley D E. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg. 2005;42(04):600–607. doi: 10.1016/j.jvs.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 20.Velazquez O C, Woo E Y, Carpenter J P, Golden M A, Barker C F, Fairman R M. Decreased use of iliac extensions and reduced graft junctions with software-assisted centerline measurements in selection of endograft components for endovascular aneurysm repair. J Vasc Surg. 2004;40(02):222–227. doi: 10.1016/j.jvs.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.WINDOWS trial participants . Marzelle J, Presles E, Becquemin J P. Results and factors affecting early outcome of fenestrated and/or branched stent grafts for aortic aneurysms: a multicenter prospective study. Ann Surg. 2015;261(01):197–206. doi: 10.1097/SLA.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 22.Budtz-Lilly J, Wanhainen A, Eriksson J, Mani K. Adapting to a total endovascular approach for complex aortic aneurysm repair: outcomes after fenestrated and branched endovascular aortic repair. J Vasc Surg. 2017;66(05):1349–1356. doi: 10.1016/j.jvs.2017.03.422. [DOI] [PubMed] [Google Scholar]
- 23.Verhoeven E L, Katsargyris A, Bekkema F et al. Editor's Choice - ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49(05):524–531. doi: 10.1016/j.ejvs.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Gallitto E, Gargiulo M, Freyrie A et al. Endovascular repair of thoracoabdominal aortic aneurysm in high-surgical risk patients: fenestrated and branched endografts. Ann Vasc Surg. 2017;40:170–177. doi: 10.1016/j.avsg.2016.07.096. [DOI] [PubMed] [Google Scholar]
- 25.Cochennec F, Kobeiter H, Gohel M S et al. Impact of intraoperative adverse events during branched and fenestrated aortic stent grafting on postoperative outcome. J Vasc Surg. 2014;60(03):571–578. doi: 10.1016/j.jvs.2014.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eagleton M J, Follansbee M, Wolski K, Mastracci T, Kuramochi Y. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg. 2016;63(04):930–942. doi: 10.1016/j.jvs.2015.10.095. [DOI] [PubMed] [Google Scholar]
- 27.Gasper W J, Reilly L M, Rapp J Het al. Assessing the anatomic applicability of the multibranched endovascular repair of thoracoabdominal aortic aneurysm technique J Vasc Surg 201357061553–1558., discussion 1558 [DOI] [PubMed] [Google Scholar]
- 28.Greenberg R, Eagleton M, Mastracci T.Branched endografts for thoracoabdominal aneurysms J Thorac Cardiovasc Surg 2010140(6, suppl):S171–S178. [DOI] [PubMed] [Google Scholar]
- 29.Gallitto E, Gargiulo M, Freyrie A et al. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66(03):696–7.04E7. doi: 10.1016/j.jvs.2016.12.129. [DOI] [PubMed] [Google Scholar]
- 30.Baba T, Ohki T, Kanaoka Y et al. Clinical outcomes of spinal cord ischemia after fenestrated and branched endovascular stent grafting during total endovascular aortic repair for thoracoabdominal aortic aneurysms. Ann Vasc Surg. 2017;44:146–157. doi: 10.1016/j.avsg.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Schurink G W, De Haan M W, Peppelenbosch A G, Mess W, Jacobs M J.Spinal cord function monitoring during endovascular treatment of thoracoabdominal aneurysms: implications for staged procedures J Cardiovasc Surg (Torino) 201354(1, suppl 1):117–124. [PubMed] [Google Scholar]
- 32.Oderich G S, Fatima J, Gloviczki P. Stent graft modification with mini-cuff reinforced fenestrations for urgent repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2011;54(05):1522–1526. doi: 10.1016/j.jvs.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Arnaoutakis D J, Cuneo M R, Arnaoutakis G J. Endovascular repair of a thoracoabdominal aortic aneurysm using a physician-modified four-vessel fenestrated endograft. Ann Cardiothorac Surg. 2022;11(01):65–67. doi: 10.21037/acs-2022-taes-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweet M P, Starnes B W, Tatum B. Endovascular treatment of thoracoabdominal aortic aneurysm using physician-modified endografts. J Vasc Surg. 2015;62(05):1160–1167. doi: 10.1016/j.jvs.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 35.Starnes B W. Physician-modified endovascular grafts for the treatment of elective, symptomatic, or ruptured juxtarenal aortic aneurysms. J Vasc Surg. 2012;56(03):601–607. doi: 10.1016/j.jvs.2012.02.011. [DOI] [PubMed] [Google Scholar]


