Abstract
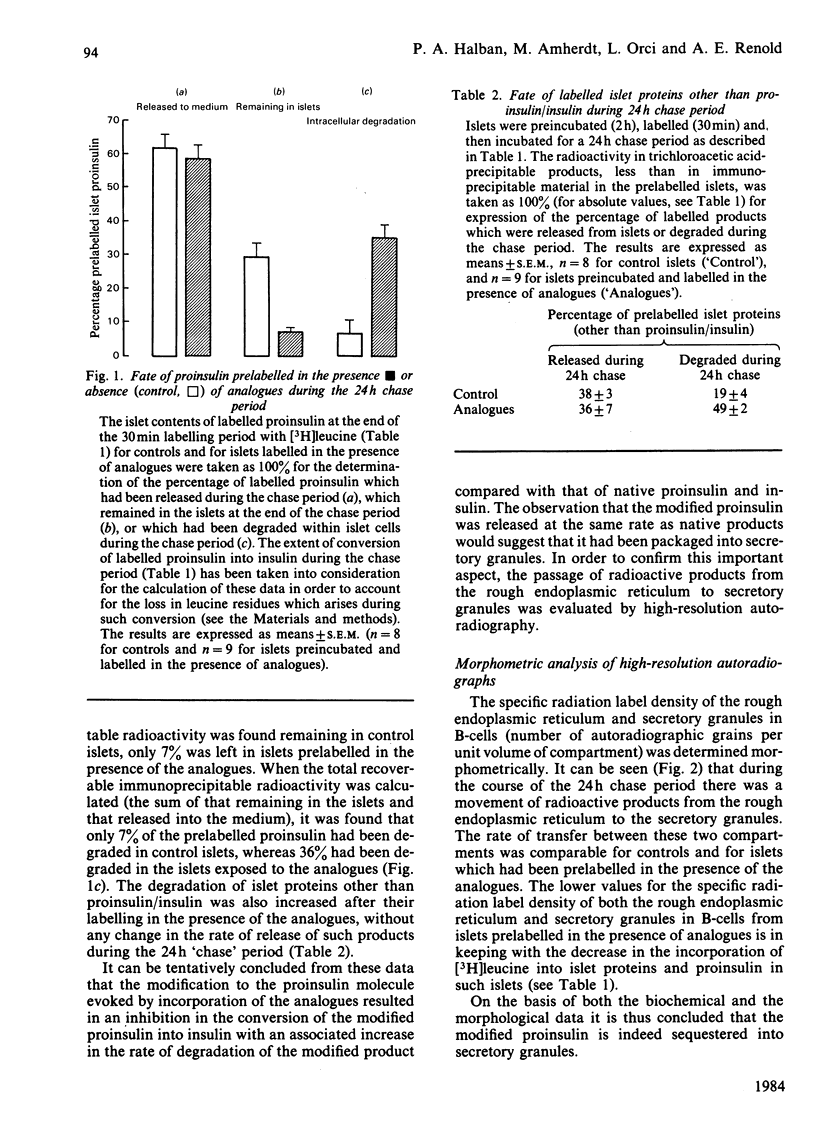
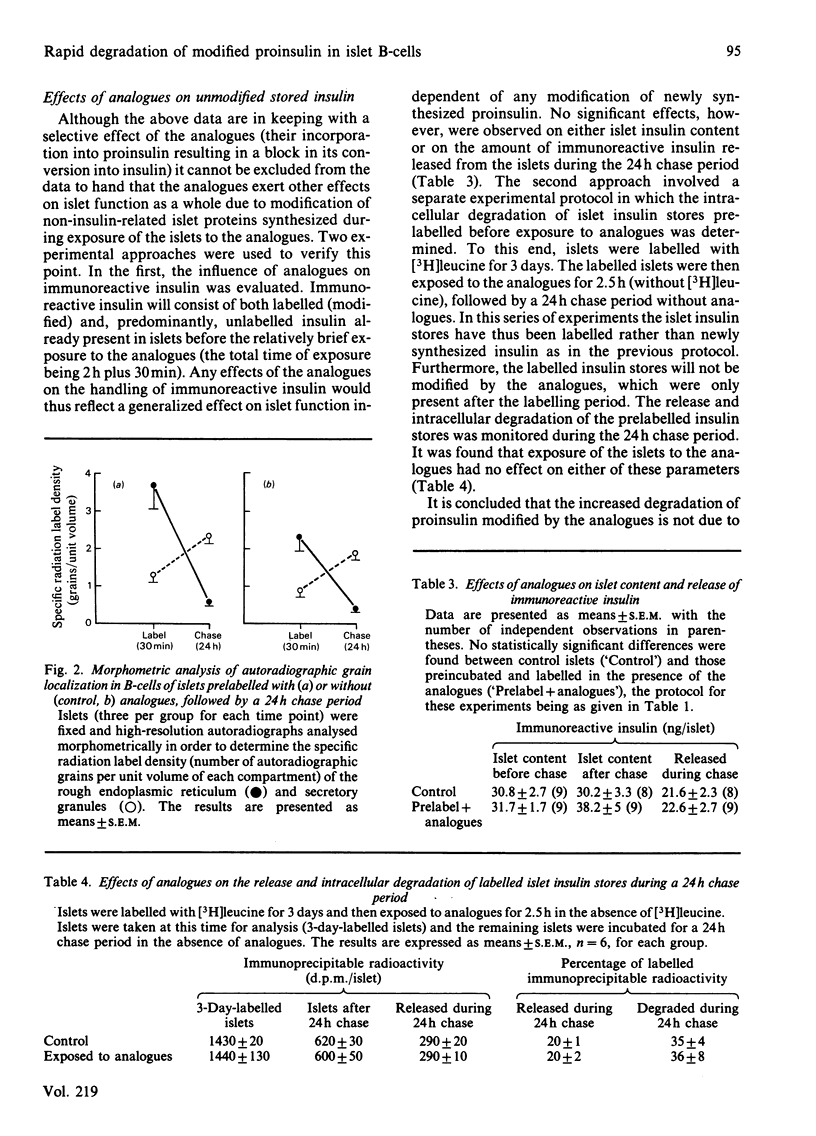
Modified cytosolic proteins are known to be degraded more rapidly than their native counterparts. In order to determine whether the same applies to a modified protein within the potentially protective environment of secretory granules, rat islets were labelled [( 3H]leucine) in the presence or absence (controls) of 3 mM-canavanine and 3 mM-thialysine (analogues of arginine and lysine respectively), followed by a 24h 'chase' period without analogues. The results showed the following. (1) Incorporation of the analogues into newly synthesized labelled proinsulin inhibited its conversion into insulin during the chase period. (2) Despite this block in conversion, the modified proinsulin was released from islets at the same rate as native proinsulin and insulin from control islets. (3) Morphometric analysis of high-resolution autoradiographs showed that products labelled in the presence of analogues were sequestered into secretory granules at the same rate as native products in control B-cells. (4) Only 7% of prelabelled proinsulin had been degraded within islet cells during the chase period in control islets, compared with 36% for proinsulin prelabelled in the presence of analogues. (5) Control experiments showed that the analogues had no effect on the release or intracellular degradation of unmodified stored insulin (present in islets before exposure to the analogues). (6) Despite sequestration into secretory granules, modified proinsulin, if not released from B-cells, is thus degraded more rapidly than native products.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski R. S. Intracellular degradation of newly synthesized secretory proteins. Biochem J. 1983 Jul 15;214(1):1–10. doi: 10.1042/bj2140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow B. S. The role of lysosomes and proteases in hormone secretion and degradation. Endocr Rev. 1981 Spring;2(2):137–173. doi: 10.1210/edrv-2-2-137. [DOI] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H., Bergenstal R. M., Wolff J., Mako M. E., Rubenstein A. H. Familial hyperproinsulinemia: partial characterization of circulating proinsulin-like material. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2881–2885. doi: 10.1073/pnas.76.6.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G., Gishizky M. L., Grodsky G. M. Evidence that glucose "marks" beta cells resulting in preferential release of newly synthesized insulin. Science. 1982 Oct 1;218(4567):56–58. doi: 10.1126/science.6181562. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Boches F. S. Oxidized proteins in erythrocytes are rapidly degraded by the adenosine triphosphate-dependent proteolytic system. Science. 1982 Feb 26;215(4536):1107–1109. doi: 10.1126/science.7038874. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Greider M. H., Howell S. L., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. II. Ultrastructure of the beta granule. J Cell Biol. 1969 Apr;41(1):162–166. doi: 10.1083/jcb.41.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A. Differential rates of release of newly synthesized and of stored insulin from pancreatic islets. Endocrinology. 1982 Apr;110(4):1183–1188. doi: 10.1210/endo-110-4-1183. [DOI] [PubMed] [Google Scholar]
- Halban P. A. Inhibition of proinsulin to insulin conversion in rat islets using arginine and lysine analogs. Lack of effect on rate of release of modified products. J Biol Chem. 1982 Nov 25;257(22):13177–13180. [PubMed] [Google Scholar]
- Halban P. A., Renold A. E. Influence of glucose on insulin handling by rat islets in culture. A reflection of integrated changes in insulin biosynthesis, release, and intracellular degradation. Diabetes. 1983 Mar;32(3):254–261. doi: 10.2337/diab.32.3.254. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B., Blondel B., Renold A. E. Long-term exposure of isolated pancreatic islets to mannoheptulose: evidence for insulin degradation in the beta cell. Biochem Pharmacol. 1980 Oct 1;29(19):2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980 Jul 10;255(13):6003–6006. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Klemes Y., Etlinger J. D., Goldberg A. L. Properties of abnormal proteins degraded rapidly in reticulocytes. Intracellular aggregation of the globin molecules prior to hydrolysis. J Biol Chem. 1981 Aug 25;256(16):8436–8444. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Gunn J. M., Hanson R. W., Ballard F. J. Increased degradation rates of protein synthesized in hepatoma cells in the presence of amino acid analogues. Biochem J. 1975 Mar;146(3):595–600. doi: 10.1042/bj1460595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnert K. D., Jahr H., Schmidt S., Hahn H. J., Zühlke H. Demonstration of insulin degradation by thiol-protein disulfide oxidoreductase (glutathione-insulin transhydrogenase) and proteinases of pancreatic islets. Biochim Biophys Acta. 1976 Feb 13;422(2):254–259. doi: 10.1016/0005-2744(76)90136-4. [DOI] [PubMed] [Google Scholar]
- Meda P. Lysosomes in normal pancreatic beta cells. Diabetologia. 1978 May;14(5):305–310. doi: 10.1007/BF01223021. [DOI] [PubMed] [Google Scholar]
- Noe B. D. Inhibition of islet prohormone to hormone conversion by incorporation of arginine and lysine analogs. J Biol Chem. 1981 May 25;256(10):4940–4946. [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Blix P. M., Rubenstein A. H., Kanazawa Y., Kosaka K., Tager H. S. A human proinsulin variant at arginine 65. Nature. 1981 Jun 25;291(5817):679–681. doi: 10.1038/291679a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J., Weibel E. R. The proliferative response of hepatic peroxidomes of neonatal rats to treatment with SU-13 437 (nafenopin). J Cell Biol. 1977 Sep;74(3):665–689. doi: 10.1083/jcb.74.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble E. R., Halban P. A., Wollheim C. B., Renold A. E. Functional differences between rat islets of ventral and dorsal pancreatic origin. J Clin Invest. 1982 Feb;69(2):405–413. doi: 10.1172/JCI110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]


