Abstract
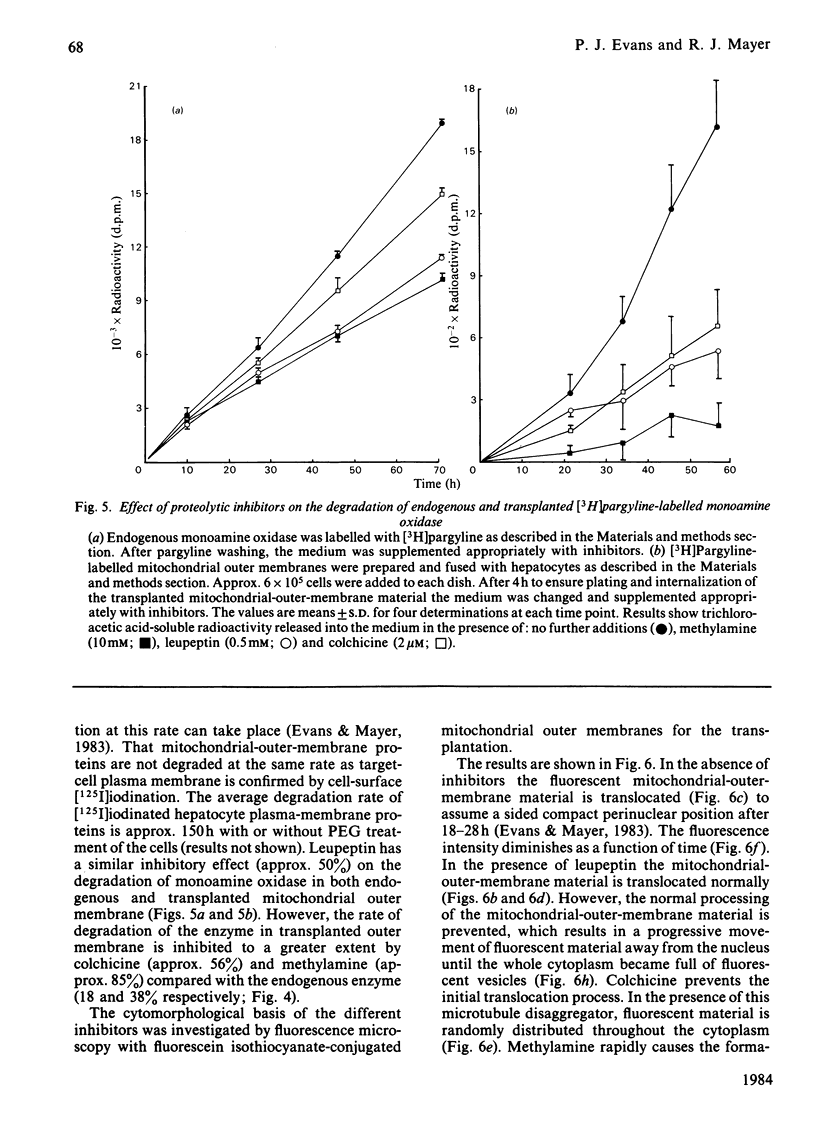
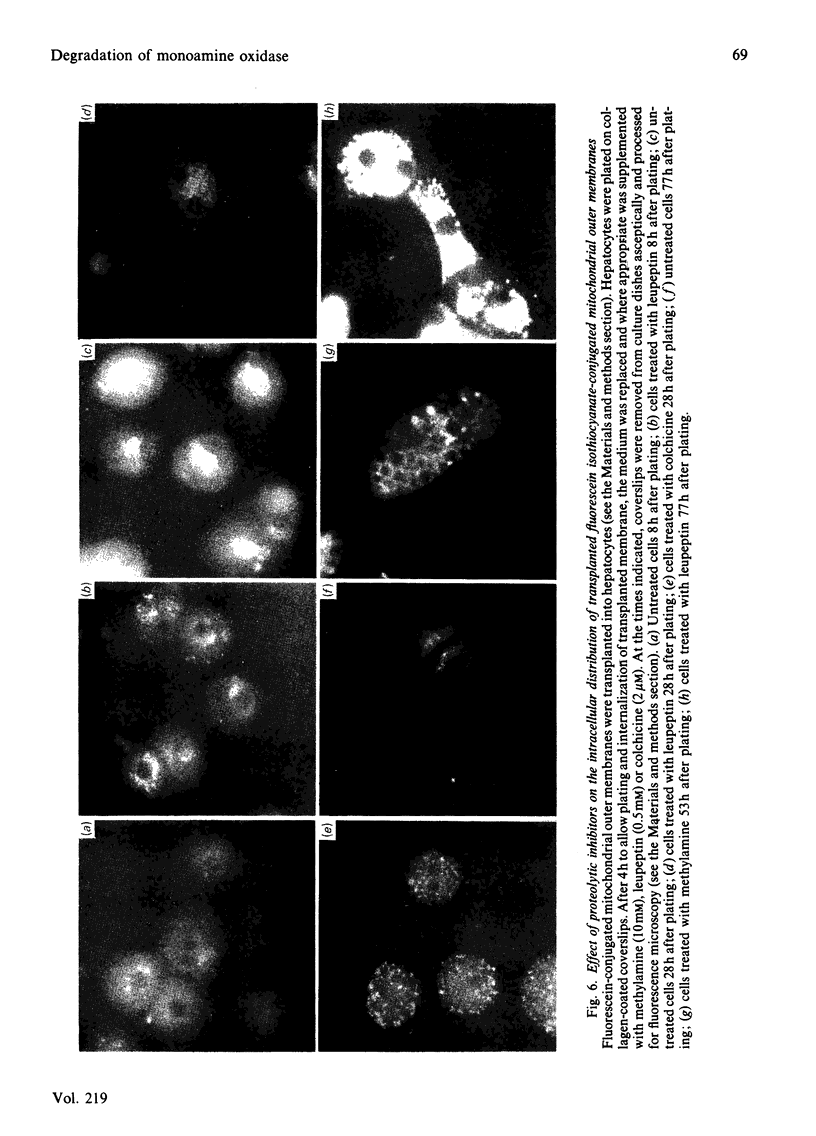
The degradative fate of monoamine oxidase in endogenous and transplanted mitochondrial outer membrane has been compared in rat hepatocyte monolayers. Monoamine oxidase was specifically irreversibly radiolabelled by the suicide inhibitor [3H]pargyline. Hepatocyte monolayers were cultured in conditions in which rates of protein catabolism like those in vivo are maintained [Evans & Mayer (1983) Biochem. J. 216, 151-161]. Incubation of hepatocyte monolayers for 17 h with [3H]pargyline specifically radiolabels mitochondrial monoamine oxidase, as shown by Percoll-gradient fractionation of broken hepatocytes. Monoamine oxidase is degraded at a similar rate to that observed in liver in vivo (t1/2 approx. 63 h). The effects of leupeptin, methylamine and colchicine on the degradation of endogenous radiolabelled enzyme has been studied over prolonged culture periods. Culture of hepatocytes for periods of up to 80 h with inhibitors was not cytotoxic, as demonstrated by measurements of several intrinsic biochemical parameters. Leupeptin, methylamine and colchicine inhibit the degradation of endogenous monoamine oxidase by 60, 38 and 18% respectively. Monoamine oxidase in mitochondrial-outer-membrane vesicles introduced into hepatocytes by poly(ethylene glycol)-mediated vesicle-cell transplantation is degraded at a similar rate (t1/2 55 h) to the endogenous mitochondrial enzyme. Whereas leupeptin inhibits the degradation of endogenous and transplanted enzyme to a similar extent, methylamine and colchicine inhibit the degradation of transplanted enzyme to a much greater extent (85 and 56% respectively). Fluorescence microscopy (with fluorescein isothiocyanate-conjugated mitochondrial outer membrane) shows that transplanted mitochondrial outer membrane undergoes internalization and translocation to a sided perinuclear site, as observed previously with whole mitochondria [Evans & Mayer (1983) Biochem. J. 216, 151-161]. The effects of the inhibitors on the distribution of transplanted membrane material in the cell and inhibition of proteolysis show the importance of cytomorphology for intracellular protein catabolism.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. H., Singer S. J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1982 Jan;79(1):123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Tolleshaug H. The effects of ammonium ions and chloroquine on uptake and degradation of 125I-labeled asialo-fetuin in isolated rat hepatocytes. Biochem Pharmacol. 1980 Mar 15;29(6):917–925. doi: 10.1016/0006-2952(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Bernier-Valentin F., Rousset B. Interaction of tubulin with rat liver mitochondria. J Biol Chem. 1982 Jun 25;257(12):7092–7099. [PubMed] [Google Scholar]
- Cawthon R. M., Breakefield X. O. Differences in the structures of monoamine oxidases A and B in rat clonal cell lines. Biochem Pharmacol. 1983 Feb 1;32(3):441–448. doi: 10.1016/0006-2952(83)90521-x. [DOI] [PubMed] [Google Scholar]
- Chuang H. Y., Patek D. R., Hellerman L. Mitochondrial monoamine oxidase. Inactivation by pargyline. Adduct formation. J Biol Chem. 1974 Apr 25;249(8):2381–2384. [PubMed] [Google Scholar]
- Clementi F., Sher E., Erroi A. Acetylcholine receptor degradation: study of mechanism of action of inhibitory drugs. Eur J Cell Biol. 1983 Jan;29(2):274–280. [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. Organelle-cytoskeleton relationships in fibroblasts: mitochondria, Golgi apparatus, and endoplasmic reticulum in phases of movement and growth. Eur J Cell Biol. 1982 Apr;27(1):47–54. [PubMed] [Google Scholar]
- Crie J. S., Ord J. M., Wakeland J. R., Wildenthal K. Inhibition of cardiac proteolysis by colchicine. Selective effects on degradation of protein subclasses. Biochem J. 1983 Jan 15;210(1):63–71. doi: 10.1042/bj2100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Rennke H. G., Cotran R. S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J Cell Sci. 1981 Jun;49:69–86. doi: 10.1242/jcs.49.1.69. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Mayer R. J. Degradation of transplanted mitochondrial proteins by hepatocyte monolayers. Biochem J. 1983 Oct 15;216(1):151–161. doi: 10.1042/bj2160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Mayer R. J. Organelle membrane-cell fusion: destruction of transplanted mitochondrial proteins in hepatocyte monolayers. Biochem Biophys Res Commun. 1982 Jul 16;107(1):51–58. doi: 10.1016/0006-291x(82)91668-0. [DOI] [PubMed] [Google Scholar]
- Evans P. J. The regulation of hepatic tyrosine aminotransferase. Biochim Biophys Acta. 1981 Nov 5;677(3-4):433–444. doi: 10.1016/0304-4165(81)90257-9. [DOI] [PubMed] [Google Scholar]
- Furuno K., Ishikawa T., Kato K. Appearance of autolysosomes in rat liver after leupeptin treatment. J Biochem. 1982 May;91(5):1485–1494. doi: 10.1093/oxfordjournals.jbchem.a133840. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Olsson R. A. A simple, specific, radioisotopic assay for 5'-nucleotidase. Anal Biochem. 1975 Apr;64(2):624–627. doi: 10.1016/0003-2697(75)90478-9. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Seglen P. O. Autophagic sequestration of [14C]sucrose, introduced into rat hepatocytes by reversible electro-permeabilization. Exp Cell Res. 1982 Nov;142(1):1–14. doi: 10.1016/0014-4827(82)90402-5. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami E., Hashida S., Khairallah E. A., Katunuma N. Sequestration of cytoplasmic enzymes in an autophagic vacuole-lysosomal system induced by injection of leupeptin. J Biol Chem. 1983 May 25;258(10):6093–6100. [PubMed] [Google Scholar]
- Kovács A. L., Reith A., Seglen P. O. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin or a lysosomotropic amine. Exp Cell Res. 1982 Jan;137(1):191–201. doi: 10.1016/0014-4827(82)90020-9. [DOI] [PubMed] [Google Scholar]
- McCauley R. 7(14C)pargyline binding to mitochondrial outer membranes. Biochem Pharmacol. 1976 Oct 1;25(19):2214–2216. doi: 10.1016/0006-2952(76)90136-2. [DOI] [PubMed] [Google Scholar]
- Nelson B. D. Hepatic lysosome and serum enzyme alterations in rats exposed to high altitude. Am J Physiol. 1966 Sep;211(3):651–655. doi: 10.1152/ajplegacy.1966.211.3.651. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Burgess R. J., Mayer R. J. Protein degradation in rat liver during post-natal development. Biochem J. 1980 Oct 15;192(1):321–330. doi: 10.1042/bj1920321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Davey J., Mayer R. J. The vectorial orientation of human monoamine oxidase in the mitochondrial outer membrane. Biochem J. 1979 Jul 1;181(1):7–14. doi: 10.1042/bj1810007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Mayer R. J. Degradation of transplanted rat liver mitochondrial-outer-membrane proteins in hepatoma cells. Biochem J. 1983 Oct 15;216(1):163–175. doi: 10.1042/bj2160163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim A. E., Seglen P. O. Cellular and lysosomal uptake of methylamine in isolated rat hepatocytes. Biochem J. 1983 Mar 15;210(3):929–936. doi: 10.1042/bj2100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth K., Hedin U., Thyberg J. Endocytosis, intracellular transport, and turnover of anionic and cationic proteins in cultured mouse peritoneal macrophages. Eur J Cell Biol. 1983 Jul;31(1):15–25. [PubMed] [Google Scholar]
- Summerhayes I. C., Wong D., Chen L. B. Effect of microtubules and intermediate filaments on mitochondrial distribution. J Cell Sci. 1983 May;61:87–105. doi: 10.1242/jcs.61.1.87. [DOI] [PubMed] [Google Scholar]
- Tweto J., Friedman E., Doyle D. Proteins of the hepatoma tissue culture cell plasma membrane. J Supramol Struct. 1976;4(2):141–159. doi: 10.1002/jss.400040202. [DOI] [PubMed] [Google Scholar]
- Warren R., Doyle D. Turnover of the surface proteins and the receptor for serum asialoglycoproteins in primary cultures of rat hepatocytes. J Biol Chem. 1981 Feb 10;256(3):1346–1355. [PubMed] [Google Scholar]
- Widnell C. C. Purification of rat liver 5'-nucleotidase as a complex with sphingomyelin. Methods Enzymol. 1974;32:368–374. doi: 10.1016/0076-6879(74)32037-x. [DOI] [PubMed] [Google Scholar]



